Introduction
Guillain–Barré syndrome (GBS) is the most frequent acute polyradiculoneuropathy and the first cause of acute flaccid paralysis worldwide. Reference Shang, Zhu, Baker, Feng, Zhou and Zhang1–Reference Martic, Bozovic and Berisavac4 It is characterized by varying degrees of limb and cranial nerve-innervated muscles weakness associated with decreased or absent muscle reflexes. Sensory and autonomic symptoms can also be present. Reference Rajabally and Uncini5,Reference Panda and Sharawat6 Incidence varies from 0.62 to 2.66 per 100,000 person-years, depending on the population and age group. Reference Sejvar, Baughman, Wise and Morgan7 A 70% of all GBS cases have the antecedent of systemic infections, such as respiratory and gastrointestinal infections. Reference Domínguez-Moreno, Tolosa-Tort and Patiño-Tamez8–Reference de Andrade da Silva, Cremaschi, Rebello Pinho, de Oliveira and Coelho15
The GBS neurophysiological spectrum includes acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and acute motor sensory axonal neuropathy (AMSAN). Reference Hadden, Cornblath and Hughes16,Reference Liu, Wang and Huang17 A less frequent variant is the Fisher syndrome (also known as the Miller Fisher syndrome) that is characterized by ataxia, areflexia, and ophthalmoplegia. Reference Prada, Massa and Salerno18
The in-hospital case fatality rate (CFR) of GBS varies from 1.61% to 15%. Reference Domínguez-Moreno, Tolosa-Tort and Patiño-Tamez8–Reference de Andrade da Silva, Cremaschi, Rebello Pinho, de Oliveira and Coelho15,Reference Korinthenberg and Sejvar19,Reference Walteros, Soares and Styczynski20,Reference Berisavac, Arsenijevic and Bozovic21 In Mexico the in-hospital GBS-associated CFR is nowadays deemed high. Reference Domínguez-Moreno, Tolosa-Tort and Patiño-Tamez8 The unpredictable clinical evolution and potential life-threatening complications often require admission to the intensive care unit. Respiratory failure requiring invasive mechanical ventilation (IMV) is a common ominous short-term complication with a reported incidence of about 20–35%. Reference Hadden, Cornblath and Hughes16,Reference Lawn, Fletcher, Henderson, Wolter and Wijdicks22–Reference Umer, Nisa, Kumari, Abbas, Mahesar and Shahbaz27 Early prediction of IMV is important to enable clinicians tailoring supportive care and individualized treatment. To our knowledge, there is no published study dedicated to determining the predictors of IMV in a Latin American population. Reference Green, Baker and Subramaniam28
Methods
Patients and Study Design
The Research Ethics Committee approved this retrospective observational study. Signed informed consent was obtained from the patients upon hospitalization. We retrospectively reviewed all the existing medical records and neurophysiologic studies of adult patients admitted with a GBS diagnosis according to Asbury and Cornblath’s diagnostic criteria Reference Asbury and Cornblath29 between January 1999 and March 2020 at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, a tertiary referral hospital in Mexico City. Patients with other specific types of acute or chronic polyneuropathy (i.e., diabetic neuropathy, neuropathies associated with drugs or industrial substances, poliomyelitis, polyneuropathy associated with HIV infection, porphyria, and other), and without electrodiagnostic procedures performed or available were excluded. We also excluded cases with equivocal neurophysiological classification of GBS subtypes for whom no serial tests were performed to attain final subtyping. This was of particular importance given that one of the main interests of this study was to assess whether the axonal variants enable a poor short-term respiratory failure prognosis. Members of the Department of Neurology examined every patient and ascertained diagnosis. Electrophysiological diagnostic criteria were met in all cases. Reference Hadden, Cornblath and Hughes16,Reference Asbury and Cornblath29
Data Collection
We collected information derived upon hospital arrival of GBS patients, as well as the clinical and paraclinical information that was obtained first in the following days after hospital arrival. Demographic and clinical features analyzed were age, sex, history of antecedent infections (e.g., upper respiratory tract infection and gastrointestinal infection), time from motor symptom onset to admission (the onset of GBS was defined as the occurrence of motor symptoms), onset of limb weakness, clinical severity assessed by the GBS disability score (scale ranging from 0 to 6, where 0 = healthy state and 6 = death), Reference Hughes, Newsom-Davis, Perkin and Pierce30 the modified Erasmus GBS outcome score (mEGOS, scale ranging from 0 to 9, with higher scores meaning a worse functional prognosis) at hospital admission, Reference van Koningsveld, Steyerberg, Hughes, Swan, van Doorn and Jacobs31 Erasmus GBS respiratory insufficiency score (EGRIS, scale ranging from 0 to 7, with higher scores meaning a worse respiratory failure prognosis), Reference Walgaard, Lingsma and Ruts32 Medical Research Council (MRC) sum score (scale ranging from 0 to 60, where 0 = tetraplegia and 60 = normal strength), presence of hyporeflexia or areflexia, cranial nerves involvement, inability to lift the head and/or bulbar dysfunction, sensitive symptoms such as neuropathic pain or paresthesias, autonomic dysfunction (fluctuating blood pressure, spontaneous severe bradycardia or spontaneous tachycardia), presence of protein-cytologic dissociation in cerebrospinal fluid (CSF) (defined as elevated protein in CSF without abnormal leukocytes count), total number of days for which ventilation support was needed, and hospital stay in days and outcome. Electrophysiological information was classified according to definitions of Hadden et al. as primary demyelinating, primary axonal, or equivocal. Reference Hadden, Cornblath and Hughes16 Respiratory failure was defined as a need for IMV within the first 30 days of admission. We also registered for analysis the treatment with intravenous immunoglobulin (IVIg), plasma exchange (PLEX), or supportive management.
Statistical Analysis
Categorical data are presented as relative frequencies in the form of proportions, while continuous data are presented as means with standard deviations (SD) or medians with their respective interquartile ranges (IQR), depending on the distribution. The Kolmogorov–Smirnov test was performed to assess the equality of continuous probability distributions. Pearson chi-square or Fisher exact tests are used to compare proportions in nominal variables. To compare the distribution of quantitative variables between two groups, Student t test or Mann–Whitney U test was performed in parametric and non-parametric variables, respectively. To find independent predictors of respiratory failure with need for IMV, multivariate analyses were constructed using the forward stepwise logistic regression models. A selection step process was performed with a p set at <0.1 in bivariate analyses. Adjusted odds ratios (OR) with 95% CIs are provided. We calculated corrected OR to approximate to the actual relative risks, with the equation proposed by Zhang and Yu Reference Zhang and Yu33 as follows: Corrected OR = Multivariate OR/[(1 − Incidence of the outcome in the nonexposed group) + (Incidence of the outcome in the nonexposed group * multivariate OR)]. The fitness of the model was assessed with the Hosmer–Lemeshow goodness-of-fit test, which was considered as reliable if p > 0.2. All p values are calculated two-sided and considered significant when p < 0.05. SPSS version 24.0 for iOS (IBM Inc., USA) was used for all calculations.
Results
After excluding cases with no neurophysiological tests performed or available (n = 4), patients with equivocal neurophysiological classification (n = 7), or incomplete clinical information (n = 3), a total of 86 adult patients were analyzed (Table 1). The mean age at diagnosis was 42.5 ± 19.8 years (median: 40, IQR: 26–53.5 years), with a male preponderance (60.5%, male-to-female ratio: 1.53). Most patients (65%) had a clear infectious antecedent preceding the onset of the weakness within the last 2 weeks, being the most frequent a gastrointestinal infection (34% of the total or 40.6% among patients with preceding infection).
Table 1: Characteristics of the population hospitalized with Guillain–Barré syndrome
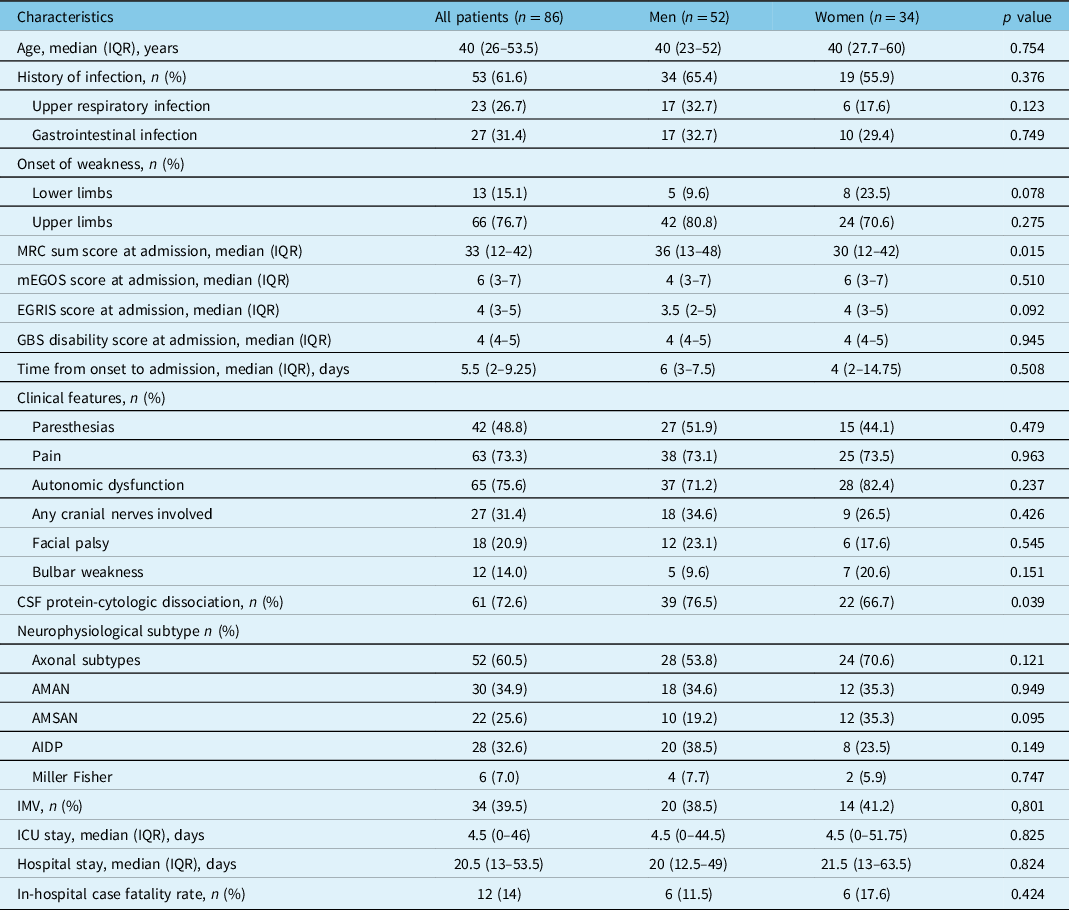
AIDP = acute inflammatory demyelinating polyneuropathy; AMAN = acute motor axonal neuropathy; AMSAN = acute motor sensory axonal neuropathy; CSF = cerebrospinal fluid; EGRIS = Erasmus GBS respiratory insufficiency score; IMV = invasive mechanical ventilation; ICU = intensive care unit; IQR = interquartile range; mEGOS = modified Erasmus Guillain–Barré syndrome outcome score; MRC = Medical Research Council.
At admission, 38% of patients had a MRC sum score <30 (32.7% vs. 47.1%, in men and women, respectively p = 0.180), with a higher median score among women, compared with men. GBS disability score >3 at hospital arrival occurred in 77.9% of patients, with mEGOS >6 in 62.8%, EGRIS >4 in 34.9%, and a MRC sum score <30 in 38.4%. The median (IQR) time elapsed since symptoms onset to hospital admission was 5.5 (2–9.5) days, from onset to CSF analysis 7 (5–12) days, and from onset to neurophysiological assessment 12 (7–12) days. There was a predominance (60.5%) of axonal GBS subtypes (i.e., AMAN/AMSAN), with AMAN being the most prevalent (34.9%). Notably, 15.1% of patients had a GBS onset in upper limbs. There was also a high frequency of dysautonomia (75.6%) and pain (73.3%).
In all, 74 (86%) received either IVIg (9.3%) or PLEX (74.4%). The median (IQR) time from onset to treatment was 11 (6–14) days. A total of 34 (39.5%) patients developed respiratory failure and received IMV, with a median (IQR) duration of 48.5 (31.75–89) days. Even when AMAN was the most frequent GBS variant, patients with AMSAN had the highest relative frequency of IMV (Figure 1).
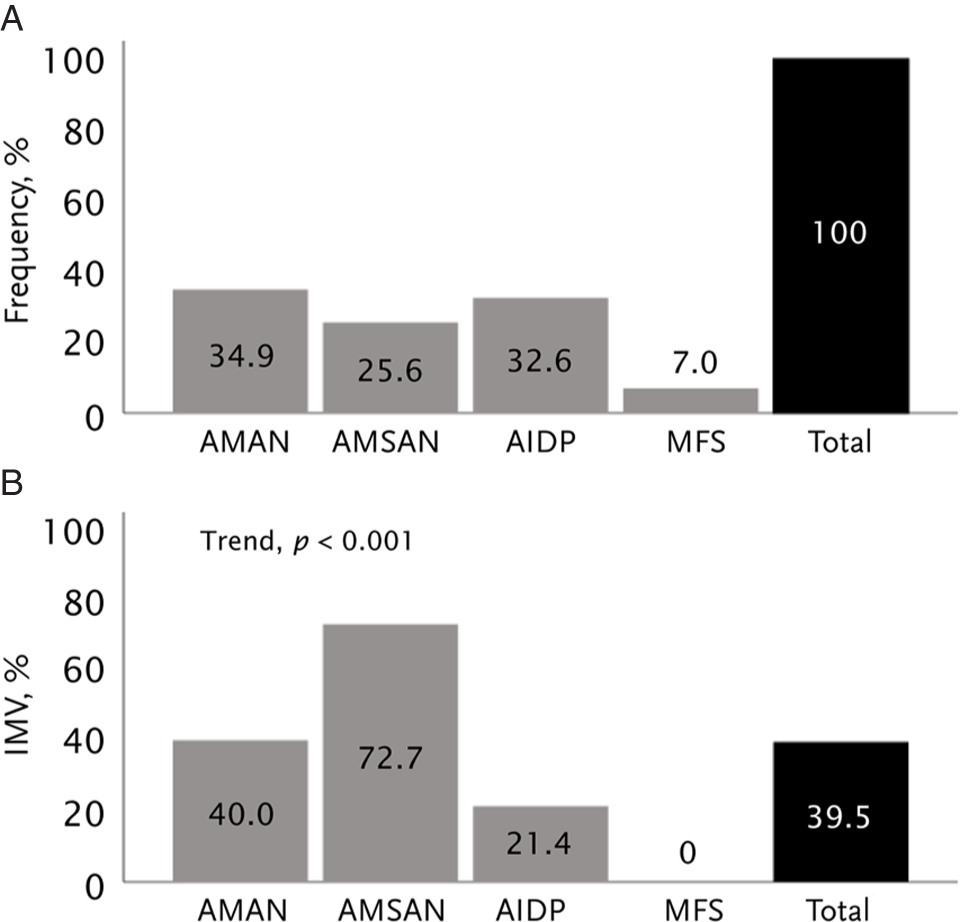
Figure 1: Relative frequency of electrophysiological subtypes among the 86 patients with Guillain–Barré syndrome (A). Rate of invasive mechanical ventilation according to electrophysiological subtypes (B). AIDP = acute inflammatory demyelinating polyneuropathy; AMAN = acute motor axonal neuropathy; AMSAN = acute motor sensory axonal neuropathy; MFS = Miller Fisher syndrome.
The median (IQR) total hospital stay was 20.5 (13–53.5) days, with an in-hospital CFR of 14% (n = 12), 9.3% of these deaths due to sepsis or septic shock. In bivariate analyses to select potential variables associated with the occurrence of respiratory failure and the need for IMV, MRC sum score, mEGOS, EGRIS, GBS disability score, onset of weakness, dysautonomia, bulbar weakness, and axonal GBS variants were factors potentially associated (Table 2). Two distinctive multivariable models were constructed to find independent predictors for IMV, one without considering mEGOS, EGRIS, or the GBS disability score, and the other entering these prognostic systems (Figure 2). The model without published scores yielded the time since GBS clinical features onset to hospital admission <15 days, axonal GBS variants, MRC sum score <30, and bulbar weakness. The second model including grading scales yielded the onset in lower limbs, EGRIS >4, and autonomic dysfunction as independent predictors for respiratory failure.
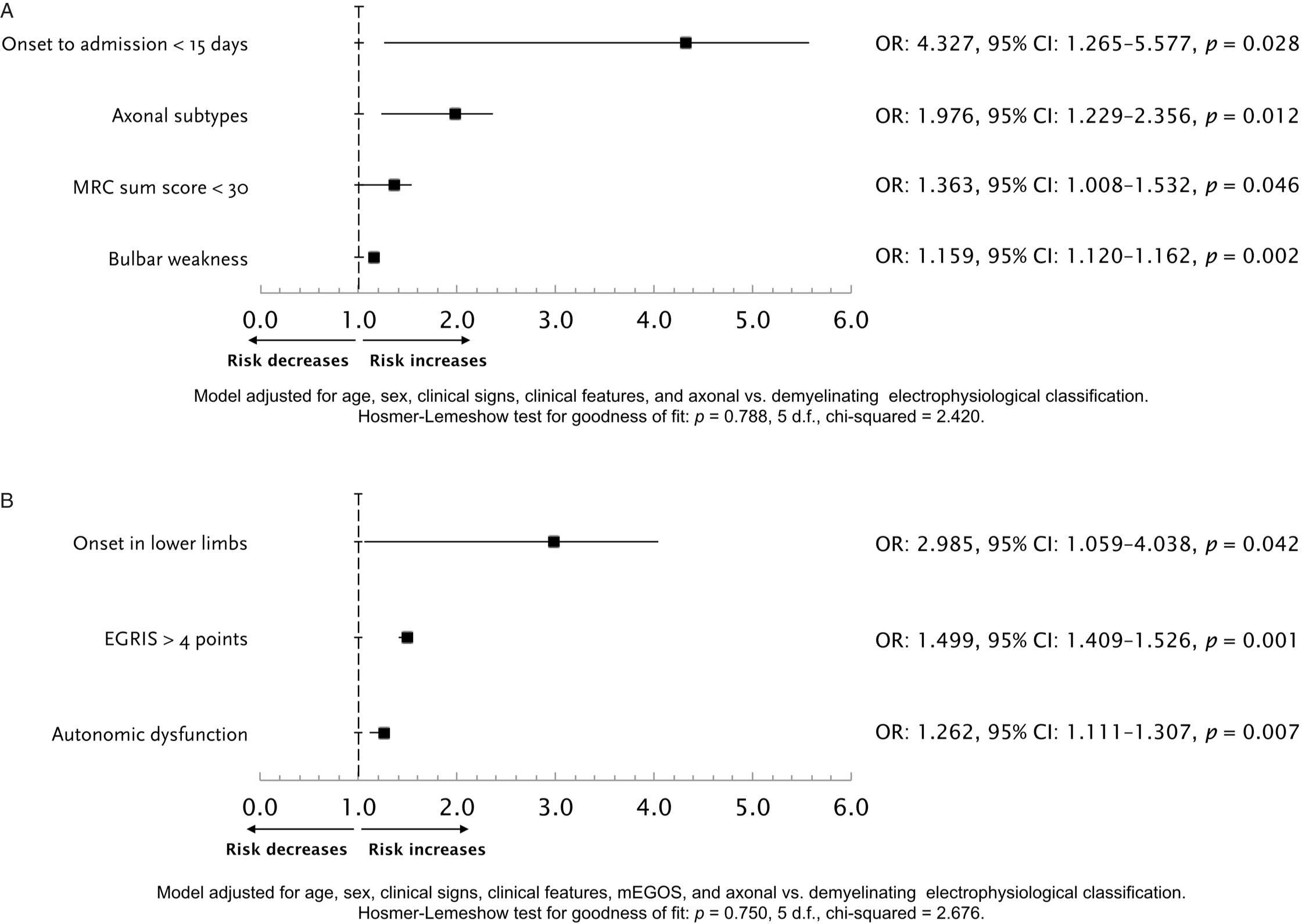
Figure 2: Logistic regression model for the prediction of respiratory failure, without including mEGOS or EGRIS as potential independent variables (A). Logistic regression model for the prediction of respiratory failure, including mEGOS or EGRIS as predictors (B). EGRIS = Erasmus GBS respiratory insufficiency score; MRC = Medical Research Council.
Table 2: Differences between patients with or without invasive mechanical ventilation (IMV)
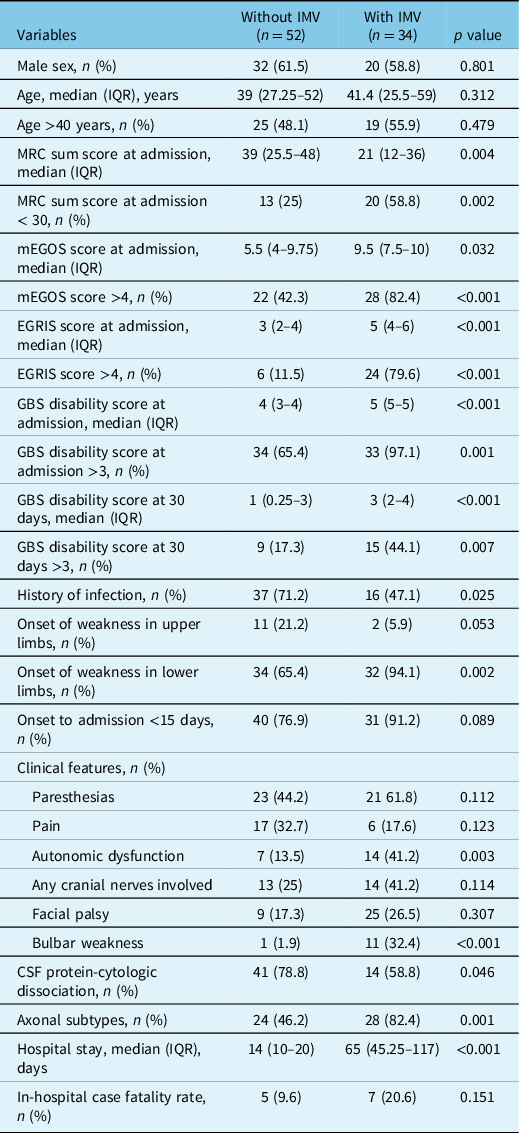
CSF = cerebrospinal fluid; EGRIS = Erasmus GBS respiratory insufficiency score; IMV = invasive mechanical ventilation; ICU = intensive care unit; IQR = interquartile range; mEGOS = modified Erasmus Guillain–Barré syndrome outcome score; MRC = Medical Research Council.
Discussion
In this data set with a predominance of axonal GBS electrophysiological subtypes, EGRIS was a relevant clinical tool to predict the need for IMV, irrespective of IVIg or PLEX treatments. Most published papers regarding diagnosis, prognosis, and treatment of patients with GBS derive from either exclusive or predominant cohorts with AIDP, Reference Leonhard, Mandarakas and Gondim2,Reference Hadden, Cornblath and Hughes16,Reference Hughes, Newsom-Davis, Perkin and Pierce30–Reference Walgaard, Lingsma and Ruts32 which might be a limitation when extrapolating results in Asian or Latin American populations regarding treatment response and outcomes. Reference Choi, Hong, Kim, Shin and Sung9–Reference de Andrade da Silva, Cremaschi, Rebello Pinho, de Oliveira and Coelho15,Reference Shalman, Savir and Mechnik Steen34–Reference Tiwari, Alam and Kanta36 Therefore, there is an urgent need to study whether treatment responses and outcomes apply in the same way as it has been described in patients with the classical AIDP. In our present report, we demonstrate that the clinical presentation is of utmost importance when classifying the risk of respiratory failure in GBS patients with a high frequency of AMAN and AMSAN variants. When analyzing the predictors with separate variables without including EGRIS, mEGOS, and GBS disability score at hospital admission, clinical information that can be obtained upon Emergency Department arrival (i.e., days elapsed from onset to admission, strength assessment, and bulbar weakness), and electrophysiological data (i.e., axonal variants), conform a set of early predictors of the need for IMV. When predicting scores were included in the model, EGRIS emerged as predictor, but other characteristics such as autonomic dysfunction and onset of weakness were also important. The fact that muscle strength, time from onset to admission, and bulbar weakness disappeared in the second model is predictable since these are variables included in EGRIS, but the disappearing of AMAN/AMSAN as prognosticator may be due to the relative power of a composite variable (i.e., EGRIS) that may also reflect the phenomenology of the axonal loss, such as skeletal muscle and bulbar weakness. It is also noticeable the high frequency of dysautonomia, taking into account the high frequency of axonal variants, since autonomic dysfunction has been considered more prevalent in AIDP. Reference Shahrizaila, Lehmann and Kuwabara3 This has also been challenged in a recent Mexican study. Reference López-Hernández, Colunga-Lozano and Garcia-Trejo10 This might also contribute to the high CFR observed in this study, together with the high proportion of AMAN/AMSAN and a high latency since symptoms onset to advanced medical care. During the study time, no differences were found with respect to CFR, and thus, no plausible effect on changes in access to technology or the quality of care can be addressed.
Respiratory failure of neuromuscular origin and access to immunotherapy is one of the major predictors influencing morbidity and mortality, Reference Shang, Zhu, Baker, Feng, Zhou and Zhang1,Reference Leonhard, Mandarakas and Gondim2,Reference Beydoun, Beydoun, Hossain, Zonderman and Eid37,Reference Ganesh, Reis, Varma, Patry and Cooke38 although GBS can also be self-limiting and the majority of patients recover completely. However, respiratory failure requiring IMV is a serious complication of GBS with an incidence of 14.8–50.9%. Reference Wu, Li and Zhang26,Reference Durand, Porcher and Orlikowski39 The incidence of respiratory failure requiring IMV in our study was roughly 40%, similar with the incidence reported in India. Reference Zifko, Chen, Remtulla, Hahn, Koopman and Bolton40 In North America and Europe, < 10% of patients have axonal subtypes of GBS, Reference Hadden, Cornblath and Hughes16 which contrasts with the present relative frequency of 60% of the cases. This frequency appears to be more comparable to what was observed in non-Western populations such as Central and South America, and Asian countries, in that axonal subtypes account for 30–56% of cases. Reference Choi, Hong, Kim, Shin and Sung9–Reference de Andrade da Silva, Cremaschi, Rebello Pinho, de Oliveira and Coelho15,Reference Shalman, Savir and Mechnik Steen34–Reference Tiwari, Alam and Kanta36
Despite not being an independent predictor when including in the multivariate model to published grading scales, the axonal variants had more cases of respiratory failure than the classical GBS, AIDP. This finding contrasts with other reports that patients with AIDP needed IMV significantly more frequently than those without AMAN/AMSAN. Reference Visser, van der Meché and Meulstee41 Similar to ours, a retrospective study in a tertiary care center in Pakistan found that patients with the axonal variant had significantly more strength impairment at hospital presentation, with the lowest scores for arm and leg strength at presentation, compared with AIDP. Reference Hadden, Cornblath and Hughes16 This was also reported in a recent Mexican report. Reference López-Hernández, Colunga-Lozano and Garcia-Trejo10
Our study confirms others’ findings that respiratory failure in GBS is associated with shorter time from onset to admission. Reference Lawn, Fletcher, Henderson, Wolter and Wijdicks22,Reference Sharshar, Chevret, Bourdain and Raphaël23,Reference Walgaard, Lingsma and Ruts32,Reference Durand, Porcher and Orlikowski39 This independent factor, included in EGRIS, may be directly linked to the requirement for IMV due to a rapid clinical worsening. Other clinical presentations have been identified as predictors for IMV in other studies. These include facial palsy Reference Lawn, Fletcher, Henderson, Wolter and Wijdicks22,Reference Sharshar, Chevret, Bourdain and Raphaël23 and simultaneous onset of motor weakness in the four limbs as the initial symptom. Successful management of GBS mandates anticipation of respiratory failure and timely intervention to reduce the risk of complications while improving the patients’ outcome.
This study has limitations, such as being a retrospective analysis of a relatively small number of patients, as well as the fact that it was performed in a single referral medical center, which partly limits the generalization of the study results. Also, in our study no data were available on the inability to lift the head, Reference Shafqat, Khealani, Awan and Abedin12 decreased vital capacity, Reference Shafqat, Khealani, Awan and Abedin12,Reference Lawn, Fletcher, Henderson, Wolter and Wijdicks22 abnormalities in diaphragmatic needle electromyography, Reference Zifko, Chen, Remtulla, Hahn, Koopman and Bolton40 and recent cytomegalovirus infection, Reference Visser, van der Meché and Meulstee41 all previously reported to be predictors of respiratory insufficiency. Another limitation is the lack of biomarkers and serial electrodiagnostic studies, which might improve the predictive models. Nevertheless, we think that our results can be easily extrapolated to other regions of the world with limited resources in which axonal GBS variants are seen with a high frequency.
Conclusion
In summary, our results suggest that EGRIS retains its ability as a good prognosticator of the need for IMV in GBS patients with axonal electrophysiological subtypes as the predominant variant, but our findings also suggest that in these particular populations, other early clinical data should also be considered. We wish to suggest that a bigger research effort should be made to perform a multinational study aimed to assess patients’ outcomes and response to treatments for the axonal GBS subtypes.
Acknowledgements
The authors are thankful to the healthcare personnel of the Intensive Care Unit, Emergency Department, Internal Medicine, and Neurology of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán for the full commitment in caring for patients with neuromuscular disorders.
Funding
None.
Conflict of Interest
Dr Erwin Chiquete has received speaker honoraria from LFB, CSL Behring, Lilly, Novartis, Bayer, Merck, Ferrer, ApoPharma, Asopharma, and Silanes. The rest of the authors have no competing interests to declare.
Statement of Ethics
This study is consistent with the Declaration of Helsinki. This study’s protocol was approved by the Ethics Committee at the First Affiliated Hospital of Harbin Medical University (No. 201314). Every patient who participated in the experiment signed informed consent before entering the experiment. Informed consent for publication was obtained from the patients.
Statement of Authorship
Conceptualization: Anaclara Michel-Chávez, Erwin Chiquete, Alfonso Gulías-Herrero, Diego Luis Carrillo-Pérez. Data curation: Anaclara Michel-Chávez, Diego Luis Carrillo-Pérez, José Aceves-Buendía, Eduardo Ruiz-Ruiz, Tatiana Bliskunova, Jennefer Portillo-Valle, Emmanuel Aguilar-Salas, Rafael Cobilt-Catana, Jorge Alberto Ortiz-Quezada, Julio Macías-Gallardo. Formal analysis: Erwin Chiquete, Emmanuel Aguilar-Salas, Salvador Durán-Coyote, Anaclara Michel-Chávez, Antonio Olivas-Martínez. Supervision: Alfonso Gulías-Herrero, Bruno Estañol, Guillermo García-Ramos, Carlos Cantú-Brito. Writing – original draft: Anaclara Michel-Chávez, Alfonso Gulías-Herrero, Diego Luis Carrillo-Pérez. Writing – review & editing: Erwin Chiquete, José Aceves-Buendía, Eduardo Ruiz-Ruiz, Salvador Durán-Coyote, Guillermo García-Ramos, Rafael Cobilt-Catana, Jorge Alberto Ortiz-Quezada, Carlos Cantú-Brito.
Data Availability Statement
The data set generated for the present analysis is available from the corresponding author on reasonable request.








