Clinical recovery in severely brain-injured patients with disorder of consciousness (DoC) could be enhanced by amantadine, a dopaminergic agent.Reference Giacino, Whyte and Bagiella 1 – Reference Gosseries and Whyte 3 Unfortunately, severe side effects such as seizures or QT-interval enlargement on electrocardiogram (ECG) could limit the use of amantadine.Reference Estraneo, Pascarella, Moretta, Loreto and Trojano 2
Selegiline, a dopaminergic drug that acts as an irreversible selective monoamine oxidase type B (MAOB) inhibitor, is used in early Parkinson’s disease and is usually well tolerated at a low dosage (10 mg daily).Reference Macleod, Counsell, Ives and Stowe 4 More recently, selegiline has been shown also to exert clinical positive effects on apathy in patients with severe traumatic brain injuryReference Moutaouakil, Otmani, Fadel and Slassi 5 and on cognitive performance in stroke patients.Reference Bartolo, Zucchella, Capone, Sandrini and Pierelli 6
The aim of this open study was to assess possible clinical effects of selegiline administration in patients with prolonged DoC who showed contraindication or relevant side effects to amantadine administration. For this purpose, we screened all severely brain-injured patients with DoC consecutively admitted to the neurorehabilitation unit from May 2015 to June 2017. Inclusion criteria for this study were as follows: (i) clinical diagnosis of vegetative state (VS), minimally conscious state plus (MCS+) or minimally conscious state minus (MCS−) according to standardized clinical diagnostic criteria;Reference Schnakers, Edlow, Chatelle and Giacino 7 (ii) ≥6 months from traumatic, anoxic or vascular severe brain injury; (iii) age ≥18 years; and (iv) contraindication (e.g. severe cardiac arrhythmia, frequent seizures) or development of relevant side effects to amantadine administration. Exclusion criteria were as follows: (i) premorbid history of psychiatric or neurodegenerative diseases; (ii) changes in the clinical diagnosis on repeated clinical evaluations in the 4 weeks before study entry, assessed using the Italian version of Coma Recovery Scale-Revised (CRS-R);Reference Estraneo, Moretta and De Tanti 8 (iii) severe medical instability that might influence clinical diagnosis, such as severe respiratory insufficiency or abundant sporadic epileptiform activity or abundant/continuous periodic epileptiform dischargesReference Hirsch, LaRoche and Gaspard 9 on electroencephalogram (EEG) recordings; and (iv) contraindication to selegiline administration (e.g. renal or hepatic insufficiency; association with sympathomimetic drugs or opiates). 10
Selegiline was administered via percutaneous gastrostomy at a dose of 5 mg/day for 1 week, and then it was titrated to 10 mg/day for 9 weeks, for a total administration of 10 weeks.
Patients’ clinical diagnosis, consciousness level and arousal level were assessed using CRS-R at study entry, once a week during selegiline administration, at the end of 10 weeks of selegiline administration and during the month after drug discontinuation (also in case of selegiline withdrawal), by a neuropsychologist blind to selegiline administration. At the same time points, general clinical evaluation, standard electrocardiogram and routine biochemical blood analysis were performed for the detection of possible side effects. 10
During the entire study, no change in the administration of psychoactive medications (e.g. antiepileptic drugs) was made.
This study was conducted after approval of the Institutional Review Board and written informed consent was obtained from the patient’s legal guardians.
For exploratory purposes, we also compared demographic and clinical features in subgroups of patients as a function of change in clinical diagnosis (“improved” vs. “non-improved”), by non-parametric Mann-Whitney U-test for continuous variables and Fisher’s exact test for nominal variables, as appropriate. All analyses were performed with SPSS statistical package (version 19.0).
We screened 42 patients with prolonged DoC: 18 showed contraindications or relevant side effects to amantadine administration, but eight of them had to be excluded from the study (Figure 1). The enrolled ten patients (five females; mean age=42.4±19.1; time post onset=19±23.2 months) were in VS (n=6) or in MCS (n=4; MCS−=2; MCS+=2). Nine of the enrolled patients could not assume amantadine because of arrhythmias or seizures, whereas case 5 had to discontinue amantadine treatment because of psychomotor agitation (Table 1).
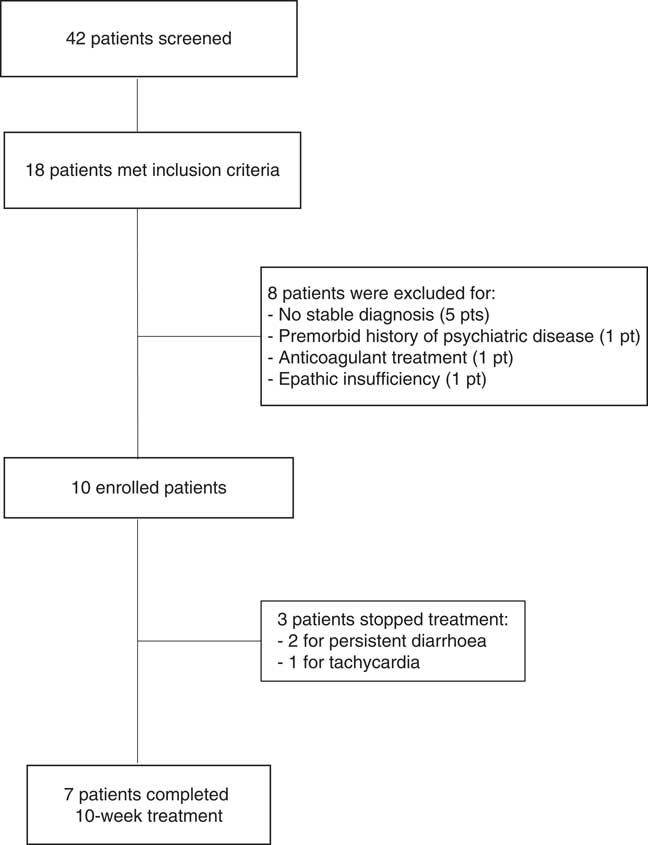
Figure 1 Flow chart summarizing patient selection.
Table 1 Demographic, anamnestic, neuroimaging findings and clinical characteristics, including diagnosis and CRS-R total score and subscores at study entry, during the treatment and at the 1-month follow-up

BG=basal ganglia lesion; CA=primary cardiac arrest (due to myocardial infarction or severe arrhythmia); CRS-R=coma recovery scale-revised; DA=diffuse atrophy; F=frontal lesion; IH=intracerebral hemorrhage; IVH=intraventricular hemorrhage; MCS−=minimally conscious state minus; MCS+=minimally conscious state plus; O=occipital lesion; OHI=open head injury; P=parietal lesion; RA = respiratory arrest; r/l/b=right/left/bilateral; SAH=subarachnoid hemorrhage; T=temporal lesion; VE=ventricular enlargement; VS=vegetative state.
Changes of clinical diagnosis and of Coma Recovery Scale-Revised total and sub-score with respect to baseline are highlighted in bold.
* Patient whose clinical diagnosis changed during the study period.
** Patient who showed adverse side effect and did not complete 10-week selegiline treatment.
In three enrolled patients selegiline administration was stopped because of protracted diarrhea (case 7 after 3 weeks and case 8 after 4 weeks of selegiline administration) or of persistent supraventricular tachycardia (case 4 after 4 weeks). One of these patients (case 4) emerged from VS to MCS+ after 2 weeks of treatment, and was still in MCS+ 4 weeks after selegiline withdrawal (see Appendix). In case 8, arousal increased after 3 weeks of treatment and persisted one month later (Table 1).
Seven patients completed the 10-week protocol without relevant side effects. Three patients with vascular etiology showed improvements in clinical diagnosis after 3-8 weeks of selegiline treatment. One patient in MCS+ (case 3) recovered full consciousness. Two patients evolved from MCS− to MCS+ (cases 1 and 2) and one of them (case 1) eventually emerged from MCS+ at 10 weeks of selegiline administration, as she regained functional communication (Table 1). The change in clinical diagnosis persisted during the 1-month follow-up (see Appendix).
Two patients in VS with vascular (case 10) or anoxic (case 9) etiology showed an increase in arousal level, as assessed by CRS-R, after 2 and 9 weeks of selegiline treatment, respectively, and until 1 month later.
One post-traumatic VS patient with ventriculo-peritoneal shunt malfunction showed a decrease in CRS-R total score.
The four patients who showed an improvement in clinical diagnosis did not significantly differ from patients who did not improve for age (51.7±18.6 vs. 36.2±18.2; p=0.89), CRS-R total score at study entry (7.7±2.5 vs. 6.7±2.3; p=0.31), and time post injury (9±2.9 vs. 26±28.7 months; p=0.98); also gender, etiology, neuroimaging findings or diagnosis at study entry did not differ in the two groups (all p>0.05), although relevant and persisting clinical improvements were observed in patients with vascular etiology only.
This open study suggested that selegiline might facilitate clinical recovery in patients with prolonged DoC. Indeed, we observed an improvement of clinical diagnosis in three patients who completed the 10-week selegiline treatment, and in one patient who discontinued the treatment because of possible side effects. All of them showed concurrent increase in arousal level (as assessed by CRS-R); clinical improvements persisted during the 4 weeks of selegiline washout. Three additional patients presented an increase in their arousal level (persisting after selegiline withdrawal), but without changes in clinical diagnosis.
These possible positive behavioral effects might remind the results from a recent randomized controlled study showing that 6-week selegiline administration was associated with persisting improvements of executive functions and attention in stroke patients, probably reflecting an improved global cortical arousal.Reference Bartolo, Zucchella, Capone, Sandrini and Pierelli 6 The persistence of improvements at the 1-month follow-up in our patients might be related to Selegiline irreversible inhibition of MAOB, which is thought to exert clinical effects to (at least) 30 days after withdrawal.Reference Fowler, Volkow and Logan 11
Selegiline enhances the release of dopamine and inhibits dopamine re-uptake of presynaptic dopamine receptors in basal ganglia, thus modulating dopaminergic fronto-striatal circuits. These circuits (the so-called “anterior mesial circuit”), which are likely to be involved in mediating arousal and attentional functions, have been proposed as the target of pharmacological treatment (amantadine) and deep brain stimulation in DoC.Reference Gosseries and Whyte 3
In this study no demographic or clinical variables were significantly associated with improvement in clinical diagnosis, although patients who improved tended to have shorter time post onset, and this finding might suggest caution in data interpretation. The present data are substantially consistent with a recent study on post-traumatic DoCReference Giacino, Whyte and Bagiella 1 in which the benefit of amantadine was independent from time since brain injury or clinical diagnosis (VS or MCS). However, our observation would support the effectiveness of dopaminergic drugs (such as selegiline) even in patients with DoC of non-traumatic etiology, who usually have poorer prognosis than post-traumatic patients, in keeping with two previous case reports showing positive effects (emergence from MCS) of dopaminergic drug (amantadine) in patients with anoxic or vascular etiology.Reference Estraneo, Pascarella, Moretta, Loreto and Trojano 2 Here, selegiline seemed to exert positive effects even in patients with chronic stabilized DoC (case 1), in whom late recovery is infrequent.Reference Estraneo, Moretta, Loreto, Santoro and Trojano 12 It is worth mentioning that prudentially we ended treatment in three patients for possible side effects (protracted diarrhea and persistent supraventricular tachycardia); although these clinical conditions are not frequently associated with selegiline, 10 they are quite frequent in patients with DoC, and occurred several weeks after starting selegiline.
This study has several limitations. We enrolled a small number of patients, which was not sufficient to draw reliable inferences on the effect of selegiline and on the features most likely associated with positive responses to the drug. Moreover, our study lacked a control group, although it is important to stress that our selection criteria aimed at enrolling patients in stabilized clinical status at least 6 months after onset, and at excluding patients with obvious trends toward spontaneous improvement on repeated behavioral evaluations in the 4 weeks before study entry. Last, neuroimaging performed in our patients did not specifically assess the structural and functional integrity of cerebral dopaminergic networks that could be the target of selegiline action. For instance, it has been suggested that a relative structural integrity of the dopaminergic circuits and sufficient endogenous production of dopamine in the neuronal network involved in recovery of consciousnessReference Gosseries and Whyte 3 could serve as eligibility criteria for dopaminergic treatment. However, this open pilot study aimed at ascertaining possible effectiveness of selegiline without a priori selection based on neurofunctional criteria for positive response to dopaminergic treatment.
In conclusion, our pilot study suggested that selegiline might be a relatively safe option to enhance arousal and promote recovery of consciousness in patients with DoC, and in patients with contraindications for or relevant side effects due to amantadine administration (about 40% of the patients screened for our study). This suggestion should be verified by systematic, formally controlled trials on larger patient samples followed up for longer time.Reference Gosseries and Whyte 3
Acknowledgments
The authors are profoundly indebted to all patients and their relatives for their participation in the project.
Disclosures
The authors have nothing to disclose.
Statement of Authorship
OM contributed to study concept and design and analysis and interpretation of data; LT performed acquisition of data, statistical analysis and interpretation of data; VL and PM were involved in acquisition of data and analysis and interpretation of data; AE contributed to study concept and design and critical revision of the manuscript for intellectual content.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.315






