SARS-CoV-2 has shown to result in a spectrum of symptoms ranging from mild to severe respiratory failure, as well as life-threatening pneumonia and multi-organ failure. Reference Satriano, Scioscia and Cagnazzo1 A few case reports have been published showing that SARS-CoV-2 is also rarely associated with chylothorax. Reference Satriano, Scioscia and Cagnazzo1,Reference Bezirganoglu and Okur2 Chylothorax results from thoracic duct damage with chyle leakage from the lymphatic system into the pleural space. Reference McGrath, Blades and Anderson3,Reference Agrawal, Chaddha, Kaul, Desai, Gillaspie and Maldonado4 SARS-CoV-2 is known to cause a cytokine storm with consequent hyper-inflammation and an arterial and venous vasculopathy associated with a prothrombotic state. Reference Satriano, Scioscia and Cagnazzo1 The theory is that a prothrombotic state leads to venous circulation endothelial damage and then thoracic duct damage ultimately causing chylothorax. Reference Satriano, Scioscia and Cagnazzo1 Patients with Noonan syndrome are known to have chylothorax either congenitally or as a spontaneous event that can also occur after a surgical intervention, typically a cardiac repair. Reference C.H.A.N. and Ho5–Reference Fisher, Weiss, Michals, DuBrow, Hastrieter and Matalon8 Even though seemingly spontaneous, chylothorax in patients with Noonan syndrome is usually due to underlying lymphatic dysplasia in approximately 20% of the patients and involves the pulmonary and intestinal lymphatics. Reference Satriano, Scioscia and Cagnazzo1,Reference Roberts, Allanson, Tartaglia and Gelb9 We present a case where SARS-CoV-2 infection triggered the underlying tendency of a patient with Noonan syndrome to develop chylothorax.
Case report
An 11-year-old male with a history of Noonan syndrome and pulmonary valve replacement (5 years prior to presentation) presented with 7 days of worsening cough, nasal congestion, shortness of breath, fever, and right-sided chest pain. A clinical diagnosis of Noonan syndrome was made based on the facial and cardiac phenotype at birth. Eventually, genetic confirmation was obtained by panel testing at 18 months of age and showed a pathogenic variant in RIT1: c.246T > A (p. Phe82Leu), a variant previously reported in other patients with Noonan syndrome. Reference Aoki, Niihori and Banjo10 He reported that his chest pain worsened with coughing and taking deep breaths. He had a positive at-home SARS-CoV-2 rapid antigen test, which was obtained 5 days before admission. On physical exam, he was haemodynamically stable without any additional respiratory distress. On auscultation, he had decreased breath sounds in the right lower lobe and a grade 2 out of 6 crescendo-decrescendo systolic murmur at the left upper sternal border. SARS-CoV-2 real-time polymerase chain reaction done at the hospital was positive. Chest X-ray showed extensive right middle lobe consolidation and right pleural effusion. The differential diagnosis included viral pneumonia secondary to SARS-CoV-2 infection with concern for secondary bacterial pneumonia, empyema, chylothorax, pulmonary embolism, or pulmonary tuberculosis. He was started on ceftriaxone due to concern for secondary bacterial pneumonia.
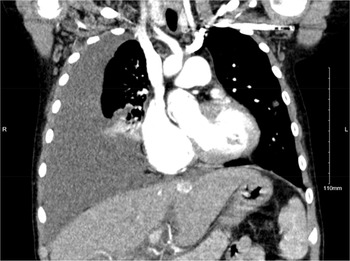
Infectious diseases, cardiology, and intensivist services were consulted. Chest CT with contrast showed a large right pleural effusion, right lower lobe opacity, and multiple bilateral pulmonary nodular opacities with ill-defined margins and ground-glass attenuation (Fig. 1). Other pertinent initial serum laboratory test results are as follows: normal procalcitonin (0.08 ng/mL, reference: <0.5 ng/mL), normal leukocytes (3.2 10*9/L, reference: 4–11 10*9/L), normal albumin (4.3 g/dL, reference: 3.5–5.0 g/dL), peripheral blood culture with no growth, and disseminated intravascular coagulation screen within normal limits.

Figure 1. Chest CT coronal view obtained before chest tube placement showing a large right pleural effusion with right lower lobe capacity.
A chest tube was then placed, which initially drained blood-tinged cloudy fluid and it turned milky over 3 days; this raised concern for chylothorax. Repeat chest CT with angiography showed decreased pleural effusion but with loculations for which the patient received pleural tissue plasminogen activator. Of note, chest CT with angiography did not show a pulmonary embolism. Serum laboratory tests performed within a day of chest tube placement showed pleural fluid to serum (pleural fluid: serum) total protein and lactate dehydrogenase ratios of 0.98 and 0.64, respectively. An echocardiogram was not concerning for an acute cardiac aetiology. It showed mild to moderate residual pulmonary stenosis with moderate to severe insufficiency (pulmonic valve mean gradient: 22 mmHg, peak, and peak gradient: 47 mmHg). The echocardiogram also noted a left anterior mitral valve leaflet with mild stenosis and no insufficiency, as well as systolic anterior motion of mitral valve. It also revealed normal left ventricle size with hyperdynamic function and mild subaortic thickening. There was no evidence of elevated right atrial pressures; as evidence by these findings, the right atrium was normal sized and the inferior vena cava was not dilated and had more than 50% collapse with respiration. The patient did not have a central line placement to permit a measurement of central venous pressure. Chest fluid cytology was negative for malignant cells. Chest fluid analysis also showed hypertriglyceridaemia (687 mg/dL). Chest fluid bacterial culture and acid-fast bacilli smear were negative. Pleural effusion met Light’s criteria for being exudative based on pleural fluid: Reference Light, Macgregor, Luchsinger and B.A.L.L.11 serum total protein and lactate dehydrogenase ratios being greater than 0.5 (value: 0.98) and 0.6 (value: 0.64), respectively. It was further determined to be chylothorax given pleural hypertriglyceridaemia and chest fluid drainage with milky appearance.
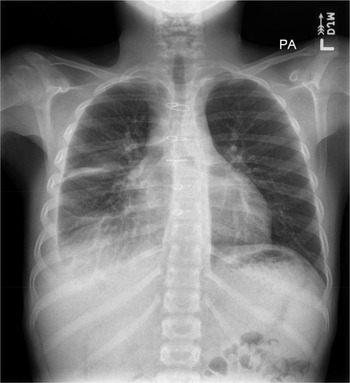
The patient then received tissue plasminogen activator for 3 days to break up the loculations. He was also started on a low-fat diet. Based on repeat chest X-rays, right pleural effusion showed interval improvement in the initial 7 days after chest tube placement and then remained stable. After 9 days, the chest tube was removed when a goal of less than 35-mL drainage within 12 hours was reached. On outpatient follow-up, the pleural effusion remained stable for the next 4 months, and it was labelled as moderate-sized on chest X-ray (Fig. 2), without any new signs of respiratory distress.

Figure 2. Follow-up chest X-ray in 4 months showing a moderate right-sized pleural effusion.
Discussion
Chylothorax is a rare type of pleural effusion in the paediatric population, wherein the lymphatic fluid is found in the pleural space. Chylothorax can be caused by several broad categories, including congenital chylothorax associated with lymphatic malformations or syndromes, traumatic chylothorax following invasive procedure or other trauma, chylothorax related to elevated central venous pressure, chylothorax associated with tumours, or other miscellaneous causes. Reference Dubin, King and Gallagher12–Reference Van Aerde, Campbell, Smyth, Lloyd and Bryan14 Chylothorax is associated with many syndromes, including Down syndrome, Noonan syndrome, and Turner syndrome. Reference C.H.A.N. and Ho5–Reference Fisher, Weiss, Michals, DuBrow, Hastrieter and Matalon8,Reference Yamamoto, Koeda and Tamura15 In Noonan syndrome, chylothorax may present as a congenital or spontaneous event and is also commonly seen in patients with Noonan syndrome after cardiothoracic surgical intervention. Reference C.H.A.N. and Ho5–Reference Fisher, Weiss, Michals, DuBrow, Hastrieter and Matalon8 In one study of paediatric cardiac surgery admissions, patients with Noonan syndrome were 90% more likely to have postoperative chylothorax. Reference Kriz, Flores, Villarreal, Bronicki and Loomba16 It is also interesting that our patient only had right-sided effusion, which may suggest a regional lymphatic abnormality or lymphatic system leak that occurred in the setting of SARS-CoV-2 infection. Recent literature using magnetic resonance lymphangiography has shown that the side of abnormal pleural perfusion does not always correlate with the side of chylothorax, which highlights the complex pathophysiology of lymphatic anatomy and physiology in patients with Noonan syndrome. Reference Pieper, Wagenpfeil and Henkel17
Chylothorax is identified by testing pleural fluid for triglycerides, like our case study. While not available for our patient, dynamic contrast magnetic resonance lymphangiography has been shown to be useful for patients with Noonan syndrome or chylous effusions to further assess the lymphatic system. Reference Pieper, Wagenpfeil and Henkel17–Reference Nakano, Rankin and Annam19 Assessment of abnormal pulmonary lymphatic perfusion via magnetic resonance lymphangiography allows the detection of pulmonary lymphangiectasia, which may be present in patient with chylothorax, although there have been patients with a chylothorax with normal underlying pleural lymphatic perfusion. Reference Pieper, Wagenpfeil and Henkel17 Once identified, chylothorax can be treated with a low-fat diet, diuresis, and, if respiratory status dictates, fluid drainage. Reference Agrawal, Chaddha, Kaul, Desai, Gillaspie and Maldonado4 Octreotide use is also practised at paediatric centres, Reference Agrawal, Chaddha, Kaul, Desai, Gillaspie and Maldonado4 although it was not used for our patient. In some circumstances of persistent chylothorax, lymphatic interventions may be indicated. In other cases, like Noonan syndrome, refractory chylothorax may be approached with a more targeted therapy like mitogen-activated protein kinase inhibition with trametinib. Reference Dori, Smith and Pinto18–Reference Gordon, Moore and Van Zanten20 Notably, due to the underlying complex lymphatic dysplasia and chylothorax pathophysiology among patients with Noonan syndrome, treatments such as lymphatic drainage, low-fat diet, and diuretics can be particularly ineffective, which is precisely why mitogen-activated protein kinase inhibition has been trialled in this patient population. Reference Nakano, Rankin and Annam19,Reference Gordon, Moore and Van Zanten20
SARS-CoV-2 is a novel coronavirus that has caused a broad clinical spectrum from asymptomatic to critical illness with acute respiratory distress syndrome and even death. SARS-CoV-2 infections are rarely associated with chylothorax. Reference Satriano, Scioscia and Cagnazzo1,Reference Bezirganoglu and Okur2 In one case report, a young infant with spontaneous chylothorax was subsequently diagnosed with acute SARS-CoV-2 infection and died at 2 months of age due to pulmonary hypertensive crisis and respiratory failure. Reference Bezirganoglu and Okur2 In another published case, a 78-year-old man diagnosed with acute SARVS-CoV-2 infection was found to have right pleural effusions later diagnosed as chylous. Reference Satriano, Scioscia and Cagnazzo1 He had developed partial thrombosis in the superior vena cava, and the distal right subclavian vein was thought to be the underlying cause of his chylothorax with resultant poor lymphatic drainage. Reference Satriano, Scioscia and Cagnazzo1
To our knowledge, this is the first reported case of a patient with Noonan syndrome having chylothorax related to acute infection with SARS-CoV-2. It is also important to note that our patient had normal estimated right atrial pressure based on an echocardiogram. While patients with Noonan syndrome can have chylothorax congenitally or spontaneously related to acute infectious processes or cardiothoracic surgery, it has not been associated previously with this specific infectious disease. Also curious in this case is that this patient has had cardiothoracic surgeries without prior history of chylothorax. There is no clear history of thrombus impairing lymphatic drainage, but the lymphatic malformations associated with Noonan syndrome coupled with systemic inflammation and cytokine storm shown to be underlying SARS-CoV-2 may have been enough to lead to chylothorax. This case demonstrates that while patients with Noonan syndrome are known to have a higher risk of chylothorax, the exact reason why a patient with Noonan syndrome will have a chylothorax in response to one process and not others is still not predictable.
Conclusion
Spontaneous chylothorax can occur in patients with a predisposition, including those with Noonan syndrome, during an acute respiratory infection such as SARS-CoV-2. An index of suspicion for chylous effusion should exist in Noonan syndrome patients with respiratory infections such as SARS CoV2 who develop effusions. Those not responding to traditional therapy that includes diuretic, low-fat diet, and octreotide should consider addition of mitogen-activated protein kinase inhibitor, particularly with ongoing need for chest tube drainage and respiratory support.
Acknowledgements
None.
Author contributions
Lubaina Ehsan and Jessica A. Thoe are contributed equally.
Drs. Ehsan and Fakhoury conceptualised the brief report.
Drs. Ehsan, Thoe, Parent, and Fakhoury were involved in the acquisition and interpretation of data for the report.
Drs. Ehsan, Thoe, Parent, and Fakhoury were involved in drafting the article and revising it critically for important intellectual content.
Drs. Ehsan, Thoe, Parent, and Fakhoury provided final approval of the version to be published.
Dr Fakhoury supervised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Financial support
None.
Competing interests
None.