Introduction
Psyllids are phytophagous insects belonging to the superfamily Psylloidea (Hemiptera: Sternorrhyncha) (Burckhardt et al. Reference Burckhardt, Ouvrard and Percy2021). There are approximately 4000 described psyllid species (Burckhardt et al. Reference Burckhardt, Ouvrard, Queiroz and Percy2014), with diversity across geographic regions. The life history of most psyllid species is unstudied outside of their host–plant associations. Most knowledge of psyllid biology and ecology is restricted to a few species that are economically important as pests of major crops. These include the important pest species Diaphorina citri Kuwayama (Hemiptera: Liviidae) (Tsai and Liu Reference Tsai and Liu2000) and Bactericera cockerelli (Šulc) (Hemiptera: Psylloidea) (Butler and Trumble Reference Butler and Trumble2012a), and the tree pests Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae) (Brennan et al. Reference Brennan, Hrusa, Weinbaum and Levison2001), Blastopsylla occidentalis Taylor (Hemiptera: Aphalaridae) (Burckhardt and Elgueta Reference Burckhardt and Elgueta2000), and Acizzia uncatoides (Hemiptera: Psylloidea) (Dreistadt and Hagen Reference Dreistadt and Hagen1994). Although the damage and symptoms associated with psyllid infection can vary, some symptoms are relatively common across species. These include distorted terminal and lateral growth and galled leaves (Hodkinson Reference Hodkinson1974). In particular, infestations with Psyllopsis (Hemiptera: Psylloidea) species are associated with pseudogalled leaves (Hodkinson Reference Hodkinson1974), termed “pseudo” because no cellular proliferation occurs.
The physiological mechanisms underlying the plant response that creates these psyllid-associated symptoms are not clear. In some literature, symptoms have been attributed to an “unknown toxin in the saliva”; however, no such toxin has been isolated (Prager and Trumble Reference Prager, Trumble, Wakil, Brust and Perring2018; Mauck et al. Reference Mauck, Sun, Meduri and Hansen2019). Economic damage from psyllids is associated not only with feeding but also with their ability to vector phytopathogenic bacteria in both the groups Candidatus Phytoplasma (Acholeplasmataceae) (Jarausch et al. Reference Jarausch, Tedeschi, Sauvion, Gross, Jarausch, Bertaccini, Weintraub, Pratap Rao and Mori2019) and Candidatus Liberibacter (Rhizobiaceae) (Haapalainen Reference Haapalainen2014).
Because of their association with Liberibacters, the potato psyllid (Bactericera cockerelli) and the Asian citrus psyllid (Diaphorina citri) are well studied with respect to the integrated pest management of psyllids. Studies include sampling methods (Hall et al. Reference Hall, Hentz and Ciomperlik2007; Tsai and Liu Reference Tsai and Liu2000; Prager et al. Reference Prager, Butler and Trumble2013a, Reference Prager, Butler and Trumble2014), spatial distributions (Costa et al. Reference Costa, Barbosa, Yamamoto and Leal2010; Butler and Trumble Reference Butler and Trumble2012a), biological control (Butler and Trumble Reference Butler and Trumble2012b; Monzó and Stansly Reference Monzó and Stansly2020), and chemical control (e.g., Mann et al. Reference Mann, Rouseff, Smoot, Rao, Meyer and Lapointe2013; Prager et al. Reference Prager, Lewis and Nansen2013b, Reference Prager, Vindiola, Kund, Byrne and Trumble2013c; Qureshi et al. Reference Qureshi, Kostyk and Stansly2014; and many others). Asian citrus psyllid is currently the most heavily managed psyllid species, and most of this management is accomplished through the application of insecticides, particularly neonicotinoids (Mann et al. Reference Mann, Rouseff, Smoot, Rao, Meyer and Lapointe2013; Tiwari et al. Reference Tiwari, Killiny and Stelinski2013) and abamectin/avermectin (Vanaclocha et al. Reference Vanaclocha, Jones, Tansey, Monzó, Chen and Stansly2019). Management of B. cockerelli is accomplished with a variety of insecticides in weekly calendar-based rotation (Guenthner et al. Reference Guenthner, Goolsby and Greenway2012).
Psyllopsis discrepans (Flor) (Hemiptera: Psyllidae), sometimes referred to as the “cottony ash psyllid,” is an insect that feeds on several species of Fraxinus (Oleaceae). Originally endemic to Europe, there are multiple reports of the insect in North America (Hodkinson Reference Hodkinson1988), including in Nova Scotia, Canada, and various American states, dating to the 1900s (Hodkinson Reference Hodkinson1988). The literature, however, contains no apparent references to economic damage caused by this insect in either Europe or North America.
Recently, urban forestry staff in western Canadian cities have reported declines in trees infested with P. discrepans; these observations were noted in the Alberta localities of Red Deer, Calgary, Edmonton, and Grande Prairie, as well as in Winnipeg, Manitoba, and in Saskatoon, Saskatchewan (Saunders et al. Reference Saunders, Wartedbe and Barr2004; Fry et al. Reference Fry, Saunders, Barr and Tinning2008). This phenomenon has also been reported in Montana, South Dakota, and North Dakota, United States of America (North Dakota Department of Agriculture, no date). In these locations, psyllids have been observed feeding on Fallgold black ash (Fraxinus nigra var. Fallgold), Mancana ash (Fraxinus mandshurica var. Mancana), and their hybrids, Northern Gem (Fraxinus nigra × mandshurica) and Northern Treasure (Fraxinus nigra × mandshurica) (Culliney and Koop Reference Culliney and Koop2005; Fauske et al. Reference Fauske, Knott, Nelson and Kangas2005). The loss of the susceptible trees has had a considerable impact on municipal urban forestry programmes (Geary Reference Geary2018; Tank Reference Tank2018). For example, the City of Red Deer, Alberta, had an estimated 1000 susceptible ash before the introduction of P. discrepans and have less than a dozen of these trees remaining. This infestation came at a cost of about $CDN 40 000 for tree removal but with greater costs in lost tree value and replacement (Susan Katzell, Urban Forester City of Red Deer, personal communication).
How these psyllids cause ash decline is not understood, although it is assumed to be associated with feeding. One possibility is an unknown toxin, as has been proposed for other psyllid-associated diseases (Prager and Trumble Reference Prager, Trumble, Wakil, Brust and Perring2018; Mauck et al. Reference Mauck, Sun, Meduri and Hansen2019). However, Wamonje et al. (Reference Wamonje, Zhou, Bamrah, Wist and Prager2021) have detected the plant pathogenic, psyllid-vectored bacteria Candidatus Liberibacter solanacearum in P. discrepans collected from F. mandschurica in Saskatoon. This bacterium is closely related to the species that causes Huánglóngbìng (citrus greening disease) in citrus (Haapalainen Reference Haapalainen2014). This suggests that the observed decline in ash may also be the result of Liberibacter infection.
In Saskatoon, approximately 6650 publicly owned trees are susceptible to P. discrepans. Periodic outbreaks of P. discrepans such as those in 2005 to 2008 (City of Saskatoon, unpublished data) that are followed by subsequent population crashes have been attributed to sustained, extreme winter cold periods that destroy overwintering eggs (City of Saskatoon, unpublished data). A recent outbreak of P. discrepans in Saskatoon was observed in 2015 (City of Saskatoon, unpublished data). In 2015, the number of psyllids was highest in the city’s central business district. The following year, the highest populations were concentrated in the central business district with increasing psyllid populations in all Saskatoon neighbourhoods (City of Saskatoon, unpublished data). In Saskatoon, ash decline follows a similar pattern to that in other localities, with bud failure, followed by a halo of leaves remaining on the tree perimeter, and eventually, tree death. Trees typically progressed from healthy to dead in less than one season in this recent outbreak.
In response to the declining ash populations, acephate (Orthene® 75% Soluble Powder Systemic Insecticide; Arysta Life Science Corp., Cary, North Carolina, United States of America) was granted emergency-use status for tree injections in Edmonton in 2005 and 2006 and in Saskatoon in 2007. Orthene is now registered in Canada for the control of P. discrepans (Health Canada, https://pr-rp.hc-sc.gc.ca/1_1/view_label?p_ukid=218237936). Orthene is applied by stem injection but can only be applied once every two years. Due to the potential of Orthene to persist in injected trees, tissues such as flowers and pollen should be analysed for insecticide traces the year following injections to assess the risk to pollinators and beneficial insects. Acephate has been previously shown to impact nontarget arthropod populations when used in a foliar application (Stanley and Preetha Reference Stanley and Preetha2016). Therefore, some risks to nontarget organisms occur with application, and any potential benefits of its use must be weighed against the negative consequences of application of Orthene for P. discrepans control. Currently, no peer-reviewed literature assesses the efficacy of acephate to reduce P. discrepans populations to stop or slow tree decline or to prevent tree loss.
TreeAzin® is an azadirachtin-based systemic insecticide for treating trees threatened by insects in urban forests and environmentally sensitive areas. Currently, TreeAzin is registered in the United States of America and Canada for use against insect pests, including emerald ash borer (Coleoptera: Buprestidae), European elm scale (Hemiptera: Eriococcidae), Lymantria dispar (Lepidoptera: Noctuoidea), tent caterpillars (Lepidoptera: Lasiocampidae), spruce budworm (Lepidoptera: Tortricidae), jack pine budworm (Lepidoptera: Tortricidae), arborvitae leaf miners (Lepidoptera: Yponomeutidae), and sawflies (Hymenoptera). As a mimic of the hormone ecdysone, one of the modes of action of azadirachtin is to interrupt the moulting process and kill larval nymphs as they attempt to moult. This mode of action has been shown to have toxicity against potato psyllids Bactericera cockerelli (Page-Weir et al. Reference Page-Weir, Jamieson, Chhagan, Connolly and Curtis2011). There is, therefore, potential for TreeAzin to work in the control of P. discrepans because the lifecycle of this psyllid begins with feeding on newly emerging leaf tissue by young nymphs (Fry et al. Reference Fry, Saunders, Barr and Tinning2008). Early spring injections of TreeAzin might concentrate in leaf tissues to coincide with the hatching of the first generation of P. discrepans nymphs and could result in high nymphal mortality.
Here, we evaluate both TreeAzin and Orthene for uptake and Orthene for potential efficacy and control against P. discrepans in urban trees in Saskatoon. The use of insecticides might prevent tree decline and can also help to delimit insect-related mortality of the host trees from other causes of mortality.
Materials and methods
Psyllopsis discrepans
Fry et al. (Reference Fry, Saunders, Barr and Tinning2008) described the lifecycle of P. discrepans in Edmonton, Alberta, in detail. In brief, they described two generations of P. discrepans, beginning with overwintering eggs that hatch before bud break. Nymphal feeding on the emerging leaflets is associated with curling of the leaflet edges towards the midrib, discolouration, and the formation of pseudogalls. Pseudogalling can affect individual leaflets, entire leaves, or all the leaf tissues from a single bud. Nymphs develop through five instars within the pseudogalled leaflets and complete development in late June. First-generation adults are active in late-June and early July. Eggs are laid in early July, often on the midrib of ungalled leaflets. Second-generation nymphs complete development by the end of July, and second-generation adults are present in August and September. Second-generation adult psyllids lay eggs in the early fall along the edges of bud scales, behind lateral buds, and in budscale scars.
Tree selection
All experiments in the present study were conducted on trees located within the municipal boundaries of the City of Saskatoon, Saskatchewan, Canada. The study was conducted exclusively on city-owned black ash (Fraxinus nigra) trees in the street-tree inventory. The greatest distance between trees was 370 m, and the closest trees were 10 m apart. The entire city was treated as a single site, with individual trees treated as replicates. Trees were included in the study if, on visual inspection, approximately 15–40% of their leaves displayed sign of pseudogalling and if the trees were located in neighbourhoods with significant cottony ash psyllid populations, as determined by Saskatoon surveys that included every city-owned black ash tree in Saskatoon. The trees selected had no defoliation, no digging in the root zone, nor wounds on the trunk. Study trees were included only if they were at least 15 cm in diameter at breast height due to concerns about the impact of drill holes on smaller trees. The average diameter at breast height was 20.7 cm +/− 0.45 (
![]() $$\bar x$$
+/− standard error), with height ranging from 9 to 13 m. Based on these criteria, a total of 150 trees were selected.
$$\bar x$$
+/− standard error), with height ranging from 9 to 13 m. Based on these criteria, a total of 150 trees were selected.
In the year following selection, trees were randomly assigned to the control group (no injection), to the group to receive an injection with Orthene, or to the group to receive an injection with TreeAzin. Trees were evaluated in the fall when leaves were present but were not assigned to treatments until the following spring. Because of this lag, some further canopy damage may have occurred in this period.
Tree injections
The timing of insecticide applications was selected based on each product’s label, including that of the Ecoject Microinjection System® (BioForest Technologies Inc., Sault Ste. Marie, Ontario, Canada). This resulted in different application times for the two insecticides but reflects when the products would be applied under real-world conditions. The injections of Orthene occurred from 2 to 4 May 2017, before leaf flush on the target trees, according to label recommendations at a rate of 0.3 mL per 2.5 cm diameter at breast height, after mixing at a rate of 286 g per 100 mL. TreeAzin injections occurred per label directions using the Ecoject Microinjection System at rates of 3 mL per centimetre diameter at breast height and at 5 mL per centimetre diameter at breast height. TreeAzin injections occurred on 19 May 2017 and continued until 6 June 2017 once leaves had flushed on trees. Due to rapid decline, whereby much of the crown failed to flush, many of the host trees would not take up TreeAzin, and consequently only 19 of 50 (38%) trees in the TreeAzin treatments were successfully injected. Notably, those 19 trees that successfully took up TreeAzin had more foliage and were healthier overall before injection than the 31 TreeAzin treatment–group trees in which injection failed. Because of the bias of having healthy trees and the low number in this treatment group, the 19 trees that were injected with TreeAzin were excluded from analysis. The trees in the control treatment were unmodified.
Evaluation of Orthene concentrations
In order to determine concentrations of Orthene in the flowers of treated trees, five flowers were collected from each of two black ash trees in the Orthene injection group. The flowers were pooled into one sample of five flowers per tree for two trees. The samples were collected two weeks after injection. Orthene-injected trees were also evaluated one year after injection, in early June 2018 (n = 5 trees) in the same manner. The concentrations of acephate and an associated breakdown product, methamidophos, were determined using the QuEChERRS LC/MS/MS method by A and L Laboratories, London, Ontario.
Canopy assessment
The canopy of each tree was visually assessed in June 2017, in August 2017, and in late-May 2018 and 2019. Canopy assessment occurred after bud break. Sampling was timed to account for bud break and P. discrepans phenology. Bud break in Saskatoon typically occurs in mid-May, whereas the first-generation adult psyllids are typically active in late-June and the second generation of P. discrepans is typically active in August. The June assessment was to determine leaf loss related to first-generation nymphs, and the August assessment was to determine leaf loss related to second-generation nymphs. To estimate crown foliage, a visual assessment based on Flower et al. (Reference Flower, Knight, Rebbeck and Gonzalez-Meler2013) was employed. Specifically, the crown was visually divided into four quadrants, and in each of the quadrants, the percentage of healthy foliage was recorded. The quadrants were visually partitioned with the observer standing on the street facing the tree and evaluating 25% of the canopy in each quadrant. Once evaluated, all four quadrants were pooled into a single value for each tree. The canopy cover measurements were then partitioned into categories of 0–25%, 26–50%, 51–75%, and 76–100% cover for presentation and statistical purposes.
Pseudogalling
Black ash leaves are compound and have 7–13 leaflets per leaf, with 11 leaflets per leaf being most common in the “Fallgold” variety. Leaves and new branches flush from lateral buds each spring. In the present study, the extent of pseudogalling on each leaf flush (bud) was visually assessed on injected and control trees by applying a rating scale of class 0, 1, 2, or 3 (Fig. 1) for each bud. “Class 0” was assigned (Fig. 1A) when there was no incidence of pseudogalling. A rating of “class 1” (Fig. 1B) was assigned when most leaves were healthy but some rolling of the margins of leaflets was evident. A rating of “class 2” (Fig. 1C) was assigned when some of the leaves were untouched by psyllid damage, but other leaves were entirely distorted. A rating of “class 3” (Fig. 1D) indicated evidence of the most extreme pseudogalling, presented as severe rolling of all the leaf tissue from one bud so that no leaves or leaflets were distinguishable.

Fig. 1. Categorical ratings of pseudogalling damage to black ash buds caused by the cottony ash psyllid, from least severe (class 0) to most severe (class 3).
During the canopy assessment, the most common ontogenetic stage of pseudogalling was identified per quadrant. For analyses purposes, galls were classified and recorded as either class 0, 1, 2, or 3. Trees were visually assessed from the ground and classified based on the proportion of galls that were designated as class 3. Stage 3 galls were used because these are distinct and represent significant damage. Psyllid damage could be identified from the ground throughout the crown. As with the canopy assessment trees, the quadrants were visually partitioned, with the observer standing on the street facing the tree and evaluating the most frequently observed gall type (stages 1, 2, or 3) and proportion of stage 3 galls. Once evaluated, all four quadrants were averaged into a single value for each tree. Additionally, the percentage of stage 3 pseudogalling was recorded in each quadrant. Pseudogalling was recorded only in 2017 because psyllid activity decreased in 2018 and 2019.
Pre- and post-injection egg counting
Overwintering eggs laid by the second generation of P. discrepans on the ends of branches, including the eggs laid on the terminal and two adjacent lateral buds (n = 20/tree), were counted and recorded. Buds were sampled haphazardly throughout the lower crown. The same process was applied before and after treatment. The pre-treatment egg counts occurred in November 2016, and the post-treatment egg counts occurred in November 2017.
Adult monitoring
To monitor the numbers of adult psyllids, two yellow Asian citrus psyllid sticky traps (13.97 × 20.32; Alphascents Inc., West Linn, Oregon, United States of America) were placed in the canopy of each of 10 trees. The traps were secured between branches, with one card suspended in the lower crown in a randomly chosen location and the second trap placed on the opposite side of the crown. The initial round of trapping began 16 June 2017 and continued until 23 June 2017. Traps were placed in the tree before adult psyllids emerged and were left in the tree until after adults finished developing, based on the prior years’ assessment of the psyllid lifecycle. The traps remained in the trees throughout the first generation of adult psyllids and were removed in the first week of August before the second-generation adults emerged.
At the end of the trapping period, the sticky traps were removed, wrapped in cellophane, and stored in a refrigerator at 4 °C until the adult psyllids on the cards were counted. Because the labour and time required to count every insect on the cards would have been prohibitive, a subsampling strategy was employed. Counts of the adults were performed using a 32-square grid placed over the trap, with each square measuring 3.5 × 3.5 cm. The grid was divided into 32 zones, with the X-axis labelled “1” to “4” and the Y-axis labelled “A” to “H”. Eight zones per grid were counted on each side of the trap. The total number of psyllids that fell within the boundary of each target zone was recorded. The total number of insects in the eight squares was averaged and then extrapolated to estimate the total number of psyllids per trap. An insect was counted if its thorax was present: the thorax has two thoracic strips that identify it as a cottony ash psyllid.
Statistical analyses
All analyses were performed in R, version 3.5.3 (R Core Team 2019). For all analyses, individual trees were treated as replicates. Before analyses, data were checked for deviations from normality and overdispersion, and probability distributions were consequently chosen as appropriate. Egg numbers were examined using a generalised linear model fit using the Poisson distribution. The model contained the total number of eggs on each tree (all buds summed) as a response and fixed factors for each insecticide treatment used, and time of count (pre- or post-treatment), and the interaction of treatment time with the type of treatment. The number of adult psyllids on sticky cards was examined using a generalised linear model with a negative binomial probability distribution and a model that included fixed effects for the side of the sticky card, insecticide treatment, and the interaction of the fixed effects. Foliar canopy damage was analysed using a repeated-measures ordinal logistic regression in the repolr function in the repolr package (Parsons Reference Parsons2016). The model included the foliar canopy damage category (class 1–4) as a response, a fixed effect of treatment, and an interaction term for the repeated subject (time) and treatment.
Results
Insecticide uptake
The uptake of TreeAzin was poor, presumably because of the rapid decline of the host trees. This was demonstrated by the injectable liquid that remained in the injector system on trees with poor canopy cover. Overall, of the 50 trees in the treatment group, only 19 could be injected. The 19 trees able to take up the TreeAzin were the healthier trees of the sample group at the time of injection. Because of this bias, these trees and the TreeAzin treatment were excluded from further analysis.
There was, however, evidence of Orthene uptake (Table 1). In the 2017 samples, all trees tested positive for Orthene and methamidophos. In 2018, no Orthene or methamidophos was detected in the flowers of the five trees assessed.
Table 1. Concentration of Orthene and methamidophos in five flowers collected from two black ash trees after injection with Orthene and in five flowers collected from five trees one year after injection.
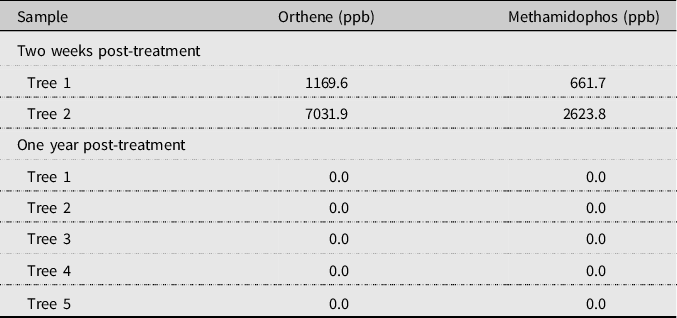
ppb, parts per billion.
Canopy
Canopy category (amount of canopy) differed significantly among treatments (χ 2 = 19.9, df = 1,6, P < 0.001). Foliage category also differed among the time points (χ 2 = 122.3, df = 3,6, P < 0.001), and significant interaction of time and treatment occurred (χ 2 = 8.4, df = 1,6, P = 0.003). This interaction indicates a change in foliage with respect to insecticide application (Fig. 2). Collectively, this result suggests that both treated and untreated trees lost some canopy cover over time but that less loss occurred in trees treated with Orthene.
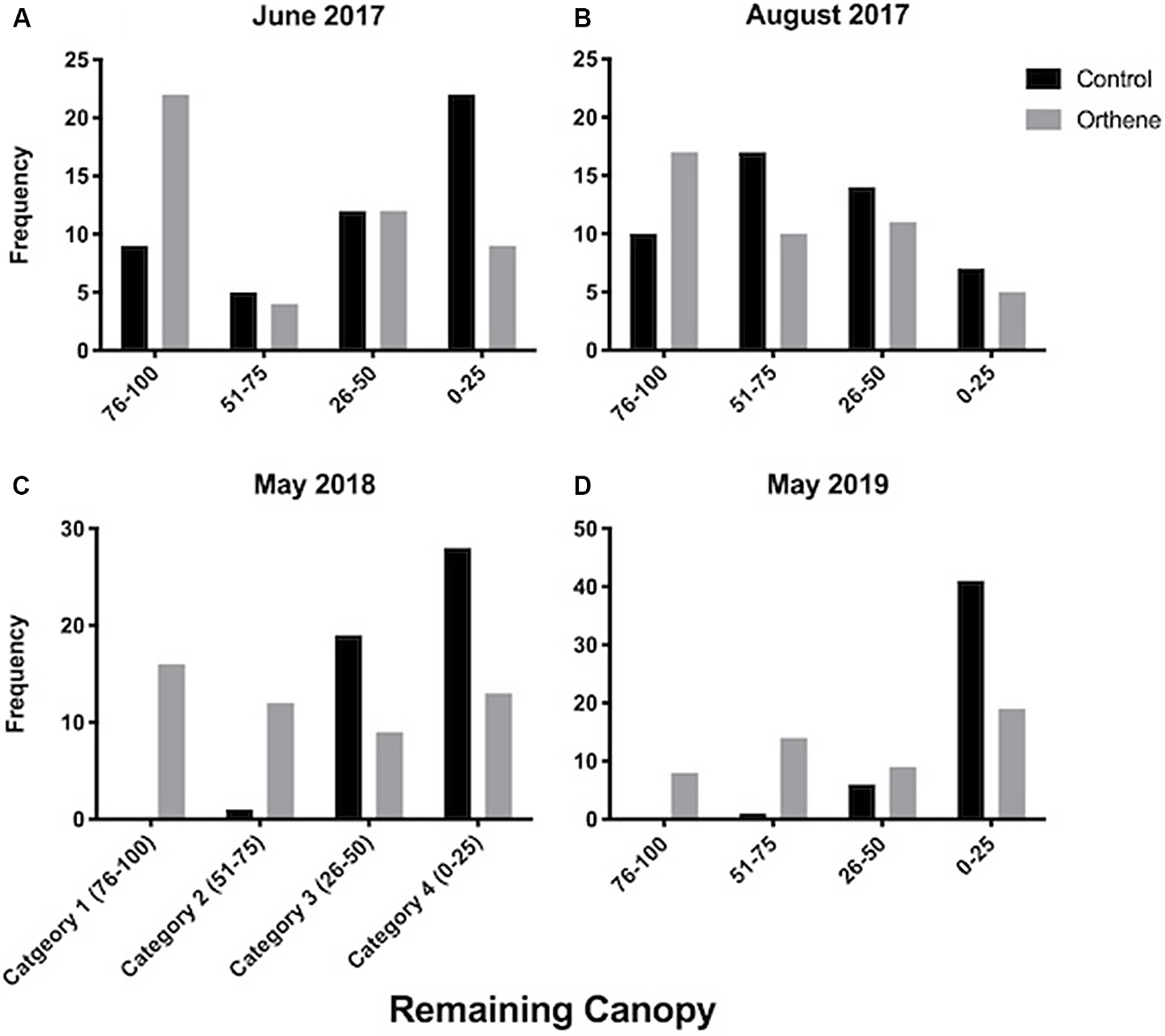
Fig. 2. The frequency of trees in each defoliation category in Orthene-treated and untreated (control) trees when evaluated at four time points post-injection. Note that the selection was based partially on a full canopy and all trees were selected having near 100% canopy.
Trees treated with TreeAzin were excluded from statistical analysis due to the biased and limited samples. However, it is noteworthy that, one year after injection, only three of 19 (15%) treated trees had more than 50% of their foliage remaining compared to 28 of 50 trees (56%) in the Orthene-treated group. Furthermore, in an additional 20 healthy trees with 75–100% of canopy remaining that were treated with TreeAzin, 100% (20 of 20) were subsequently removed.
Pseudogalling
With respect to galls, trees treated with Orthene more often scored as class 1, whereas the control trees (without insecticide treatment) more frequently scored as class 3. These score distributions differed significantly (Chi-square: χ 2 = 11.22, df = 7, P < 0.01; Fig. 3). Furthermore, when the proportion of class 3 galls was examined, the distributions also differed significantly (Chi-square: χ 2 = 14.14, df = 7, P < 0.001; Fig. 4). This difference was primarily due to the greater number of trees that had been treated with Orthene that had no class 3 galls and, conversely, the greater number of trees with a high proportion of class 3 galls in the untreated control trees.
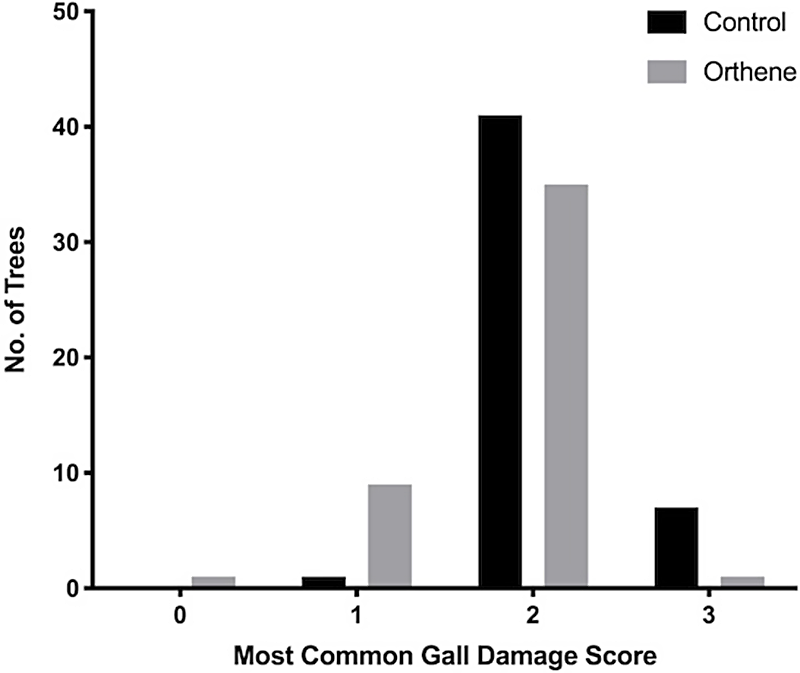
Fig. 3. The frequency (number of trees) of untreated control and Orthene-treated trees in which the most common gall score was class 0, 1, 2, or 3.
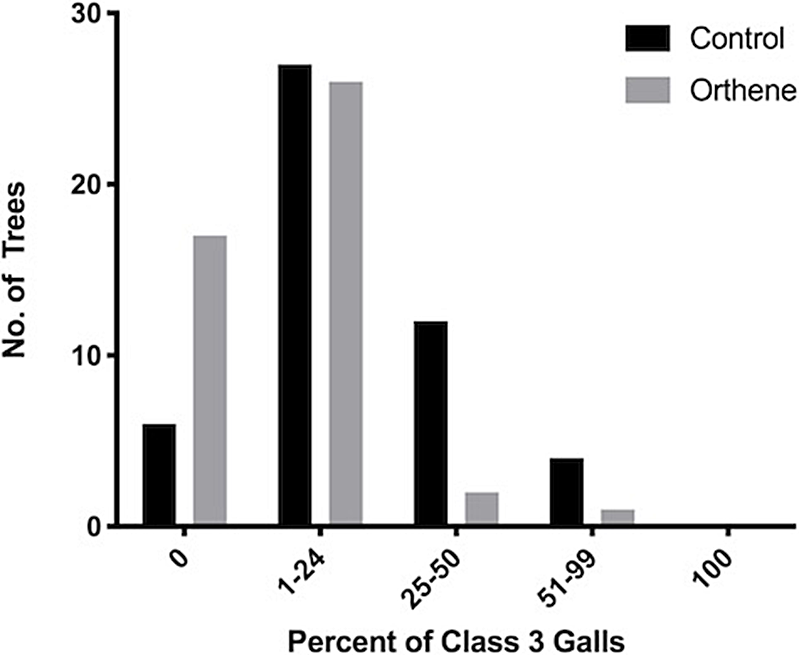
Fig. 4. The frequency (number of trees) of untreated control and Orthene-treated trees with 0–100 percent of galls classified as class 3.
Eggs
In the model, the interaction between treatment and time of count was significant (pre- or post-treatment application; χ 2 = 427.3, df = 1, P < 0.001; Fig. 5). This is the expected result if an insecticide has an effect and is compared to a control. The effects of the treatment factor (χ 2 = 79.9, df = 1, P < 0.001) and of the time factor (pre- or post-treatment application; χ 2 = 259.4, df = 1, P < 0.001) were also significant. Overall, trees injected with Orthene had 32% more overwintering eggs when counted in the fall following the spring application.
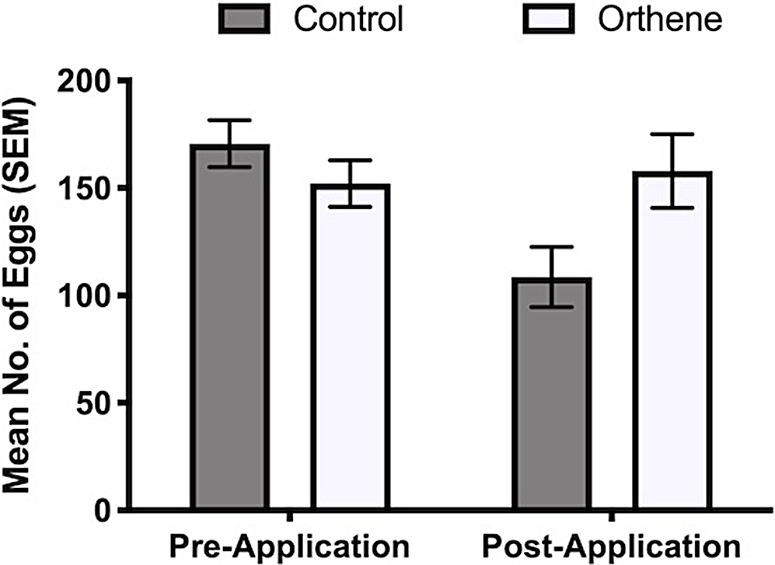
Fig. 5. The number of overwintering eggs laid by second-generation cottony ash psyllids on the ends of branches counting terminal and two adjacent lateral buds in untreated control and Orthene-treated trees before and after Orthene treatment.
Adults
No statistically significant differences occurred between the number of adults on sticky cards (χ 2 = 2.8, df = 1,76, P = 0.09) in control and Orthene-treated trees. A trend towards more psyllids in control trees (558 ± 608) versus Orthene-treated trees (356 ± 608) was detected, but each treatment was associated with substantial variation.
Discussion
Trunk injections of Orthene into black ash result in some level of the insecticidal material reaching flowers and, presumably, other terminal structures, such as the buds that psyllid nymphs feed upon. Unfortunately, uptake of the insecticide by a tree’s tissues appears to depend on the tree’s health and, in particular, the presence of enough leaf cover to result in sufficient upwards transport of the insecticidal material in the phloem. Poor tree health and its associated effects hampered the TreeAzin treatment sufficiently that the treatment group had to be discarded. Despite the greater canopy cover in the 19 trees injected with TreeAzin, only three trees had more than 50% canopy remaining one year after the injection. When trees were treated with Orthene, leaf canopy was significantly preserved compared to those of untreated trees. Similarly, the Orthene treatment reduced pseudogalling damage compared to control trees, but pseudogalling and canopy losses were still present in Orthene-treated trees. Unfortunately, the canopy decreased over time in both treatments, and trees were lost eventually despite the insecticide treatment used. In fact, even with treatment, the canopy was often completely lost within two years after injection. This rate of loss is faster than the rate of canopy loss associated with both emerald ash borer (Agrilus planipennis) (Herms and McCullough Reference Herms and McCullough2014) and Dutch elm disease (D’Arcy Reference D’Arcy2000).
Orthene-injected trees did not differ from control trees in the number of adult psyllids captured on yellow sticky cards. However, more overwintering eggs were collected on Orthene-injected trees following the spring injection. This somewhat counterintuitive result can be attributed to the greater canopy that was maintained in the injected trees. Presumably, Orthene results in a short-term reduction in psyllid population through nymphal death, as was demonstrated by the reduced pseudogalling on the treated trees, but this reduction is followed by rapid recolonisation of the trees by second-generation adults, leading to increased oviposition on the next year’s buds. This outcome suggests that black ash trees with a larger and healthier canopy are more attractive to adult psyllids. However, the specific factors that might be responsible for this increased attraction were not evaluated. Further investigation is warranted, as both colour (Paris et al. Reference Paris, Allan, Udell and Stansly2017) and volatiles (Patt and Sétamou Reference Patt and Sétamou2010) are known to influence behaviour in some psyllid species. Regardless, second-generation adults likely preferred Orthene-treated trees for recolonisation and oviposition because those trees had maintained a greater amount of healthy leaf tissue. Conversely, Orthene application may have also stimulated insect reproduction through exposure to sublethal doses of insecticide (hormoligosis), thereby leading to increased oviposition and egg numbers (Cohen Reference Cohen2006). Hari and Mahal (Reference Hari and Mahal2008) showed that Helicoverpa armigera (Lepidoptera: Noctuidae) lays more eggs on Orthene-treated cotton.
Acephate has been investigated as a control for other tree-attacking psyllid species. Downer et al. (Reference Downer, Koehler and Paine1991) reported that acephate-treated Australian bush cherry performed better against the eugenia psyllid (Trioza eugeniae Froggat) (Hemiptera: Triozidae) than controls did but was less effective than pyrethroids. When Tjosvold and Chaney (Reference Tjosvold and Chaney1996) tested acephate for control of blue gum psyllid (Ctenarytaina eucalypti Maskell) (Hemiptera: Psyllidae) on eucalyptus, they found acephate demonstrated control effects for 61 days but was not substantially more effective than other materials tested. In addition, Casey and Cranshaw (Reference Casey and Cranshaw1995) found that when acephate was soil-applied to hackberry, Celtis occidentalis (Cannabaceae), for control of the hackberry nipplegall maker (Pachypsylla celtidismamma (Fletcher) (Hemiptera: Aphalaridae)) and hackberry blistergall psyllids (Pachypsylla celtidisvesiculum (Riley) (Hemiptera: Aphalaridae)), no difference in galling of acephate-treated trees compared to untreated trees occurred.
Studies of the Asian citrus psyllid have demonstrated sensitivity to an array of insecticidal materials, including organophosphates, pyrethroids, carbamates, horticultural oil, and lipid synthesis inhibitors (Grafton-Cardwell et al. Reference Grafton-Cardwell, Stelinski and Stansly2013, and references within). Currently, however, successful management of this species centres on the use of systemic neonicotinoids (Grafton-Cardwell et al. Reference Grafton-Cardwell, Stelinski and Stansly2013), which are often soil-applied (Sétamou et. al. Reference Sétamou, Rodriguez, Saldana, Schwarzlose, Palrang and Nelson2010) and also exhibit long periods of protection compared to other materials and to foliar application (Grafton-Cardwell et al. Reference Grafton-Cardwell, Stelinski and Stansly2013; Byrne et al. Reference Byrne, Daugherty, Grafton-Cardwell, Bethke and Morse2017). Similarly, multiple insecticidal materials have been shown to be effective against potato psyllid (Bactericera cockerelli) (Echegaray et al. Reference Echegaray, Vinchesi, Rondon, Alvarez and McKinley2017), with soil-applied neonicotinoids proving particularly effective (Prager et al. Reference Prager, Butler and Trumble2013a). In the present study, trunk injection of acephate was examined to control psyllids, and thus tree loss, with limited success. It should be noted that psyllid nymphal stages, which are more susceptible to insecticides and therefore are the preferred target stages in other psyllids, were not examined in the present study, in which pseudogalling was instead used as a proxy for first-generation nymphal activity. The next examination of the effectiveness of insecticidal control of P. discrepans on black ash trees should focus on soil-applied neonicotinoids if the dripline of the tree is available and if neonicotinoids remain registered in Canada.
Although urban forests contribute great value, and damage from pests is a substantial cost to communities, the value of that loss differs from the direct and more easily quantifiable loss of yield in orchard crops such as citrus. Both managers of urban forests and of orchards must consider factors in addition to loss, such as costs of treatment (including labour or outsourcing to contractors) and environmental impacts, but the public's perception of which pest management methods are acceptable and the subjective value the public places on urban forests can increase the challenge of managing pests in urban environments. Therefore, the repeated soil application or trunk injection of insecticides may not be a realistic management method in the urban forest context. Instead, cultural control methods may need to be employed, such as diversified plantings, to prevent widespread buildup of psyllid populations. Urban foresters have implemented the diverse planting approach since at least the arrival of Dutch elm disease in western Canada; black ash is one of the tree species that urban foresters have turned to to increase urban forest diversity.
In summary, trunk injection of trees with Orthene resulted in detectable insecticide concentrations in the trees’ flowers two weeks after injection but not one year after injection. TreeAzin trunk injections resulted in detectable concentrations two weeks after treatment but was limited in uptake and therefore will not be effective as a control method for psyllids. Orthene treatment reduced pseudogalling damage by nymphal psyllids and slowed canopy loss. However, treatment did not reduce the numbers of adult psyllids and was associated with an increase in overwintering egg numbers. Foliar applications of acephate can be associated with harm to beneficial insects and, although effects on beneficial insects were not considered in this study, the potential for harm relative to the limited efficacy shown in the present study should be considered. The mixed results reported here indicate that, although tree injection with Orthene may be a short-term solution to black ash loss, when factors such as cost of treatment and a label restriction limiting application to once every two years are considered, Orthene is probably not a viable long-term solution to preventing losses of black ash trees to cottony ash psyllid invasions.
Acknowledgements
The authors wish to thank BioForest Technologies Inc. for their partnership on the TreeAzin injections and Arbour Crest Tree Service for performing the tree injections with Orthene and TreeAzin. This work was conducted by the City of Saskatoon Urban Biological Services section, and the work of city staff is gratefully acknowledged in the psyllid population monitoring. The authors thank the University of Saskatchewan Entomology Journal Club for their helpful comments on this manuscript.









