CLINICIAN’S CAPSULE
What is known about the topic?
Age-adjusted D-dimer thresholds have been proposed to improve specificity of diagnostic testing for thromboembolism in patients ages 50 and over.
What did this study ask?
What is the diagnostic accuracy of an age-adjusted D-dimer threshold in a population of patients undergoing investigations for suspected pulmonary embolism (PE)?
What did this study find?
Age-adjusted D-dimer cut-offs improved specificity but at the expense of a slightly higher risk of missed PE.
Why does this study matter to clinicians?
Use of age adjusted D-dimer thresholds, in combination with validated risk scores, may reduce CT utilization in older patients.
INTRODUCTION
The D-dimer assay is a high-sensitivity, low-specificity blood test used to rule out pulmonary embolism (PE) in patients determined to be at low risk for PE.Reference Wells 1 An individual with a low pretest probability and a D-dimer concentration lower than the conventional 500 ng/ml cut-off is considered to have had PE ruled out. A patient with a D-dimer concentration exceeding that threshold typically undergoes imaging with either a computed tomography pulmonary angiography (CTPA) or ventilation-perfusion (VQ) scan to establish a definitive diagnosis.
D-dimer concentrations rise in healthy individuals as they age, often well above the conventional cut-off, in the absence of a clinically important disease.Reference Hager and Platt 2 , Reference Harper, Theakston, Ahemd and Ockelford 3 This lowers the test’s specificity in older patients and leads to avoidable imaging utilization. Given the time, cost, and radiation exposure associated with CT imaging,Reference Brenner and Hall 4 along with potential adverse events associated with intravenous contrast, it would be advantageous to identify an age-adjusted D-dimer cut-off that would increase the test’s specificity in an older population while minimizing the number of additional cases of a missed PE.
Previous studies have demonstrated that an age-adjusted threshold (patient age × 10 ng/ml) increases D-dimer specificity in patients over the age of 50 years with inconsequential reductions in sensitivity.Reference Douma, le Gal and Söhne 5 - Reference Righini, Van and Den Exter 8 In particular, the ADJUST-PE study demonstrated improved diagnostic accuracy and safety when an age-adjusted D-dimer strategy was used for the management of patients with a suspected PE.Reference Righini, Van and Den Exter 8
Our objective was to evaluate the test characteristics of an age-adjusted D-dimer cut-off concentration in a cohort of patients undergoing workup for PE. We compared sensitivity, specificity, and false-negative risk of this age-adjustment formula with the conventional cut-off (500 ng/ml) and the local laboratory reference standard (470 ng/ml). We also estimated downstream effects on CT imaging utilization if an age-adjusted cut-off were used.
METHODS
Setting and subject selection
This observational study used prospectively collected administrative data from four adult urban emergency departments (EDs) in Calgary, Alberta, Canada (population 1.2 million), which have a combined annual ED census of 325,000 visits. These four hospitals share a common, linked ED information system (EDIS) and administrative database. The study period was from July 2013 to January 2015.
We included all ED patients over the age of 50 years presenting with standardized triage complaint codes of chest pain, shortness of breath, or syncope, and who underwent D-dimer testing (Figure 1). This sample was chosen to allow estimate test characteristics of an age-adjusted D-dimer threshold in patients to whom an age-adjusted D-dimer threshold would be applied. We believed that the inclusion of younger patients would bias findings towards higher sensitivity. Patients with a pre-existing diagnosis of PE made in the 90 days prior to presentation were excluded from the analysis.

Figure 1 Patient inclusion and outcomes.
A high-sensitivity latex turbidimetric D-dimer assay was used by the participating EDs (HemosIL HS 500, Instrumentation Laboratory Canada, Richmond Hill, ON). The local laboratory uses an upper reference limit of 470 ng/ml rather than the manufacturer’s recommended 500 ng/ml cut-off, to achieve optimal sensitivity based on the findings of a local quality improvement study. All D-dimer assays were ordered by physicians (either ED physicians, trainees, or consultants) in the course of clinical care. Although formal risk stratification is encouraged prior to D-dimer ordering, there is no formal institutional PE diagnostic protocol.
This study was approved by the University of Calgary Conjoint Health Research Ethics Board without the need for informed consent.
Measurements and data verification
The primary outcome was a diagnosis of PE within 30 days (including on the index ED visit). A diagnosis of PE was ascertained by an electronic search of hospital administrative databases for an ICD-10-CA code indicative of PE (I26.9 and I26.0) or a free text diagnosis of PE. Medical records of patients with an ICD-10 code or a free text ED diagnosis of PE were reviewed by two reviewers (KS, KB) to confirm the diagnosis. The reference standard for a PE diagnosis included a positive CT or VQ scan, or Doppler leg ultrasounds in unstable patients or those with contraindications to chest imaging. A prior validation of ICD-10 coding for PE in this study population ensured that no patients with a PE were misclassified based on erroneous ICD-10 coding,Reference Burles, Innes and Senior 9 thus the outcomes (diagnoses among patients with and without PE) were verified manually. A second round of outcome adjudication was performed by a trained research coordinator and an emergency physician to verify outcomes in discrepant cases or in cases with unclear imaging findings.
All patients who did not have an ED discharge diagnosis of PE on the index encounter were followed forward in the administrative data for 30 days to capture any PEs that may have been missed or miscoded at the index encounter. Reviewers were blinded to the D-dimer result of those followed forward at the time of the review. No patients were excluded due to missing data or indeterminate test values.
Analysis
The formula 10 ng/ml × patient age in years as an integer was used to calculate each individual’s age-adjusted D-dimer cut-off value. This cut-off was compared to the actual D-dimer result to assign a positive or negative test status, then related to the ED diagnosis for each encounter to generate a 2 × 2 table. Similar 2 × 2 tables were created for the 500 ng/ml cut-off and the local 470 ng/ml cut-off to assess differences in diagnostic performance. Sensitivity, specificity, likelihood ratios, and their 95% confidence intervals were calculated using statistical software XLSTAT 2016. The false-negative risk was calculated as the number of patients with false-negative D-dimer results divided by the number of all patients with a negative D-dimer result. The difference between the number of patients with a positive age-adjusted D-dimer and the number of patients with a positive 500 ng/ml or 470 ng/ml cut-off served as an estimate of the potential for reduction in CT utilization.
RESULTS
We identified 6,655 patients ages 50 and older who had D-dimer assays performed (Table 1). Of these, 242 had an imaging-confirmed PE diagnosis on initial encounter, and another 4 patients had a PE diagnosis after the index visit yielding a 30-day PE incidence of 246 (3.9%). Of these, 234 were diagnosed on a CT scan and 12 on a VQ scan. PE was diagnosed in one hemodynamically unstable patient on the basis of a positive Doppler leg ultrasound.
Table 1 Characteristics of study subjects

Among all patients, 2,708 (40.7%) had a D-dimer concentration exceeding the local (470 ng/ml) threshold, 2,557 (38.4%) had a D-dimer concentration greater than 500 ng/ml, and 1,802 (27.1%) had a D-dimer concentration greater than an age-adjusted threshold.
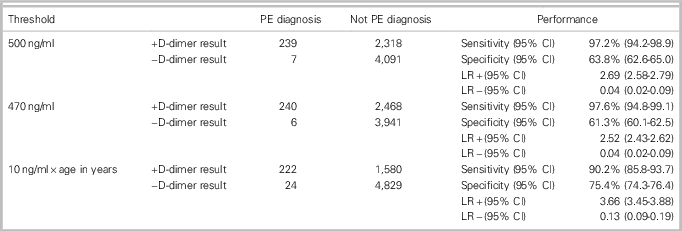
Table 2 summarizes D-dimer diagnostic performances using the three cut-off values. We found similar sensitivity and specificity for the two fixed cut-offs. The local threshold of 470 ng/ml had a sensitivity of 97.6% and a specificity of 61.3% for acute PE. The standard threshold of 500 ng/ml was 97.2% sensitive and 63.8% specific. The age-adjusted threshold was 90.2% sensitive and 75.4% specific.
Table 2 Proportion of patients with positive D-dimers and imaging utilization

The age-adjusted D-dimer cut-off had 24 false negatives, compared to 7 for the 500 ng/ml cut-off and 6 for the 470 ng/ml cut-off. In this cohort, this translates into a false-negative risk of 0.49% using an age-adjusted threshold, compared to 0.17% with a 500 ng/ml cut-off and 0.15% with a 470 ng/ml cut-off (Table 3).
Table 3 Comparison of accuracy of D-dimer test using the conventional cut-off (500 ng/ml), a local laboratory cut-off (470 ng/ml), and an age-adjusted cut-off (10 ng/ml x patient age in years) in the diagnosis of PE in ED encounters with patients ages 50 and older
DISCUSSION
In this observational cohort study, the diagnostic performance of an age-adjusted D-dimer (10 ng/ml × patient age in years) was assessed in comparison to the local laboratory cut-off (470 ng/ml) and the manufacturer’s recommended cut-off (500 ng/ml). We found that, in comparison with the local cut-off and the manufacturer’s cut-off, specificity increased from 61.3% and 63.8%, respectively, to 75.4% using an age-adjusted cut-off. However, sensitivity fell from 97.2% and 97.6% with standard cut-offs to 90.2% with an age-adjusted cut-off.
Compared with 38.4% patients using a cut-off of 500 ng/ml and 40.7% using a cut-off of 470 ng/ml, 27.1% of patients had a D-dimer concentration above an age-adjusted threshold. If all patients over the age of 50 with an elevated D-dimer underwent a CT scan, using an age-adjusted cut-off could have led to a 13.6% absolute reduction in imaging utilization compared with the 470 ng/ml local laboratory cut-off. We note, however, that only 58.0% of patients whose D-dimer exceeded the local reference standard went on to CT imaging in actual practice, so our estimate of potential imaging reduction may be optimistic, because it represents the reduction that would be seen if all patients ages 50 and over who had a positive D-dimer result underwent CT imaging. Moreover, based on the increased false-negative risk, we would have observed a small but important increase in the risk of a missed PE if an age-adjusted D-dimer strategy were used in this cohort.
These results run contrary to much of the current literature evaluating the test characteristics of the age-adjusted D-dimer. In a 2013 meta-analysis, Shouten et al. found that the age-adjusted cut-off increased specificity substantially across 13 cohorts, whereas sensitivity remained above 97%.Reference Shouten, Geersin and Koek 7 Similarly, the ADJUST-PE study, a large multicentre prospective validation study by Righini et al., found an exceedingly low failure rate for the age-adjusted D-dimer.Reference Righini, Van and Den Exter 8 However, our findings are congruent with the science of diagnostic testing. Diseased and non-diseased populations each have a distribution of test results and differing proportions of patients above and below a given test threshold. Shifting a test threshold upwards to achieve a higher specificity will by definition allow some patients with disease to fall below the new threshold and move from the true-positive group to the false-negative group. It is highly unlikely, therefore, that test cut-offs could be modified to improve specificity without affecting sensitivity. The question is not why sensitivity diminished (this is expected) but whether the decrease in sensitivity is acceptable.
It is important to note that prior studies examining the utility of age-adjusted D-dimer cut-offs studied prospective cohorts of patients being investigated for venous thromboembolism (VTE), and patients had a structured risk assessment performed prior to D-dimer testing with either the Wells’ or the Revised Geneva scores.Reference Douma, le Gal and Söhne 5 - Reference Righini, Van and Den Exter 8 Strict adherence to a diagnostic algorithm would have ensured that the D-dimer test would be obtained in patients only at low risk for PE. However, rigorous compliance to diagnostic algorithms for PE is not necessarily achieved in actual practice, with physician adherence to standardized diagnostic pathways being as low as 50% to 60% in some studies,Reference Ng and Lindstrom 10 - Reference Yin, Wilson and Fave 13 in line with our observations. Therefore, our data give an alternative perspective, by assessing the performance of the age-adjusted D-dimer in the way that the test is used in a real-world clinical setting at a major academic centre. A substantial decrease in sensitivity from 98% to 93% was also observed by Sharp et al., when evaluating the use of an age-adjusted D-dimer cut-off in retrospective observational data.Reference Sharp, Vinson and Alamshaw 14
The test characteristics of various D-dimer assays for VTE vary substantially, meaning that these findings may not be generalizable to settings using other D-dimer assays.Reference Oude Elferink, Loot and Van De Klashorst 15 - Reference Mullier, Vanpee and Jamart 17 Mullier et al. found important differences in specificity for VTE when comparing the performance of five D-dimer assays, although sensitivity appeared to be uniformly high among assays.Reference Mullier, Vanpee and Jamart 17 Thus, the loss of sensitivity we observed in our study may also be an issue in other settings using different D-dimer assays. Variation in specificity between different assays suggests caution when generalizing predicted reductions in the use of imaging by using an age-adjusted cut-off.
Previous research has indicated that the combination of formal risk stratification followed by either D-dimer testing and/or CTPA is the most cost-effective diagnostic strategy for PE,Reference Raymakers, Mayo, Marra and FitzGerald 18 but the effectiveness of this cost-saving strategy diminishes with age, especially for those 80 and older.Reference Righini, Nendaz and Le Gal 19 Use of an age-adjusted cut-off may reduce costly low-yield CT imaging in the older population. However, because the primary goal of D-dimer testing must be to maintain high sensitivity for PE, diagnostic algorithms attempting to increase specificity must not significantly compromise the sensitivity of the strategy.
LIMITATIONS
This was an observational study using administrative data. We enrolled patients with common PE presenting symptoms and, as such, may have excluded patients with atypical presentations. We undertook substantial effort to verify the reliability of the administrative data, including a manual chart review for all patients with ICD-10 codes and free text diagnoses of PE. All PE diagnoses were confirmed by imaging. Furthermore, we sought to minimize missed diagnoses of PE due to ICD-10 miscoding by manual review, the method of which is reported separately.Reference Burles, Innes and Senior 9 Sensitivity and specificity estimates may be vulnerable to random ICD-10 misclassification; however, given our efforts to verify outcomes, the effect of any misclassification is likely minimal. Random ICD-10 miscoding would not explain the reduction in sensitivity for age-adjusted D-dimer cut-offs compared to traditional cut-offs.
Our restriction to patients ages 50 years and over may have led to our observation of lower sensitivity of an age-adjusted D-dimer threshold. We note that other studies finding higher sensitivity with an age-adjusted D-dimer cut-off included patients less than 50 years in their cohorts.Reference Douma, le Gal and Söhne 5 - Reference Righini, Van and Den Exter 8 The test-characteristics of an age-adjusted D-dimer cut-off would not change in patients less than 50 years of age, and inclusion of such patients in any study would dilute the potential change in test characteristics that might be observed in older adults. We felt that it was important to restrict our analysis to the patients who both bear the risks of lower sensitivity and stand to benefit from reductions in unnecessary imaging utilization.
Importantly, our administrative data set did not record patients’ pretest probability of PE. D-dimer testing to rule out PE should be used only in patients with a low pretest probability using an evidence-based risk prediction tool. However, some clinicians may not use formal risk stratification tools for patients with suspected PE,Reference Ng and Lindstrom 10 - Reference Yin, Wilson and Fave 13 and thus our sample may have included some patients with moderate-to-high pretest probability that inappropriately underwent D-dimer testing. Therefore, our data do not estimate the test characteristics of an age-adjusted D-dimer threshold in patients with a low pretest probability (for whom D-dimer testing would be appropriate) and may underestimate sensitivity and safety of an age-adjusted D-dimer cut-off when used in the appropriate population. We note, though, that our data estimate test characteristics in all patients whose physicians ordered D-dimers, which may be more representative of actual clinical practice.
We also observed additional deviation from expected clinical practice. A large proportion of patients with positive D-dimer results did not proceed to CT or VQ imaging. Some physicians may have already incorporated an age-adjusted D-dimer strategy into their clinical practice and thus did not pursue imaging. However, some elevated D-dimer results may have been dismissed by the treating physician. Given that only 4 of 246 PE cases (from among 6,655 total patients) were diagnosed after ED discharge, we are not concerned that current clinical practice at our institution has an exceedingly high miss rate. It is also possible that the D-dimer may have been ordered inappropriately by trainees, in some cases. These cases would have biased our findings towards an increase in specificity, because results from encounters without reasonable indication for D-dimer testing would artificially increase the number of true negatives.
Finally, we considered all PE diagnoses to be clinically important. The detection of clinically irrelevant subsegmental emboli is a source of PE overdiagnosis,Reference Carrier, Righini and Wells 20 and inclusion of these cases as study events could have contributed to the apparent drop in assay sensitivity that we identified.
CONCLUSION
An age-adjusted D-dimer cut-off used in conjunction with formal risk stratification prior to testing has the potential to substantially reduce the use of CT imaging among older patients with suspected PE. However, in this administrative data set representing real-world practice, we observed an unacceptable loss of diagnostic sensitivity. Consequently, we encourage further evaluation of age-adjusted D-dimer strategies in routine clinical practice to determine whether similar losses in sensitivity are commonplace.
Acknowledgement
The authors thank Katrina Koger for assistance with the medical record review and manuscript preparation.
Competing interests: None declared.