Introduction
Around the world, more and more research participants are involved in noninvasive imaging-based studies that investigate the human body.Footnote 1 , Footnote 2 , Footnote 3 With the rise of new imaging technologies, the quality of the scans is rapidly evolving. Consequently, more details are shown on research scans, including details researchers were not looking for. Incidental findings have therefore become a rising concern among researchers.Footnote 4 Incidental findings are defined as “unexpected observations of possible clinical significance in healthy subjects or in patients recruited to research.”Footnote 5 The prevalence of incidental findings in imaging studies varies from 2.7% to 47%, depending, among other things, on the bodily region, the sequences acquired, the age of the research participants, and the research setting.Footnote 6 , Footnote 7 , Footnote 8 , Footnote 9 In approximately 3.2%–6.6% of otherwise healthy research participants, incidental findings are detected that are clinically relevant and participants need referral to a medical specialist.Footnote 10 , Footnote 11
The potential of discovering incidental findings confronts researchers with an ethical dilemma. On the one hand, the detection of incidental findings and the possibility of quick follow-up and treatment may lead to health benefit for the research participant.Footnote 12 But on the other hand, incidental findings may cause harm by giving rise to psychological burdens such as anxiety or emotional distress or even unnecessary treatment.Footnote 13 , Footnote 14 , Footnote 15 Incidental findings often lead to clinical follow-up with the associated medical risks, financial costs, and to what is referred to as “medicalization.”Footnote 16 , Footnote 17 , Footnote 18 Although the possible psychological burden is thus described in the literature, little is known about the consequences of the disclosure of incidental findings to research participants. Previous research has mainly focused on the implications of disclosure of incidental findings in the context of genomics,Footnote 19 , Footnote 20 whereas within the field of imaging studies, empirical data on the impact of incidental findings remain sparse. So far, the ethical implications of incidental findings in imaging studies have mainly been addressed from the researcher’s perspective.Footnote 21 , Footnote 22 , Footnote 23 A few research groups have looked into research participants’ preferences, mapping the preferences and expectations of research participants regarding the disclosure of incidental findings.Footnote 24 , Footnote 25 , Footnote 26 , Footnote 27 This research showed that >90% of the participants would want to be informed about an incidental finding, regardless of its clinical relevance.Footnote 28 Also, most research participants expected that if they had abnormalities, these would be discovered through research imaging, despite being informed otherwise during the informed consent process.Footnote 29 Many participants saw participation in a study as a free “health check.”Footnote 30 This is referred to as the diagnostic misconception: the mistaken belief that the research (also) aims at uncovering clinically relevant abnormalities.Footnote 31 Receiving a (free) health check was an important motivation for participants to take part in a study.Footnote 32 Although in principle, early detection of abnormalities can lead to more timely and effective treatment and thus to health benefit, approximately 76% of the referred incidental findings in brain imaging are followed by a wait-and-see policy or discharge from follow-up.Footnote 33 Schmidt et al. were the first to quantitatively assess the impact of the disclosure of incidental findings on research participants undergoing whole-body magnetic resonance imaging (MRI), and found that 9.9% of the participants to whom an incidental finding was disclosed, experienced “strong” distress and 28.6% experienced “moderate to severe” distress.Footnote 34 So far, it remains unclear whether incidental findings cause more benefit than harm. Consequently, it is still a matter of debate whether and under what conditions researchers should disclose incidental findings to research participants.
In the past few years the ethical implications of incidental findings have been identified and several groups have put forward recommendations on the handling of incidental findings in research settings. Most guidance points to an obligation for researchers to disclose clinically relevant incidental findings to the participants.Footnote 35 , Footnote 36 , Footnote 37 Furthermore, consensus exists that for all research projects involving imaging of human subjects, a protocol should be developed for the detection, management, and communication of incidental findings; also, this protocol should be communicated and agreed upon with the participants during the informed consent process.Footnote 38 , Footnote 39 Existing guidance does not stipulate, however, what a responsible protocol for the handling of incidental findings looks like. It is not clear, for instance, whether researchers should seek to avoid incidental findings (and see it as a risk or a harm of participating in research) or whether they should “hunt” for incidental findings (and see it as a potential benefit of participating in research).Footnote 40 Furthermore, there is still little guidance on practical issues, such as the best way and moment to communicate incidental findings. To answer these questions, it is relevant to know the experiences of research participants, and the impact of the disclosure of incidental findings on their lives.
Many large population-based imaging studies are being conducted across the world, such as the Framingham Heart Study in the United States and the Biobank study in the United Kingdom.Footnote 41 , Footnote 42 As the use of imaging technologies in research is becoming more widespread, it is likely that in the future many other (healthy) research participants will undergo research imaging. These participants will need to be informed beforehand about the risks and benefits of study participation. However, the risks and benefits of being informed about incidental findings are unclear, as the impact of disclosure on research participants’ lives has not yet fully been assessed. The aim of this study is to provide an in-depth qualitative analysis of the finer impacts of incidental findings on research participants’ lives by interviewing individuals who have dealt with incidental findings in the past about their experiences. This interview study was performed within the Rotterdam Study, a prospective longitudinal cohort study in the city of Rotterdam which has been designed to study causes and consequences of age-related diseases at middle and old age.Footnote 43 The Rotterdam Study has incorporated brain MRI in their study protocol since 2005 and has almost 15 years of experience with the management of incidental findings detected during research imaging of the brain.
Research Setting
Since the start of the Rotterdam Study in 1990 almost 15,000 participants aged 45 years and over have been enrolled.Footnote 44 The Rotterdam Study takes place in a single suburban neighborhood of Rotterdam, and all residents who reach the age of 40 are invited to participate in the study. Every 3–4 years, the participants undergo extensive interviews, physical examinations and tests, including cognitive testing, an ECG, and laboratory tests. At the end of the study visit, the participants receive some clinically relevant individual research results (e.g., blood pressure, lab results, and hearing results) from the study doctor. Since 2005, all participants also undergo an MRI scan of the brain as part of the Rotterdam Scan Study.Footnote 45 With a dedicated on-site scanner, the Rotterdam Scan Study was one of the first longitudinal population-based cohort studies to image a large number of healthy research participants. Acknowledging the importance of an appropriate protocol for the management of incidental findings and a certain responsibility toward the research participants, the Rotterdam Study researchers implemented an expert-defined protocol in which all research scans are reviewed by raters: physician researchers who are trained to distinguish abnormal from normal brain scans. During the informed consent process, participants are informed about the possibility of incidental findings and asked whether or not they want incidental findings to be reported, and whether they want their general practitioner to be informed. When an abnormality is flagged, the scan is reviewed by a neuroradiologist to confirm the finding. Incidental findings that are deemed clinically relevant are then disclosed and communicated with the participant. A list of clinically relevant incidental findings was established by an expert panel before the start of the study and is evaluated every 2 years. The researchers monitor the follow-up and clinical outcomes of disclosure,Footnote 46 but they do not routinely assess the psychological and ethical implications of disclosure on their research participants. The current study is meant to fill this gap and to feed into the evaluation of the incidental findings protocol.
Study Design and Study Participants
In the first approximately 10 years of the Rotterdam Scan Study, a total of 549 incidental findings on brain imaging were detected.Footnote 47 The prevalence of incidental findings was 9.5%, whereas the prevalence of incidental findings that were considered in need of clinical referral was 3.2%. All clinically relevant incidental findings were disclosed to the research participants. For this study, nine participants to whom a finding had been disclosed, were interviewed. The aim of the study was to explore and understand the experiences of research participants with incidental findings. In order to capture the more subtle impacts on research participants’ lives and relationships, a qualitative research approach was chosen. The researchers of the Rotterdam Study provided us with a list of participants with a broad range of types of incidental findings and follow-up procedures. An overview of the study participants is shown in Table 1. The participants received a letter containing detailed information about the interview study and were telephoned after 1 week to ask if they had interest in participating in the study, to answer questions, and make an appointment. Data saturation was reached after nine interviews. All participants who were approached chose to participate in the study.
Table 1. Study Participants.
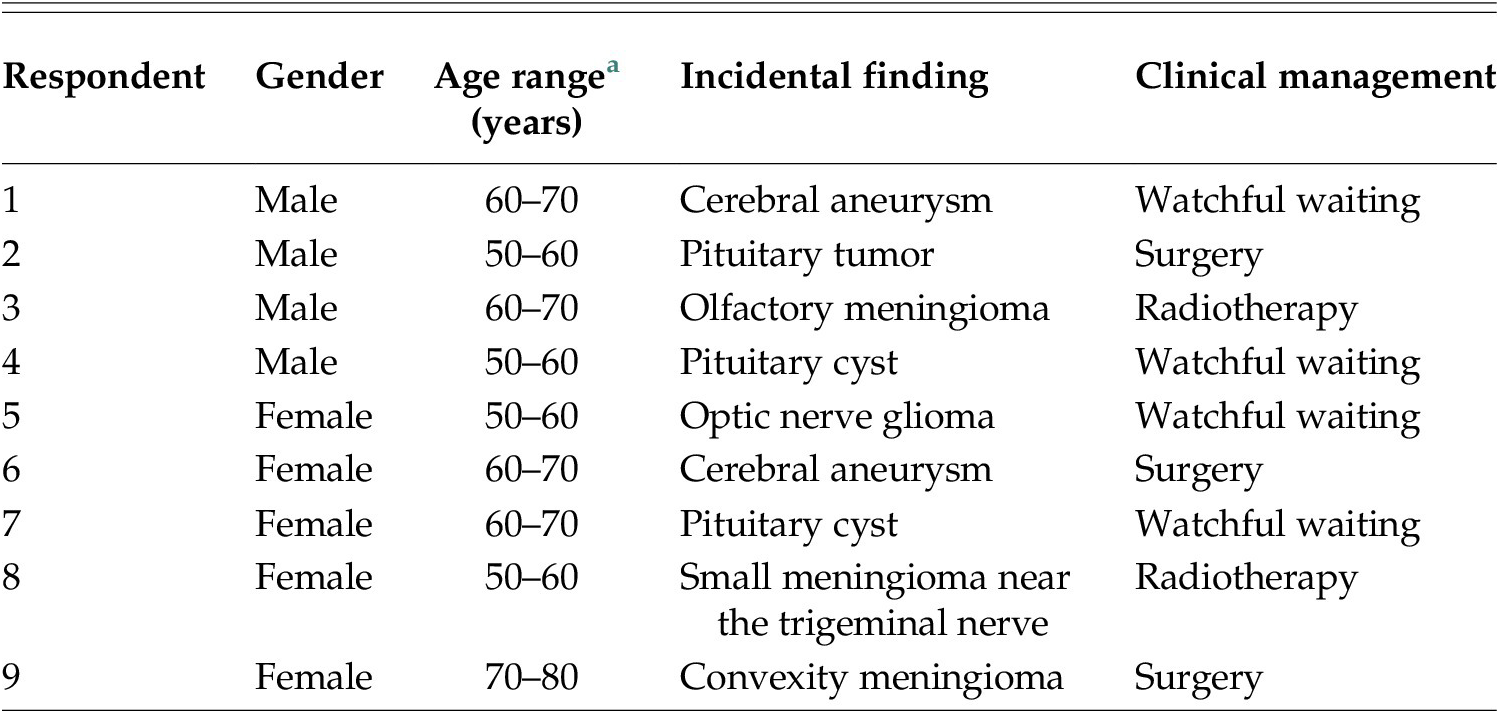
a Age provided in ranges for privacy reasons.
Interviews
Based on the literature, important themes were identified and an interview guide was developed. Themes included perception of health, informed consent, preferences concerning the communication of the incidental finding, impact of the disclosure of the incidental finding and moral attitudes. The interviews were open; participants were encouraged to speak freely and elaborate on their experiences with the incidental finding. During the interviews mainly open questions were asked. The interviews were carried out by one researcher (L.B.) in the summer of 2014 and lasted approximately one and half hour. The interviews took place in the research center or at the participants’ home, depending on the participants’ preferences. All interviews were audio-recorded and transcribed verbatim. During transcription, emotional responses and silences were noted.
Analysis
Interviews were analyzed independently by two researchers (C.B. and E.B.) using directed content analysis.Footnote 48 Codes were assigned and refined through multiple readings. If deemed necessary new codes were added. Discrepancies in coding were solved through discussion. For the coding of the data NVivo 12 qualitative data software was used.
Results
Based on our analysis of the interviews five key themes were identified. We present our results according to these key themes.
Motivations for Research Participation and Informed Consent
For the majority of the respondents, the most important reason to participate in the Rotterdam Study was to evaluate their health. It was notable that some respondents even referred to participation in terms of “taking responsibility for their health” and felt that it would be unwise not to participate.
I’m curious about my health status. And preferably I would want to hear that everything is okay, but even if it weren’t good, then I would also want to know. [participant with an olfactory meningioma, radiotherapy]
Most respondents mentioned more than one reason to participate in the study. Other reasons mentioned were: to contribute to research, to be able to help others, and to be involved in the research project because others, neighbors, or family members, were also involved.
Well, it’s two-sided. You participate in the study so researchers get a better picture of the health of the elderly, I would say. And if I look at myself, I think it’s nice to know if I’m healthy or not. [participant with a pituitary cyst, watchful waiting]
Although all respondents were primarily motivated by an interest in their own health to participate in the study, most respondents had never anticipated the possibility of an incidental finding. Respondents wanted to know about their health and also wanted to know if anything was amiss, although at the same time, they felt ill-prepared for an actual finding. Although they had not anticipated the possibility of an incidental finding, respondents shared the opinion that it would be difficult, if not impossible, for researchers to better prepare research participants in advance for the possibility of incidental findings. All respondents had previously signed an informed consent form which explicitly mentioned the possibility of being confronted with incidental findings. However, not all respondents remembered this. Even if the possibility of an incidental finding would be discussed more extensively during the informed consent process, respondents did not believe that they could have been better prepared for this outcome.
And as long as you don’t feel anything and you’re functioning fine, everyone thinks that nothing will be found. [participant with a small meningioma near the trigeminal nerve, radiotherapy]
Expectations of Research Participation
Most respondents perceived the Rotterdam Study to be a health check. Some respondents believed that if they did not receive any information back from the researchers, this meant that they were healthy. However, most respondents were aware that the absence of any disclosure of detected abnormalities did not necessarily guarantee a “clean bill of health.”
Yes, yes, I understand it’s not a total body check. Yes, I’m very aware of that.… No, I don’t let my entire health depend on the Rotterdam Study. [participant with a small meningioma near the trigeminal nerve, radiotherapy]
All respondents expected that researchers (or doctors) would check the MRI scans for abnormalities in the brain and that abnormalities would be detected and disclosed. Respondents also felt that researchers were morally obligated to search for incidental findings in exchange for their participation in the study. By contrast, some respondents felt that researchers should only look at data necessary for their research questions, and should not feel obligated to look at data beyond the scope of their research question. There was a tension between respondents’ perceptions of research as science on the one hand, and their expectations of researchers’ responsibilities for the health of participants and their perception of research as an individual health check on the other hand.
In my opinion if you [as a researcher] do something like this [an MRI], then you’re obligated to look at it very closely. Otherwise, why would you do it? [participant with an optic nerve glioma, watchful waiting]
Preferences with Regard to Disclosure
Almost all respondents wanted all incidental findings to be reported, regardless of their clinical relevance or the possibility of treatment.
If nothing can be done about it I would still want to know. Because then I would… well, maybe enjoy things more during the last period… That’s what I would think. I’m aware it’s also a burden [for partner and daughter]. But I would still want to know, because then you can take measures. [participant with a cerebral aneurysm, surgery]
Respondents offered multiple reasons why they wanted incidental findings to be disclosed. The most important reason was the possibility of health benefit and treatment. Another important reason was respect for the autonomy of the participant. Respondents felt strongly that they should be informed about anything that may be going on in their own body, regardless of the possible clinical relevance. They felt that they had an ownership over the data concerning their own body. Respondents felt that they should decide whether or not knowing about the incidental finding is relevant or useful. They also referred to a moral principle of reciprocity; in return for their participation, they have a right to know if something is going on.
Yes, openness on behalf of my health. Not openness because of the openness. But I offer my time and body for all sorts of testing, then if something comes forward which is relevant for me, then I have the right or I deserve to hear it. [participant with a meningioma, surgery]
I don’t know. I think it’s ethically incorrect, if something is wrong with someone, because that’s what we’re talking about, to withhold information from that person. I think that everyone has the right to know what’s going on. [participant with a pituitary tumor, surgery]
Most respondents were glad that the incidental finding had been reported. For some participants the disclosure of the incidental finding came as a relief.
For me it was, it sounds crazy, a relief that it had been found. Because when I said this headache, it isn’t migraine but something else…. It was a confirmation that I had been right all along. So that has been very positive. I’ve felt it right, I felt it right all along. [participant with a cerebral aneurysm, surgery]
Suppose that after all it is something abnormal? Now I’m being checked every time. Yes, the doctor also asked me, isn’t it a burden to come to the hospital every time? I told him I’d rather have 10 more MRIs, than that I wouldn’t be allowed to come for the next 5 years. [participant with an optic nerve glioma, watchful waiting]
Despite their preferences for the feedback of information on incidental findings, respondents did consider reasons not to report findings. One frequently mentioned reason was the psychological burden that comes with the disclosure of an incidental finding. Most respondents considered themselves to be down-to-earth and said that they felt competent at dealing psychologically with the disclosure of the finding, but they could imagine that other people might experience more emotional distress. Also, clinical relevance was taken into consideration; one participant believed that if an incidental finding did not have any clinical relevance, it should not be disclosed. Respondents also underlined how stressful the first period was after the incidental finding was disclosed.
If I don’t look at myself, but at other people, I think it can be very tough to live in uncertainty and fear all this time… Look, you don’t wish this for anyone; the nerves, the stress you experience before the examinations… Like I said, I wouldn’t like to repeat those first 7 months. [participant with an optic nerve glioma, watchful waiting]
Furthermore, age was mentioned as a factor which should be taken into account when reporting incidental findings. Two participants mentioned that now that they were older, they were better able to put things in perspective and that they wanted incidental findings to be disclosed, but that this might not have been the case when they were younger.
When you’re younger, and if then you already have to carry such pressure… like ‘it might turn out the wrong way for me…’ I wouldn’t know what my opinions would have been then [about the disclosure of an incidental finding]. [participant with an olfactory meningioma, radiotherapy]
Respondents thought that as part of the informed consent process, participants should be offered a choice whether or not they want incidental findings to be disclosed. Respondents felt that whether they want incidental findings to be disclosed is a personal matter that is different for every individual and that therefore that choice should always be made by the research participants themselves.
Short-Term and Long-Term Impact
Immediately after the disclosure of the incidental finding, nearly all respondents experienced shock and disbelief. They all still remember the moment they were informed very vividly. The disclosure of the incidental finding had a substantial impact on the lives of the respondents in the first period after the disclosure and some respondents experienced emotional distress in the first few months.
I was just taken off guard. I was just completely taken off guard, that’s how I experienced it. [participant with an optic nerve glioma, watchful waiting]
And also after the first phone call. Then you’re at a total loss what to do. And then you discuss it over here [in the research center]and you accept it. But afterwards I’ve been worrying the entire night. I’ve dropped out for two days with migraine headaches. [participant with a small meningioma near the trigeminal nerve, radiotherapy]
However, two respondents said that they did not experience any shock when they heard the news.
No not at all. No, why be scared if you don’t even know yet what’s going on. So no. That’s still possible afterwards [after more examination]. [participant with a pituitary tumor, surgery]
In the long term, the respondents said that the disclosure of the incidental finding had little impact on their lives. All respondents said they were able to deal with the disclosure of the incidental finding adequately, and none of the respondents said they experienced emotional distress in the long term. However, some respondents did still experience some momentary stress during follow-up examinations.
The doctor had reassured me and then I’ve stopped thinking about it. Sometimes it comes up, then I think hey there’s something going on over there [in the brain]. I could worry about it every day, but that wouldn’t help either. [participant with a pituitary cyst, discharged from follow-up]
If you know it for a few months and nothing happens, then it [the fear] fades. [participant with a cerebral aneurysm, watchful waiting]
For all respondents, the disclosure of the incidental finding did not have any effect on the perception of their health, even as they are still undergoing routine check-ups. Respondents with an incidental finding which could be treated were thankful that the incidental finding had been found. In some cases, due to early intervention, a great deal of suffering could be avoided. For that, research participants were very grateful.
Respondents were pleased with the procedure concerning the incidental finding and especially appreciated the brief waiting time (a few days at most) between the disclosure of the incidental finding and the hospital appointment for further diagnostic evaluation. Most respondents were content with the disclosure of the incidental finding by a principal investigator involved in the Rotterdam Study, some respondents would have preferred it if the incidental finding was disclosed by their general practitioner. All respondents agreed to letting their general practitioner be informed (by letter) about the incidental finding.
Impact on Respondents and Their Social Surroundings
As mentioned above, almost all respondents considered themselves to be down-to-earth and, when asked, they said that they did not experience emotional distress in the long term. However, they did offer narratives about the impact of the incidental finding on their lives that suggested otherwise. Some respondents wondered whether they might have been better off not knowing. For instance, one respondent mentioned that every time he had a headache, he wondered whether it had something to do with the finding.
Well… Every time I have a headache, I think…. it won’t have something to do with it [the incidental finding], will it? That’s a disadvantage. God, will it be that? [the incidental finding]. Yes of course that’s the case, that’s part of your world now. [participant with an olfactory meningioma, radiotherapy]
Also, multiple respondents had follow-up examinations for a few years, which did have an impact on their lives.
You do realize, but that’s an emotion of course, that well, during multiple years you will have to get a scan. You’re part of a circuit now, of which you never were part before. In fact, you have to give up your independence, you are at the mercy of the healthcare system in the hospital, and that’s a strange sensation. Yes, that’s not pleasant. [participant with an olfactory meningioma, radiotherapy]
The respondents perceived the impact of disclosure of the incidental findings on themselves as small. However, they did talk about the more serious impact on their direct social environment. Multiple respondents mentioned a serious impact on the people surrounding them.
And yes, at some time you’re able to get some peace. And then you tell it to everyone at the office and you tell your family… And strangely enough you become much calmer… While the people surrounding you are scared to death. They were much more scared then I was. [participant with a small meningioma near the trigeminal nerve, radiotherapy]
One respondent mentioned that at her daughter’s wedding, she found out how much her daughter had been affected by the news and had been worrying about her.
But also for the people surrounding you. Your children for instance. I know they have been worrying a lot. You’re their mother, and one [of my children] was going to get married, and the other one wanted to get married. And they thought ‘What if [mother] isn’t here anymore to see it?’ They told me afterwards, at their wedding, through the wedding official – my daughter wanted to say something but she couldn’t do it herself. She had been worrying so much. I found that very upsetting, because she couldn’t even… [tell me]. And that she had the feeling like, I have to hurry because maybe mom wouldn’t be able to see me in my wedding dress. [participant with an optic nerve glioma, watchful waiting]
Another respondent with a cerebral aneurysm mentioned that although she was rather calm herself, her partner had been frightened. In his family someone had died because of a ruptured aneurysm, and therefore he wanted to inform her daughter immediately.
He [her partner] said: ‘Will you please sit down?’ Well.. he knew what it [an aneurysm] was. In his family, a family member had had a ruptured aneurysm. So he was scared to death. And so he arrived and he said ‘Does [her daughter] know?’ I have one daughter. I said no. He says: ‘Put your shoes on and come!’ And I thought, I don’t understand what he’s up to. I said ‘Oh she’s coming on Thursday, I’ll tell her then’. He said ‘No, we’re going to [see her] now! Well and when we arrived, he told her what was going on and that it was rather dangerous. [participant with a cerebral aneurysm, surgery]
Discussion
Until now, qualitative studies have mainly focused on the preferences and expectations of research participants regarding the procedural aspects of the disclosure of incidental findings in imaging studies.Footnote 49 , Footnote 50 , Footnote 51 These previous findings are largely in accordance with the results of this study; participants preferred a short waiting time between the disclosure of the incidental finding and clinical follow-up, they preferred the communication of the finding to be done by a researcher who was involved in the study, and they all agreed to letting their general practitioner be informed. In accordance with those studies, our study also showed that research participants have expectations regarding imaging studies that researchers might not always be aware of. For instance, research participants expect researchers to always check for abnormalities. Moreover, research participants have a strong preference for being informed about incidental findings. Researchers and ethics committees need to be aware of participants’ preferences and expectations regarding the procedural aspects of the disclosure of incidental findings, so that they can accommodate or address these in their procedures. In this study however, we have focused not on these more procedural aspects but on understanding the impact and meaning of the disclosure of an incidental finding on the lives of research participants.
In our in-depth interviews with research participants, we noticed tensions in their narratives of what had happened to them and how they evaluated their experiences. There were tensions between their experiences at the time of hearing about the incidental finding and their valuations of knowing about the finding in the present. Learning about the finding was a shock at first, they would typically say, but now they have adjusted and are coping well. Also, on the one hand, they say that they are happy to know about the finding, on the other hand, some suggest that they might have been better off not knowing.
One of the most striking findings in our study was the discrepancy between participants’ explicit and implicit valuations of the impact of the incidental finding on themselves and on their lives. When asked by the interviewer, participants initially reported that the incidental finding had a relatively small impact on them psychologically. Further on in the interview, however, participants’ narratives revealed an evidently larger impact both on the participants themselves and on their loved ones and social environment. One dramatic example is a respondent’s story of the role the incidental finding had played on her daughter’s wedding day. The participant had been informed about a small meningioma that may very well remain asymptomatic for years—or for good—but that caused her and those surrounding her to worry deeply nonetheless. Partners, children and other loved ones of research participants may be affected, too, duly or unduly, by the implications of the incidental finding. Until now, this impact on the social environment has not fully been described in the literature. Therefore, this makes us question whether the actual impact of incidental findings on participants’ lives might be greater than has been suggested based on previous studies. These “hidden harms,” these softer impacts on the lives of research participants and their loved ones, merit further attention from psychologists and medical ethicists in the future.
Nearly all of our participants mentioned the shock they first experienced when the incidental finding was reported to them and the emotional distress it gave rise to in the first few weeks and months. Ultimately, however, all participants were satisfied with the disclosure of the incidental finding. Even those who may not have gained medical benefit were happy that they knew about the finding. This is in accordance with previous research regarding the impact of incidental findings.Footnote 52 This could be explained by a similar mechanism that is known from ethical discussions on population screening and what we would like to call the concept of the positive feedback loop. The mechanism underlying this concept has been referred to before in the context of incidental findings.Footnote 53 When participating in screening, individuals will tend to be positive about the outcome: if no abnormalities are found, individuals will be happy that they have been checked, as they now feel reassured, and if abnormalities are found, participants will be happy that the abnormality was detected early as it can now be treated or monitored and (possibly) benefit their health. Therefore, one could argue that, after overcoming the initial shock, participants will always be content with the disclosure of an incidental finding. This concept of the “positive feedback loop” might explain why at first glance, the drawbacks of incidental findings tend to remain underexposed.
Most participants wanted all incidental findings to be reported, regardless of their clinical relevance. Participants simply wanted to know anything that the researchers may have detected, even if the finding may be difficult to interpret or not actionable clinically. This is largely in accordance with previous studies,Footnote 54 , Footnote 55 , Footnote 56 but this is not in accordance with current ethical guidance, which states that only potentially clinically relevant findings should be reported.Footnote 57 Respondents cited the principle of autonomy, feeling that they should receive all information concerning their own bodies. They themselves should decide whether or not a finding is relevant, clinically or personally. They also referred to the moral principle of reciprocity: in return for their contribution to the research project, they expect researchers to look for incidental findings and to act on them. Previous research has shown that not only participants, but researchers too, recognize the principle of reciprocity.Footnote 58 Some participants also cited personal utility: even if a finding may not lead to medical benefit, it could be useful from a personal perspective, for instance, in making important life changes. This argument, however, may not apply to findings of limited clinical validity; when it is not clear what the finding means for the participant’s (future) health, there may not be any personal utility to be gained and participants might even experience harm.Footnote 59 Therefore, a principle of reciprocity may pertain to findings detected in the course of research that constitute information of potential (clinical) relevance to research participants, but it may not pertain to findings that are uninterpretable (and do not constitute information at all). However, this could change in the future. Societal trends such as large-scale data collection together with a growing belief that people should own their own health data, might give rise to changes in how we evaluate current ethical guidance.
Another important finding in our study was that although their health status was an important motivation for participation in the Rotterdam Study and respondents wanted to be informed, they were not prepared for an actual finding. Even though the possibility of an incidental finding had been explicitly discussed during the informed consent process, participants felt ill-prepared, and sometimes did not even remember that it was discussed. It is well known that participants often forget or misinterpret what has been said during informed consent processes.Footnote 60 Our respondents, however, believed that even if informed consent discussions would be more extensive, they would still not have been prepared for incidental findings. Furthermore, our study also showed that a majority of the participants regarded the research as a health check, despite being told otherwise during the informed consent process, marking the occurrence of diagnostic misconceptions.Footnote 61 This too, emphasizes the importance of future research into expectations management regarding the disclosure of individual research results and adequate communication of the possibility of incidental findings during the informed consent process.
The current study has several limitations. First, in this study, only participants who in the first place wanted incidental findings to be disclosed were interviewed. This might have caused a selection bias which could have led to a more optimistic picture of research participants’ experiences. Second, the Rotterdam Study is a large cohort with a clear, careful and rigorous protocol concerning incidental findings. In smaller cohorts with less experience with incidental findings, the experience of participants might be different. Finally, another limitation of this study is the small sample size (n = 9). However, the aim of this study was to understand the impact the disclosure of an incidental finding may have on the lives of individual research participants, and for this reason, we used extensive, in-depth qualitative interviews with a few individual research participants, rather than more cursory surveys of larger groups of participants. The interviews allowed us to extract examples, memories, after-thoughts, and tensions in their narratives. By using a narrative approach our study provided fine-grained insight into respondent’s views that might not have been obtained by asking respondents to fill out questionnaires.
Conclusion
By closely examining research participants’ narratives on the impact of the disclosure of incidental findings and the tensions that came forward during our interviews, some important lessons can be learned. First, the impact of incidental findings on the lives of participants may sometimes be greater than participants let on at first. Second, incidental findings can also have a significant impact on the social environment of the research participants. Until now, this impact on the people surrounding the research participant has never fully been described in the literature. Third, participants do not feel prepared for the disclosure of an incidental finding, even if it has been discussed during the informed consent process and even if they have consented to being informed of clinically relevant abnormalities. It is important for researchers, research managers, policymakers, and members of research ethics review committees to be aware of these existing tensions in research participants’ expectations and experiences, for this may help them to design suitable courses of action for the detection, management, and communication of incidental findings in research.
Funding
This study is a result of the research project “Previously healthy? An ethical approach of incidental findings through imaging studies,” which was funded by ZonMW, dossier number 731010004 (2013–2015). Additional funding for this project was awarded to Eline Bunnik by the WiRe Fellowship Program at the University of Münster, Germany (2019).



