An 18-year-old female presented to the emergency department after her second generalized tonic-clonic seizure in the preceding 3 months. A left parietal convexity enhancing mass was found on computed tomography (CT), and magnetic resonance imaging (MRI) revealed T1 hyperintensity further described below (Figure 1A-E). Angiography favored the mass to be extra-axial and meningioma became the leading diagnostic consideration (Figure 1F). External carotid artery feeders were embolized intraoperatively (Figure 1G), followed by gross total resection of an extra-axial mass. Pathological diagnosis was melanocytoma (Figure 2). Five-month and 1-year follow-up MRIs showed no recurrence. Seizures had not recurred with phenytoin and clobazam. The patient declined adjuvant radiation therapy.
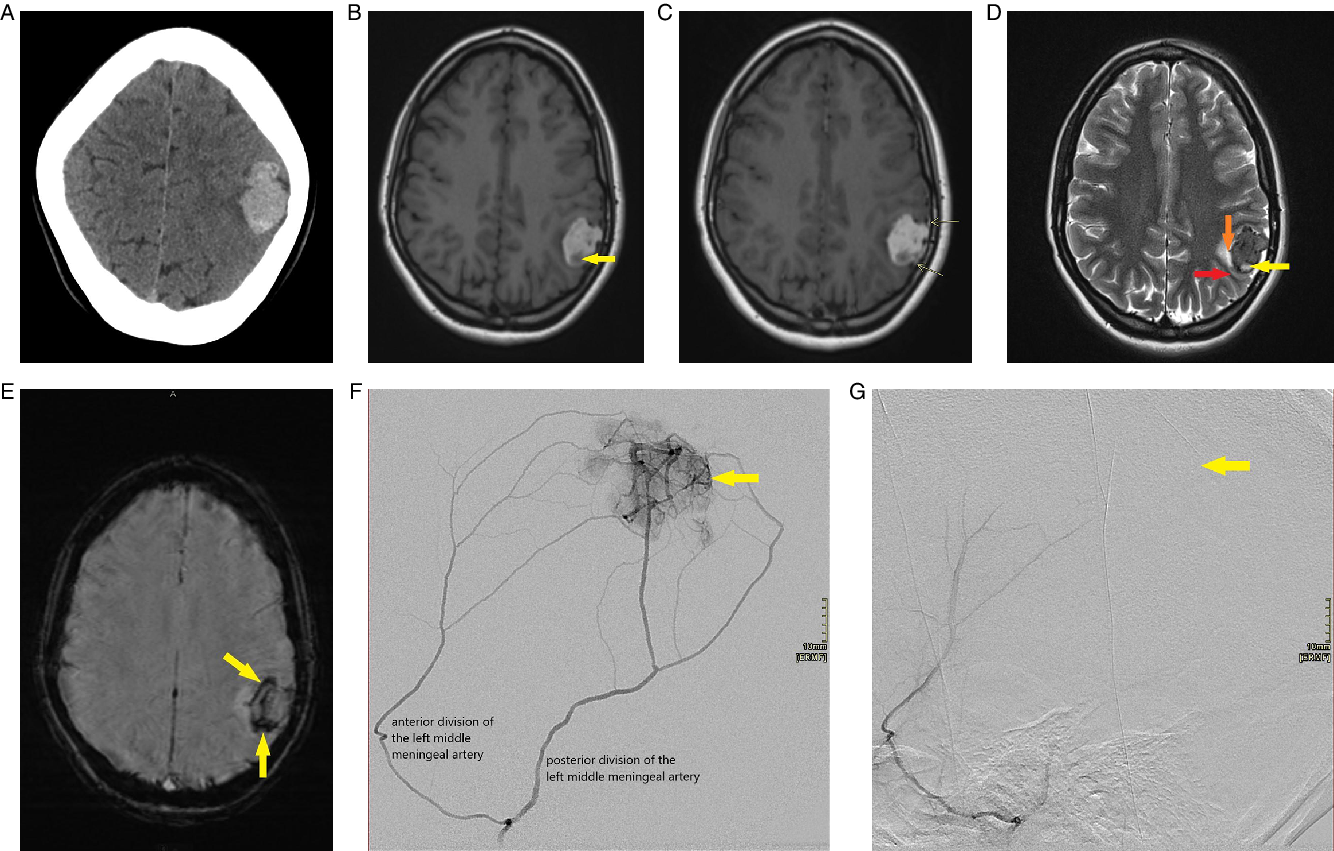
Figure 1: (A-G) Radiologic appearance of meningeal melanocytoma. (A) Axial non-contrast brain CT image demonstrates a lobulated homogenously dense left lateral parietal region mass with minimal adjacent hypodensity and mild associated local mass effect. (B) Axial T1-weighted brain MR image without contrast demonstrates a lobulated left lateral parietal region mass which is primarily hyperintense (indicating melanin as seen on histology) with a small hypointense component at the posterior aspect (arrow) representing intratumoral hemorrhage as seen on gross pathology and histology. (C) Axial T1-weighted brain MR image following contrast administration demonstrates a lobulated predominantly hyperintense left parietal region mass. There are tiny foci of enhancement within the mass. Arrows point to mild thickening and enhancement of the adjacent dura, which correlates to gross pathology. (D) Axial T2-weighted brain MR image demonstrates a lobulated hypointense left lateral parietal region mass with a small hyperintense component at the posterior aspect (yellow arrow) representing intratumoral hemorrhage as seen on gross pathology and histology. White matter buckling (red arrow) and a CSF cleft (orange arrow) suggest its extra-axial location. (E) Axial susceptibility-weighted brain MR image demonstrates significant susceptibility artifact (arrows) within the mass, in particular, at the posterior aspect and along the periphery correlating with hemorrhage as seen on pathology. (F) Selective angiogram of the left middle meningeal artery shows the tumoral blush (arrow) supplied by both the anterior and posterior divisions. (G) Selective angiogram post polyvinyl alcohol (PVA) particle embolization of the posterior and anterior divisions of the left middle meningeal artery showing lack of tumoral blush (arrow).

Figure 2: (A-H): Gross and microscopic appearance of meningeal melanocytoma. (A) Gross sections demonstrating the tumor to be lobulated with abundant dark pigment. Meninges are attached (white arrow), and the interior aspect is filled with dark red-brown gelatinous material (blue arrow), possibly hemorrhage (Bar = 1 cm). (B-D) Microscopic morphology of the well-circumscribed tumor stained with hematoxylin and eosin, demonstrating large areas of hemorrhage (B, Bar = 100 µm), lobulated and pseudopapillary architecture of cells with intravascular foreign material, likely PVA particles from embolization (C, Bar = 100 µm). The tumor cells possess well-defined borders and ample cytoplasm containing fine, light brown pigment, with large round nuclei containing prominent nucleoli (D, Bar = 20 µm). (E-G) The pigment is shown to be melanin owing to its negativity with Perls Prussian Blue staining (E, Bar = 20 µm), disappearance with melanin bleach (F, Bar = 20 µm), and positive black staining with Fontana-Masson (G, Bar = 20 µm). (H) The tumor cells show strong positivity with immunohistochemistry for HMB45 (red indicator) (Bar = 20 µm).
Meningeal melanocytomas are rare and typically benign neoplasms derived from leptomeningeal melanocytes.Reference Smith, Rushing and Smirniotopoulos1 They most often occur in the posterior fossa, Meckel’s cave, or cervical and thoracic spinal canal. Supratentorial lesions are rare.Reference Smith, Rushing and Smirniotopoulos1,Reference Rahimi-Movaghar and Karimi2 Few examples of convexity meningeal melanocytomas have been documented in the literature.Reference Smith, Rushing and Smirniotopoulos1,Reference Beseoglu, Knobbe, Reifenberger, Steiger and Stummer3,Reference Das, Nair and Jaiswal4 Annual incidence of meningeal melanocytoma is 1 per 10 million, and females have been reported to be affected twice as frequently.Reference Jellinger, Böck and Brenner5 Peak presentation is in the fourth and fifth decades, and it very rarely occurs in children.Reference Smith, Rushing and Smirniotopoulos1,Reference O’Brien, Crooks and Mallucci6 Depending on the location of the tumor, patients may present with myelopathy, radiculopathy, cranial nerve deficits, hydrocephalus, or, as in our case, seizures.Reference Bydon, Gutierrez and Mahmood7
Preoperative diagnosis of meningeal melanocytoma often proves difficult as the clinical and radiological features of the tumor are nonspecific. CT typically demonstrates a well-defined, isodense to hyperdense, homogenous, contrast-enhancing mass (Figure 1A). The MRI appearance of meningeal melanocytomas is variable, depending on the amount of melanin content present, which when abundant produces characteristic T1 hyperintensity.Reference O’Brien, Crooks and Mallucci6 Intratumoral hemorrhage may also be present (Figure 1B). Signal characteristics include isointensity or hyperintensity on T1-weighted images (Figure 1B), isointensity or hypointensity on T2-weighted images (Figure 1D), homogeneous or heterogeneous contrast enhancement (Figure 1C), and blooming on T2 * GRE sequence (Figure 1E).
Differential diagnosis on imaging includes other melanotic lesions (e.g. intracranial metastatic melanoma and melanotic meningioma), hemorrhagic lesions (e.g. hemorrhagic metastases and hemangioblastoma), and extra-axial lesions (e.g. meningioma, solitary fibrous tumors of the dura, and hemangiopericytoma). On CT, melanocytoma and meningioma will both appear dense and may enhance, but meningiomas often contain calcifications and hyperostosis of the adjacent bone not commonly seen in association with melanocytomas.Reference Chen, Hsu, Ho, Hsu, Wang and Wong8 MRI is often useful to determine whether the mass is extra-axial (Figure 1D) or intra-axial. MRI cannot clearly differentiate melanocytomas from other extra-axial neoplasms such as meningioma, hemangiopericytoma, malignant melanoma, or metastasis, which can occur in similar locations.Reference Smith, Rushing and Smirniotopoulos1 A metastatic tumor would be a consideration in older patients and in patients with a known primary malignancy. On histology, meningeal melanocytomas display minimal nuclear pleomorphism, atypia, and macronucleoli in contrast to metastatic melanomas. Moreover, mitotic figures and necrosis are typically absent in melanocytomas.
Treatment of choice is complete excision; however, this may be complicated by severe intraoperative hemorrhage.Reference O’Brien, Crooks and Mallucci6 Although typically benign, a small number of meningeal melanocytomas can demonstrate malignant activity.Reference Bydon, Gutierrez and Mahmood7 While gross total surgical resection may be curative, 22% of lesions recur within 3 years. Without gross total resection, the recurrence rate increases to approximately 50%.Reference Rades, Heidenreich, Tatagiba, Brandis and Karstens9 Therefore, adjuvant radiation therapy is often recommended, especially with incomplete resection.Reference Lin, Yang, Qu, Li and Yu10
Statement of Authorship
IYMC contributed to the case description, literature review, radiology images and descriptions, as well as the drafting and revision of the manuscript. HL contributed pathology images and descriptions. TS provided pathology case details and descriptions. RRH and MTJ provided feedback and revised the draft manuscript. All authors edited and approved the final manuscript.
Acknowledgments
The authors are grateful to Dr. Dalia A. Jadkarim, Dr. Michael S. Mayich, and Dr. Manas Sharma for their insights during the drafting of this manuscript.






