Background
Endovascular thrombectomy (EVT) improves 3-month functional outcomes in patients with large vessel acute ischemic stroke. Reference Goyal, Menon and van Zwam1 However, 4.4% of patients undergoing EVT still develop symptomatic intracranial hemorrhage (sICH). Reference Goyal, Tsivgoulis and Malhotra2 Real-world studies show even higher sICH rate at 9–16%. Reference Zaidat, Castonguay and Gupta3,Reference Hao, Yang and Wang4 Post-EVT patients who develop sICH have significantly higher morbidity and mortality. Reference Berger, Fiorelli and Steiner5 Even asymptomatic intracranial hemorrhage (ICH) in patients undergoing EVT, has been found to decrease the likelihood of attaining an excellent (MRS 0-1) functional outcome. Reference Hao, Liu and Wang6
Given the prognostic implications of post-EVT ICH, identifying factors associated with its occurrence has significant clinical relevance. In particular, we sought to determine if serum biomarkers of neuroinflammation such as neutrophil count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio had any predictive value for ICH after EVT. The goal of this study was to determine factors associated with post-EVT hemorrhage in patients undergoing EVT for large vessel acute ischemic stroke.
Methods
Patient Selection and Study Site
We performed a retrospective review of consecutive patients treated with EVT for acute large vessel ischemic stroke at our institution, a regional stroke center, from March 2011 to April 2020. The inclusion criteria were as follows: age >18 years, diagnosis of acute ischemic stroke with large vessel occlusion (LVO) of the middle cerebral artery (MCA), and/or internal carotid artery (ICA) on computed tomography angiography (CTA), treatment with standard-of-care EVT (stent retriever and/or large bore catheter aspiration), availability of post-EVT head computed tomography (CT), and pre-EVT complete blood counts. Patients with hemorrhage due to vessel perforation were excluded from the study.
The study was approved by the institutional review board. A complete blood count is done for all new acute stroke patients. The hospital uses the Sysmex XN-9000 (Kobe, Japan), a quantitative, multiparameter automated hematology analyzer. The machine uses flow cytometry for cell analysis and sheath flow direct current detection method for counting red blood cells and platelets. Image analysis is also utilized for the differential count
A CTA follows to identify an LVO. Then, the neurovascular team assesses EVT candidacy. Twenty-four hours after EVT, a plain head CT is done to screen for hemorrhage. In cases where it is difficult to delineate HT from contrast staining, a repeat CT is done after another 24 hours to establish a final radiologic diagnosis.
Outcome Measures
The primary outcome of the study is the presence of ICH on the post-EVT scan. We used four definitions Reference Yaghi, Willey and Cucchiara7 : the SITS-MOST criteria (parenchymal hemorrhage >30% of the territory of the infarcted region, with an associated NIHSS worsening of >4 points within 36 hours of stroke onset), the NINDS criteria (any clinical deterioration with any hemorrhage), asymptomatic hemorrhage (no symptoms with any hemorrhage), and overall hemorrhage (includes all types of hemorrhage).
Data Collection
Patient medical records were reviewed by study personnel. We obtained patient demographics, treatment details, and both radiologic and clinical treatment outcomes. All post-EVT cranial scans were again reviewed for the presence of ICH by interventional neuroradiologists.
Statistical Analysis
Methods Overview
Briefly to overview, we identified nonredundant predictors of outcome using backward elimination based on Akaike Information Criteria. We then assessed prediction accuracy using area under the receiver operating curve. To further assess the influence of our predictors, we implemented variable importance ranking from logistic regression models using the drop in Naegelkerke R2 with the exclusion of each predictor.
Predictive Models
The following variables were considered for the models: age, sex, comorbid conditions (atrial fibrillation, congestive heart failure, hypertension, diabetes, coronary artery disease, hyperlipidemia) NIHSS score at presentation, prior anticoagulation, prior antiplatelet use, prior ischemic stroke, prior hemorrhagic stroke, treatment with intravenous or intraarterial thrombolysis, time from symptom onset to reperfusion, degree of recanalization, use of general anesthesia, ASPECTS score on admission, exact location of clot, total number of thrombectomy passes, and serum biomarkers (WBC count, neutrophil count, lymphocyte count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio). We applied logistic regression models for predictions and to assess variable correlation with clinical outcome. For model selection, we will use Akaike information criterion with backward elimination to optimize the balance of model complexity against goodness of fit. Reference Akaike, Atkinson and Fienberg8 Predictions will be undertaken with threefold cross-validation to avoid overfitting. Reference Steyerberg, Mushkudiani and Perel9 This method has been found superior in terms of discriminatory ability, calibration, and overall accuracy to the split-sample method by the comparative study of Steyerberg et al. Reference Steyerberg, Harrell, Borsboom, Eijkemans, Vergouwe and Habbema10 Predictive performance of the different models described below will be assessed by computing the AUC and compared using DeLong’s test. Reference DeLong, DeLong and Clarke-Pearson11
Variable Importance Ranking
We used the Nagelkerke R2 value, Reference Nagelkerke12 a measure for goodness of fit, to rank variable importance. Nagelkerke R2 numerically expresses the percentage of variability attributed to a predictor. The ranking of variables was extracted from the drop in the Nagelkerke R2 value that occurs in response to excluding variables of interest from the model. We used this ranking to identify the most influential variables and include them to create a more simplistic model with fewer variables. There are recognized limitations for “pseudo” R2 methods: (1) they can be argued to give artificially high R2 scores that may suggest the model fits better than it really does, and (2) there are a variety of “pseudo” R2 measures to choose from, each of which interprets the model differently and therefore gives different results. In our study, we used the same modality of R2 value to assess the change in model fit rather than focus on the numeric value, a technique that has been applied in past papers. Reference Murray, Butcher and McHugh13,Reference Zador, Sperrin and King14
Statistical Software
Statistical analysis and modeling were carried out in R, 15 an open-source software environment for statistical programming and graphics (https://www.r-project.org/). Receiver operating curve analysis was done using the “pROC” package. Reference Robin, Turck and Hainard16 Threshold optimization was performed using packages “pROC” and “SDMTools” Reference VanDerWal, Falconi, Januchowski, Shoo and Storlie17 AUCs were compared using DeLong’s test. Reference DeLong, DeLong and Clarke-Pearson11 Nagelkerke R2 was implemented using the “fmsb” package. Reference Nakazawa18
Ethics Approval
The study was approved by our institutional research ethics board (18-397). The retrospective nature of the study precluded informed consent from the patients.
Results
Patient Characteristics
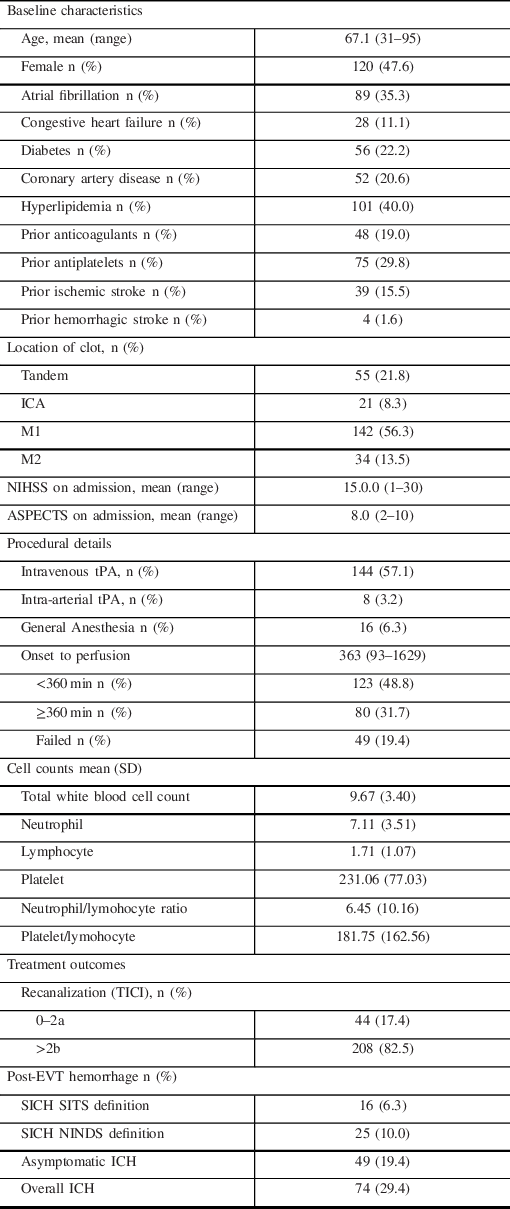
A total of 335 acute stroke patients underwent EVT, of which 252 patients met the inclusion criteria (See Online Supplement Figure 1). The baseline characteristics of the patients are summarized in Table 1.
Table 1: Baseline characteristics and treatment outcomes
Predictive Models
On logistic regression modeling, we yielded 5, 6, 7, and 6 variables significantly associated with occurrence of post-EVT intracerebral hemorrhage as described by SITS, NINDS, asymptomatic, and overall ICH definitions (Table 2) respectively. Consistent predictors across the four models were the lower white blood cell (WBC) or lymphocyte count, and number of passes. Significance values and odds ratios are summarized in Table 2.
Table 2: Table summary of confidence intervals, odds ratios, and significance levels for the post EVT intracerebral hemorrhage definitions

Predictive Performance
AUCs were 0.69 (95% CI: 0.55–0.83), 0.78 (95% CI: 0.69–0.86), 0.69 (95% CI: 0.61–0.77), and 0.72 (95% CI: 0.65– 0.79) for the SITS, NINDS, asymptomatic, and overall hemorrhages, respectively. These values reflect only fair diagnostic accuracy of the models. Predictions were carried out using threefold cross-validation approach to avoid model overfitting. Using DeLong’s test, there were no significant differences between all combinations of ROC curves.
Importance Ranking of Outcome Predictors
Based on the drop in Nagelkerke R2 values, a pseudo-R2 value used to describe goodness of model fit, we ranked the influence of each variable on the outcome of interest. While there was some overlap in significant predictors between models, the highest-ranking variables were distinct for each model as summarized in Table 3.
Table 3: Variable importance ranking

Discussion
Our study demonstrates a 6.3% (SITS) and 10.0% (NINDS) sICH rate, as well as a 19.4% asymptomatic and 29.4% overall hemorrhage rate. Serologic markers that demonstrated association with post-EVT hemorrhage were: low lymphocyte count (SITS), high neutrophil count (NINDS, overall hemorrhage), low platelet-to-lymphocyte ratio (NINDS), and low total WBC (NINDS, asymptomatic hemorrhage). Other factors that showed previous association with post EVT hemorrhage were also seen in our study: diabetes mellitus, Reference Enomoto, Shigeta and Ota19 higher number of passes, Reference Hao, Yang and Wang4,Reference Enomoto, Shigeta and Ota19 onset to perfusion time, Reference Hao, Yang and Wang4,Reference Bin, Yoon and Lee20 lower ASPECTS, Reference Hao, Liu and Wang6,Reference Boisseau, Fahed and Lapergue21 and intravenous thrombolysis. Reference Enomoto, Shigeta and Ota19,Reference Nogueira, Gupta and Jovin22 The neutrophil-to-lymphocyte ratio has previously been associated with early neurological deterioration, Reference Nam, Kim and Lee23 post-thrombolysis hemorrhagic transformation, Reference Liu, Lu and Yin24 and poor outcomes in acute ischemic stroke patients. Reference Song, Zhao and Rajah25 However, it was not associated with post-EVT hemorrhage in our study using any of the definitions.
Blood–brain barrier disruption induced by the inflammatory response to acute ischemic stroke may play a role in post-EVT hemorrhage. Neutrophils are a source of matrix metalloproteinase (MMP)-9, which play a direct role in degrading tight junction proteins. Conceivably, higher neutrophil counts may lead to higher levels of MMP-9, potentially translating to a higher risk of HT. Reference Kanazawa, Takahashi, Nishizawa and Shimohata26 The role of neutrophils is emphasized further by decrease in ischemic volume in rats undergoing middle cerebral artery occlusion after the administration of anti-neutrophil antibodies compared with those with a normal complement of neutrophils. Reference Dawson, Ruetzler, Carlos, Kochanek and Hallenbeck27 These pathomechanisms may underlie a recent finding that high neutrophil count predicts poor clinical outcomes despite recanalization in patients with large vessel occlusion. Reference Boisseau, Desilles and Fahed28 Large cohorts of patients undergoing intravenous thrombolysis Reference Maestrini, Strbian and Gautier29 and endovascular thrombectomy Reference Hao, Yang and Wang4 also demonstrated an increased risk of hemorrhagic transformation in patients with higher neutrophil counts and ratios. Circulating neutrophil transcriptomes have also been demonstrated to correlate with the presence of unruptured intracranial aneurysms. Reference Tutino, Poppenberg and Li30–Reference Poppenberg, Tutino and Li32
Diabetes has been associated with post EVT hemorrhage in past studies. Reference Enomoto, Shigeta and Ota19,Reference Nogueira, Gupta and Jovin22 Chronic hyperglycemia in diabetic mice undergoing MCA occlusion has been demonstrated to aggravate HT by inciting mitochondrial dysfunction leading to endothelial cell death. Reference Mishiro, Imai and Sugitani33 Classically, HT had been attributed to free radical damage from reperfusion injury. However, similar to our findings, a large multicenter study demonstrated that reperfusion status did not correlate with HT after EVT. Reference Boisseau, Fahed and Lapergue21 More recent basic science research has focused on downstream microvascular thromboinflammation (DMT), a phenomenon characterized by leukocyte margination (mostly neutrophils) and thrombosis in the venules after proximal large vessel occlusion. DMT by inducing vascular leakage may account for microhemorrhages and incomplete reperfusion despite large vessel recanalization. Reference Desilles, Syvannarath and Di Meglio34
Hyperglycemia has been demonstrated to precipitate infarct growth and HT by exacerbating DMT through preactivating neutrophils in rats undergoing experimental large vessel occlusion. Reference Desilles, Syvannarath and Ollivier35 Earlier studies noted that diabetic rats had a more robust neutrophil–endothelial cell interaction compared with nondiabetic ones in the setting of large vessel ischemia reperfusion injury models. Reference Ritter, Davidson and Henry36 This interplay between diabetes and neutrophils may be the pathomechanism behind the association of both factors with post-EVT ICH in our study.
Higher WBC count has already been associated with less hematoma expansion in spontaneous ICH. Reference Morotti, Phuah and Anderson37 However, its association with less post-EVT hemorrhage has not yet been described elsewhere. A higher platelet lymphocyte ratio has been associated with poorer clinical outcomes in patients undergoing thrombolysis and endovascular therapy Reference Altintas, Altintas, Tasal, Kucukdagli and Asil38,Reference Xu, He and Li39 ; counterintuitively, our study demonstrates that a lower ratio is associated with sICH (NINDS). Similarly, the association of lower lymphocyte to our outcome of interest (SITS) is also a new finding.
Limitations of our study include its retrospective nature, lack of a core laboratory, and low sample size. Next, perfusion imaging and MRI data were not included in the analysis. The model also needs validation using a different large independent cohort. Furthermore, the models obtained by the study only have moderate accuracy. Lastly, the paper is exploratory in nature. It is a proof of concept of the association of serology markers to post-EVT hemorrhage.
Conclusion
Higher neutrophil counts, low WBC counts, low lymphocyte counts, and low platelet-to-lymphoycyte ratio were baseline serology biomarkers that were associated with post-EVT hemorrhage. Our findings, particularly the association of diabetes mellitus and high neutrophil count with post-EVT hemorrhage, support experimental data on the role of thromboinflammation in hemorrhagic transformation of large vessel occlusions. Larger stroke registries with data on inflammatory biomarkers are necessary to confirm our findings.
Conflict of Interest
The authors have no conflicts of interest to declare.
Statement of Authorship
All authors fulfilled the following ICMJE criteria for authorship; substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND Drafting the work or revising it critically for important intellectual content; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Statement
The full dataset of the study is available upon reasonable request.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.197.







