Coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the WHO declared the pandemic in March 2020, more than 230 million infections and around 4·8 million deaths have been reported around the globe(1). Pending the distribution of newly developed vaccines, the health care system is still facing unprecedented pressure. Apart from being an acute respiratory disease, COVID-19 is associated with broad-spectrum symptoms of systemic inflammation and multiple organ dysfunction that leads to the persistence of symptoms and long-term complications(Reference Ayoubkhani, Khunti and Nafilyan2–Reference Nalbandian, Sehgal and Gupta4). COVID-19 is characterised by immunosuppression in the first stage (or non-severe symptomatic period) and hyper-inflammation in the second stage (or severe symptomatic period)(Reference Jacques and Apedaile5,Reference Ni, Alu and Lei6) . A well-coordinated immune response promotes recovery, while an uncontrolled systemic inflammatory response leads to complications and death(Reference Jacques and Apedaile5). SARS-CoV-2 infection activates a strong innate immune response in epithelial cells and alveolar macrophages followed by neutrophil/monocyte infiltration(Reference Jacques and Apedaile5). The SARS-CoV-2 virus appears to strongly activate respiratory burst in neutrophils, generation of reactive oxygen spaces and formation of neutrophils extracellular traps (NET)(Reference Arcanjo, Logullo and Menezes7,Reference Yang, Biermann and Brauner8) . The process of NETosis may contribute to microthrombotic events that drive organ damage in COVID-19(Reference Arcanjo, Logullo and Menezes7). Additionally, exhaustion and decrease in the number of lymphocytes, particularly T regulatory lymphocytes (Treg), are found in patients with severe COVID-19 and might add to the loss of regulatory functions and cytokine storm(Reference Chen, Zhou and Dong9,Reference Silva, Tomiotto-Pellissier and Sanfelice10) . The release of cytokines such as TNF α, IL-1β and IL-6 disrupts alveolar-capillary barrier integrity and results is in acute respiratory distress syndrome (ARDS), extensive microthrombus formation and multiple organ failure(Reference Jacques and Apedaile5,Reference Arcanjo, Logullo and Menezes7) .
The optimal status of specific nutrients is considered of uttermost importance for the proper functioning of the immune system. The European Food Safety Authority declared six vitamins (D, A, C, folate, B6 and B12) and four microelements (Zn, Fe, Cu and Se) as essential for the normal functioning of the immune system(Reference Galmés, Serra and Palou11). A recent study in COVID-19 patients showed that most of the patients with respiratory distress were vitamin deficient, whereas vitamin D and Se deficiency were the most commonly found(Reference Im, Je and Baek12). In that respect, the effect of supplementation with vitamins and microelements has been studied in various registered clinical trials up to date, sometimes with conflicting results. Besides, supplements have been commonly used as prophylaxis in healthy, or as adjunctive treatment in COVID-19 patients, even without medical prescription.
We aimed to summarise and discuss current evidence-based knowledge about the physiological role of vitamins and microelements in the immune system regulation in consideration with COVID-19 pathogenesis, as well as recent findings related to their usage and potential effects in the treatment of COVID-19 patients.
Methods
PubMed database was searched for articles on vitamins and microelements, their role in the immune system or the prevention or treatment COVID-19. Specifically, the following search terms were used: (1) COVID-19 OR SARS-CoV-2 OR CoronaVirus AND (Vitamin D OR Vitamin A OR Vitamin C OR Vitamin B6 OR Folate OR Vitamin B12 OR Selenium OR Zinc OR Copper OR Iron). This part of the search included papers published from December 2019 to May 2021. In addition, we used the following search terms to explore potential mechanisms of vitamins and microelements involved in the immune system regulation: (2) Immune system OR Immune response AND (Vitamin D OR Vitamin A OR Vitamin C OR Vitamin B6 OR Folate OR Vitamin B12 OR Selenium OR Zinc OR Copper OR Iron). This part of the search included papers published from January 2000 to May 2021. The reference lists of relevant articles were also reviewed to identify additional appropriate articles. The abstracts were evaluated by two independent pairs of reviewers (B. Djordjevic and A. Velickov; J. Milenkovic and D. Stojanovic), and papers considered not relevant were excluded. The selected studies were evaluated in full text to identify molecular and physiological mechanisms of vitamin/micronutrient actions in consideration with COVID-19 pathogenesis or crucial interventions and outcomes related to the use of vitamins/micronutrients in the prevention/treatment of COVID-19.
Vitamins against coronavirus disease 2019
Vitamin D
Vitamin D is a group of fat-soluble secosteroids that caught a lot of attention due to the potential role in the prevention and treatment of COVID-19. Beyond its well-known function in Ca homoeostasis, vitamin D3 exerts a profound effect on the immune responses, both innate and adaptive. It might have led to prompt empirical inclusion of vitamin D as an adjunctive treatment in COVID-19. However, current guidelines and several studies argue that there is still not enough evidence to support its use in the prevention and treatment of the SARS-CoV-19 infection. There is a need for well-designed RCT; hence, further investigations are encouraged(13–Reference Ali16).
Mechanisms of vitamin D immunomodulatory actions
Generally, after its synthesis or ingestion, vitamin D3 is metabolised into 25-hydroxyvitamin D3 (25(OH)D) in the liver. Circulating 25(OH)D is then activated by the 1α-hydroxylase (CYP27B1) into the 1α,25-dihihydroxyvitamin D3 (1,25(OH)2D), which exerts autocrine and paracrine functions(Reference Charoenngam, Shirvani and Reddy17,Reference Liu, Stenger and Li18) . The major circulating form is 25(OH)D, and its serum concentrations are measured to assess vitamin D status. A total 25(OH)D cut-off serum levels of ≥ 30 ng/ml (75 nmol/l) are generally considered sufficient, the levels of 20–29 ng/ml represent vitamin D insufficiency, and below 20 ng/ml (50 nmol/l) a deficiency(Reference Ali16,Reference Holick, Binkley and Bischoff-Ferrari19,Reference Grant, Lahore and McDonnell20) .
The rationale for the wide use of vitamin D in COVID-19 patients is based on its immunomodulatory and anti-inflammatory effects, which might prevent cytokine storm syndrome and severe lung damage. Many vitamin D beneficial effects on immune functions have been described so far(Reference Aygun15,Reference Charoenngam, Shirvani and Reddy17,Reference Kim, Baek and Hong21–Reference Fernández-Quintela, Milton-Laskibar and Trepiana23) . It induces potent antimicrobial effects in human monocytes that are dependent on its active form and vitamin D receptor (VDR). Following Toll-like receptor (TLR)1/2 heterodimer activation, the VDR gene is upregulated in monocytes and macrophages, and the CYP27B1 gene in dendritic cells. Therefore, there is local activation of 25(OH)D, which further upregulates defensins and microbicidal peptides such as cathelicidin LL-37 and CYP24-hydroxylase (vitamin D-inactivating enzyme)(Reference Liu, Stenger and Li18). Besides being capable of attacking intracellular microbes, cathelicidin acts against fungi and invading respiratory viruses(Reference Charoenngam, Shirvani and Reddy17). Additionally, vitamin D impairs TLR9-induced IL-6 production in human peripheral blood monocytes, whereas TLR3 (endosomal dsRNA detector) remains unaffected(Reference Dickie, Church and Coulthard24). T-cell cytokines may influence innate immune responses by modulation of vitamin D metabolism. Interferon γ (IFN-γ) upregulates CYP27B1, enhancing the conversion of 25(OH)D, while IL-4 induces CYP24 hydroxylase(Reference Edfeldt, Liu and Chun22).
Vitamin D increases IκBα mRNA and protein levels, while its receptor, VDR, decreases NF-κB activity by physical interaction with IκB kinase β (Reference Chen, Zhang and Ge25). 1,25(OH)2D3 may further increase the inhibitory activity of VDR. By blocking of NF-κB pathway, vitamin D downregulates many cytokine genes, including genes for plasminogen activator inhibitor1, renin and angiotensinogen(Reference Chen, Zhang and Ge25). Specifically, it was determined to inhibit the TNF family of genes, IFN-γ, IL-2, IL-6, IL-12, IL-17 and IL-22(Reference Aygun15,Reference Grant, Lahore and McDonnell20,Reference Kim, Baek and Hong21,Reference Allen, Kelly and Basdeo26–Reference Grant, Baggerly and Lahore28) , while increasing the anti-inflammatory IL-10 involved in producing Tregs(Reference Allen, Kelly and Basdeo26).
Many additional potentially beneficial effects of vitamin D against COVID-19 are being proposed, such as altered ACE2 receptor expression, inhibition of renin action and angiotensin II (Ang II) accumulation, maintenance of epithelial gap junctions, protective effect on the alveolar epithelium (trophic and anti-apoptotic effect) and anti-thrombotic actions(Reference Ali16,Reference Kong, Zhu and Shi29,Reference Dancer, Parekh and Lax30) . In an animal model, vitamin D attenuated acute lung injury, by the inhibition of angiopoietin-2-Tie-2 signalling, thus creating protection of vascular barrier. Vitamin D receptor is highly expressed in the lungs, and VDR-null mice exhibited more severe lung injury, with neutrophil infiltration, pulmonary inflammation, vascular leakage and increased pulmonary levels of renin and Ang II(Reference Kong, Zhu and Shi29).
The use of vitamin D in prophylaxis and therapy of coronavirus disease 2019
Two recent meta-analyses found that vitamin D supplementation is safe and effective in preventing acute respiratory infections overall, especially in those with vitamin D deficiency at baseline (< 25 nmol/l) and when taken in recommended doses for a longer period(Reference Martineau and Forouhi31,Reference Jolliffe, Camargo and Sluyter32) .
Unfortunately, the elderly with chronic diseases and patients with ventilator-acquired pneumonia are likely to have vitamin D deficiency(Reference Miroliaee, Salamzadeh and Shokouhi27,Reference Grant, Baggerly and Lahore28) . Vitamin D deficiency was also reported to be common in people who develop ARDS, especially in those who died(Reference Dancer, Parekh and Lax30). The population at higher risk for COVID-19 has the same risks as for vitamin D deficiency, such as older age, African Americans, obese, cancer patients, chronic kidney failure or liver disease, autoimmune conditions, pregnant females, healthcare workers, etc(13,Reference Charoenngam, Shirvani and Reddy17–Reference Holick, Binkley and Bischoff-Ferrari19) .
A substantial number of observational small-scale studies and systematic reviews reported the important role of vitamin D in decreasing the risk for COVID-19 and related mortality, supporting its prophylactic and therapeutic use(Reference Martineau, Jolliffe and Hooper14,Reference Ali16,Reference Charoenngam, Shirvani and Reddy17,Reference Grant, Lahore and McDonnell20,Reference Tan, Ho and Kalimuddin33–Reference Ling, Broad and Murphy37) . A recent study investigated 25(OH)D status in 287 adult COVID-19 patients and revealed an independent association between a total of 25(OH)D levels of ≥ 30 ng/dl and decreased risk of mortality in elderly and non-obese COVID-19 patients(Reference Charoenngam, Shirvani and Reddy17). Similarly, a study that correlated data of COVID-19 cases and deaths per 1 million general population (Europeans) to the vitamin D serum levels found a significant negative correlation between mean vitamin D concentrations and an incidence of COVID-19 cases, but not deaths(Reference Ali16). The meta-analysis by Kazemi et al. (Reference Kazemi, Mohammadi and Aghababaee38) indicated a significant relationship between vitamin D status and SARS-CoV-2 infection, COVID-19 composite severity and mortality, simultaneously emphasising the study limitations reflected in heterogeneity in methodological and statistical approaches.
Since a substantial number of people do not get enough vitamin D from natural sources, there is a question of whether it is possible to increase vitamin D levels quickly enough, with high bolus doses, after one gets COVID-19. Is it going to be effective and safe? Besides, the vitamin D serum threshold that would provide some protection against COVID-19 is not determined(Reference Grant, Baggerly and Lahore28).
National Institute for Health and Care Excellence recommends vitamin D supplementation of 10 μg (400 μg) a day, which would be enough to prevent serum 25(OH)D from falling below 10 ng/dl (< 25 nmol/l). The tolerable UL for adults is 100 μg (4000 μg) a day and should not be exceeded(13). USA National Academy of Medicine and the European Food Safety Authority supports a target blood level of vitamin D of at least 20 ng/ml (50 nmol/l), which requires supplementation of 800 μg a day(Reference Griffin, Hewison and Hopkin39). The Endocrine Society’s Clinical Guidelines suggest that raising 25(OH)D levels above 30 ng/ml may require at least 1500–2000 μg a day, while the maintenance tolerable UL should not exceed 4000 μg a day(Reference Holick, Binkley and Bischoff-Ferrari19).
However, people who become SARS-CoV-2 infected might need higher vitamin D doses, especially those already deficient(Reference Grant, Lahore and McDonnell20,Reference Fernández-Quintela, Milton-Laskibar and Trepiana23) . There are several recommendations regarding vitamin D supplementation, but the goal should be to raise 25(OH)D levels above 40 ng/ml (100 nmol/l)(13,Reference Holick, Binkley and Bischoff-Ferrari19,Reference Grant, Lahore and McDonnell20,Reference Griffin, Hewison and Hopkin39) . According to Heaney et al (Reference Heaney, Davies and Chen40), when starting from 25(OH)D baseline level of around 20 ng/ml (70 nmol/l), it takes about a month to reach 60 ng/ml with 10 000 μg a day and about three months with 4000 μg a day. According to the Endocrine Society Clinical Practice Guidelines, adults who are vitamin D deficient should be treated with 50 000 μg once a week for 8 weeks or 6000 μg a day to achieve a level of 25(OH)D above 30 ng/ml, followed by the maintenance therapy of 1500–2000 μg/d(Reference Holick, Binkley and Bischoff-Ferrari19).
There are no clear results on whether short-term high doses of vitamin D might improve outcomes in critically ill patients(Reference Ling, Broad and Murphy37,Reference Amrein, Scherkl and Hoffmann41,Reference Ginde and Brower42) . In the VITdAL-ICU study, vitamin D was given enterally once at a dose of 540 000 μg, followed by monthly maintenance doses of 90 000 μg, to critically ill patients with vitamin D deficiency. There was no general reduction in hospital length of stay nor mortality, except for in-hospital mortality of those who were severely vitamin D deficient (≤ 12 ng/ml)(Reference Amrein, Scherkl and Hoffmann41). Similarly, a single dose of 200 000 μg of vitamin D did not affect hospital length of stay in patients with moderate to severe COVID-19(Reference Murai, Fernandes and Sales43). In contrast, vitamin D booster therapy in a dose of 280 000 μg in a period of up to 7 weeks appears to be associated with a reduced risk of mortality in COVID-19 patients(Reference Ling, Broad and Murphy37). In another study, vitamin D supplementation gave significant protection against respiratory infections in those receiving daily or weekly vitamin D doses, but not in those with one or more bolus doses (of at least 30 000 μg)(Reference Martineau, Jolliffe and Hooper14).
Prolonged use of bolus doses could cause wide fluctuations in 25(OH)D circulating levels, which might provoke dysregulation of vitamin D hydroxylases, with possible cellular maladaptation and impairment of respiratory epithelium(Reference Vieth44). Another concern is that high doses in a short period, which would improve serum levels may not provide and sustain overall body needs. Therefore, it might be preferable to gradually achieve sufficient and stable 25(OH)D serum levels.
When vitamin D is used in COVID-19 treatment, caution should be taken regarding adverse effects, especially hypercalcemia, and related organ dysfunctions(13,Reference Aygun15,Reference Holick, Binkley and Bischoff-Ferrari19,Reference Grant, Lahore and McDonnell20) .
It is worth mentioning that critically ill COVID-19 patients might benefit from combined vitamin D and anti-IL-6 (tocilizumab) treatment, proposed in severe forms of pneumonia(Reference Castelnovo, Tamburello and Lurati45). A single shot of vitamin D (300 000 μg) was determined to diminish increased serum IL-6 levels in previously vitamin D-deficient patients with ventilator-acquired pneumonia and was associated with reduced mortality(Reference Miroliaee, Salamzadeh and Shokouhi27). Besides, a better therapy response to tocilizumab was achieved after 6 months in those rheumatoid arthritis patients that had sufficient serum vitamin D levels (≥ 30 ng/dl)(Reference Kim, Baek and Hong21).
Taken together, since vitamin D supplementation is determined effective against acute respiratory tract infections, people with high risk for severe COVID-19 should take vitamin D to maintain their serum 25(OH)D levels in the optimal range (> 30–40 ng/dl). Daily or weekly intake of recommended tolerable doses, without bolus doses, was showed protective and safe(Reference Martineau, Jolliffe and Hooper14,Reference Grant, Lahore and McDonnell20) . Treatment that includes high bolus doses of vitamin D needs to be further investigated for efficacy and safety.
Implications for the use of vitamin D in patients that require mechanical ventilation or ICU admission
Given the suggestions for vitamin D intake in COVID-19 patients, one should consider concomitant adjustments of phosphate and Mg levels, the elements that are usually overlooked. However, they are in the middle of the complex interactions between vitamin D, hormones, acid–base balance and hypoxia. Since severe phosphate and/or Mg deficiencies lead to energy shortage and metabolic abnormalities, they might contribute to the disease progression(Reference van Kempen and Deixler46–Reference Taghizadeh-Hesary and Akbari48). SARS-CoV-2 caused acute hyperinflammatory response leads to cellular ATP depletion and immune cell dysfunction, while phosphate and Mg are required for ATP regeneration(Reference Taghizadeh-Hesary and Akbari48). Several studies have already shown a significant correlation between phosphate deficiency and COVID-19 severity. Non-survivors had lower serum phosphate levels than survivors, which correlated to lymphocyte counts and severity of lung damage(Reference van Kempen and Deixler46,Reference Javdani, Parsa and Shakeri47,Reference Xue, Ma and Zhao49) .
The risks for severe COVID-19 are the same as for hypophosphatemia and hypomagnesemia, e. g. older age, obesity, diabetes, renal or liver dysfunction(Reference van Kempen and Deixler46,Reference Seers and Davenport50) . Additional risks may emerge during the disease course, such as diminished food intake, diarrhoea, renal dysfunction, diuretics, hyperglycaemia or insulin use, antacids, corticosteroids or inadequate mechanical ventilation. Hyperventilation can easily aggravate hypophosphatemia due to respiratory alkalosis, which stimulates ATP production and consumes serum phosphates(Reference van Kempen and Deixler46,Reference Alsumrain, Jawad and Imran51) .
Symptoms of hypophosphatemia and hypomagnesemia are in line with the COVID-19 manifestations, the most common being a general weakness. Others include myopathy and cardiomyopathy, thrombocytopenia and increased platelet aggregation, coagulopathy, neurologic disturbances (encephalopathy, confusion, delirium, paresthesia and dysarthria), immunodeficiency, disturbed tubular transport, dysregulated vitamin D metabolism and failure-to-wean from mechanical ventilation. Importantly, hypophosphatemia impedes 2,3-bisphosphoglycerate in erythrocytes, which regulates haemoglobin affinity toward oxygen, and so may aggravate hypoxia(Reference van Kempen and Deixler46,Reference Taghizadeh-Hesary and Akbari48,Reference Seers and Davenport50,Reference Alsumrain, Jawad and Imran51) .
Vitamin A
Vitamin A or retinol represents a group of unsaturated monohydric alcohols that contain a beta-ionone ring to which an isoprenoid chain is attached. Foods of animal origin contain a high concentration of retinol and retinyl-esters, while fruits and vegetables are rich in provitamin β-carotene. RDA for vitamin A is 900 μg for adult males and 700 μg for females, but is higher in pregnant and lactating individuals. Deficiency is uncommon in high-income countries, but is found in vulnerable groups such as infants, children, pregnant and lactating women in low-income countries(52,53) .
Retinol is the precursor to two active metabolites: retinal, essential for the vision process, and retinoic acid, which acts as an intracellular messenger that affects gene transcription by binding nuclear retinoid receptors (RAR and RXR). The RAR-RXR heterodimer binds retinoic acid response elements that are typically found in gene promoters or enhancers regions of the DNA(Reference Penkert, Rowe and Surman54). Similarly, the VDR forms heterodimers with the retinoid × receptor (RXR). RAR-RXR and VDR-RXR heterodimers, as ligand-dependent transcription factors, bind particular regions of DNA that are responsive to vitamins A and D, respectively. Since both vitamins A and D may compete for binding to RXR and DNA, they might act as antagonists(Reference Evans and Mangelsdorf55).
Mechanisms of vitamin A anti-inflammatory actions
Apart from maintenance of vision, vitamin A regulates growth, cell differentiation, embryonic and fetal development, epithelial barrier function and immunity(Reference Tripathy, Dhaduk and Kapadiya56). Vitamin A is involved in the regulation of both innate and adaptive immune responses. Alveolar macrophages are critical to the homoeostasis of the inflammatory environment in the lung and typically exhibit hybrid M1/M2 phenotype, thus maintaining an equilibrium between immune tolerance and protective immunity in the alveolar space(Reference Mitsi, Kamng’ona and Rylance57). While infections might induce monocyte differentiation towards the M1 lineage, vitamin A promotes an M1 to M2 phenotype shift, thus inhibiting macrophages-mediated pro-inflammatory reaction(Reference Vellozo, Pereira-Marques and Cabral-Piccin58). Additionally, by releasing cytokines, macrophages may influence the degree of activity or maturation of neighbouring dendritic cells and thus influence antigen presentation in lungs(Reference Nicod, Cochand and Dreher59). Dendritic cells not only initiate the innate immune response but also present antigens to naive T cells, thus activating adaptive immunity(Reference He, Zhu and Shi60). Dendritic cells express enzymes in the vitamin A metabolic pathway that is capable of conversion of retinol into its active form, which, after being released from the cell, activates macrophage-mediated antimicrobial responses(Reference Kim, De Leon and Jiang61). Vitamin A promotes T cell migration towards the area of inflammation and T cell differentiation and poly-clonal response in a dose-dependent manner(Reference Huang, Liu and Qi62). Besides, it mediates in TGF-ß-dependent conversion of T cells into T regulatory (Treg) cells, thus preventing autoimmunity. However, in the presence of IL-6 and TGF-β may act towards autoimmunity and inflammation by inducing T helper 17 response(Reference Huang, Liu and Qi62).
Retinoic acid-inducible gene I-like receptors represent a direct link between vitamin A and viral diseases. Retinoic acid-inducible gene receptors recognise cytosolic viral RNA and mediate the transcription of type I interferon involved in antiviral host response(Reference Sarohan, Kızıl and İnkaya63). Coronaviruses including SARS-CoV-2 may suppress type I interferon antiviral responses and cause disturbance of the delicate balance between immune-suppressive Tregs and pro-inflammatory T helper 17 cells(Reference Sarohan, Kızıl and İnkaya63,Reference Trasino64) . Additionally, active forms of vitamin A have a direct effect on IgA plasma cell differentiation which affects the synthesis and secretion of IgA(Reference Pantazi, Marks and Stolarczyk65).
The use of vitamin A in the therapy of infectious diseases and implications for the use in coronavirus disease 2019
Up to date, there is no consistent evidence for the beneficial effects of vitamin A supplementation in adults and the elderly in the treatment of infections(Reference Villamor and Fawzi66). In children, positive effects in preventing lower respiratory tract infections appear to be limited to populations with acute and chronic undernutrition(Reference Chen, Zhuo and Yuan67). Vitamin A supplementation, along with vaccination, improves survival after challenge with a high dose of pneumococcus in animals and clinical trials in children have been initiated (clincaltrials.gov, PCVIT NCT03859687)(Reference Penkert, Rowe and Surman54).
Although, in theory, vitamin A supplementation might be beneficial in COVID-19, especially in the second inflammatory phase of the disease, up to date there are no data regarding its use in patients and only a few RCT have been registered(Reference Beigmohammadi, Bitarafan and Hoseindokht68). The data from the clinical trials regarding the prevention of acute lower respiratory tract infections in children certainly advise approach with caution, especially in high-income countries where vitamin A deficiency is rare(Reference Villamor and Fawzi66).
Vitamin C
Vitamin C or L-ascorbic acid is a water-soluble vitamin with a chemical structure similar to carbohydrates that cannot be synthesised in humans due to the lack of an enzyme l-gulono-γ-lactone oxidase. RDA for vitamin C has been set to 90 mg/day for males and 75 mg/d for females, whereas smokers require 35 mg/d more. Adverse effects related to vitamin C supplementation are observed when daily intake is higher than 3 g and include diarrhoea and other gastrointestinal disturbances, increased oxalate and uric acid excretion, changes in Fe, Cu and B12 absorption and erosion of dental enamel(69). In balanced diets, most of the daily intake of vitamin C comes from fruits and vegetables. Due to the transporter-mediated absorption and saturation phenomenon, the bioavailability of vitamin C after oral intake decreases with the increase of the oral dose in the range from 200 to 1000 mg, which can be bypassed by intravenous administration(Reference Kashiouris, L’heureux and Cable70).
Table 1. Vitamins and microelements and their roles and effects related to the immune system

ACE2, angiotensin-converting enzyme 2; TGF-β1, transforming growth factor β1; M1, pro-inflammatory macrophage phenotype; M2, anti-inflammatory macrophage phenotype; Treg, T regulatory cells; S1P, sphingosine 1-phosphate; NET, neutrophil extracellular traps.
Table 2. Registered interventional RCT of vitamins/microelements supplementation in COVID-19 that were completed by September 2021 (clinicaltrials.gov; covid-19.cochrane.org)

The role of vitamin C in immune system regulation implicated in coronavirus disease 2019
Vitamin C is a highly effective antioxidant and a co-factor in the process of collagen and neurotransmitter/hormone synthesis. Therefore, it is essential for the barrier function of skin/mucosa, blood vessel integrity and cardiovascular response to severe infection(Reference Carr, Shaw and Fowler71,Reference Carr and Maggini72) . It contributes to the proper function of both the innate and adaptive immune systems. Vitamin C accumulates in neutrophils and enhances chemotaxis, phagocytosis and apoptosis of the neutrophils, thereby decreasing NETosis and excessive tissue damage(Reference Carr and Maggini72). Additionally, it accumulates in lymphocytes and affects lymphocyte proliferation, including Treg cells. The effect on the immune cells, especially the decrease in NETosis, could be beneficial for COVID19 patients since SARS-CoV-2 can stimulate extracellular neutrophils traps (NET) and activate NETosis in neutrophils that contributes to multiorgan failure(Reference Arcanjo, Logullo and Menezes7).
Vitamin C in the therapy of coronavirus disease 2019
Although it was previously shown that oral administration of vitamin C in doses of 1–3 g/d reduces the length of ICU stay, hospital stay and the duration of mechanical ventilation in ICU patients(Reference Hemilä and Chalker73,Reference Hemilä and Chalker74) , the benefits of intravenous/oral high dose administration of Vitamin C in ARDS and COVID-19 patients was not shown up to date in RCT(Reference Fowler, Truwit and Hite75–Reference Thomas, Patel and Bittel77). No significant benefit was reported in the reduction of symptoms, hospitalisations, SpO2 at discharge, the length of ICU stay and mortality in COVID-19 patients who were administered Vitamin C in high doses(Reference JamaliMoghadamSiahkali, Besharat and Koolaji76,Reference Thomas, Patel and Bittel77) . Besides, high doses of Vitamin C did not significantly improve organ dysfunction scores, inflammation marker levels and vascular injury in sepsis and ARDS(Reference Fowler, Truwit and Hite75). However, ambulatory treated COVID19 patients who received 8 g/d of vitamin C reported adverse effects such as nausea, diarrhoea and stomach cramps in a higher proportion than non-treated individuals(Reference Thomas, Patel and Bittel77).
The available data suggest that there is no benefit in supplementing vitamin C in high doses in the treatment of COVID-19 patients. However, high doses of vitamin C have been associated with pro-oxidative effects especially through the interaction with transition metal ions such as Fe and Cu(Reference Kaźmierczak-Barańska, Boguszewska and Adamus-Grabicka78). Since Fe overload has been implicated in the pathogenesis of COVID-19(Reference Habib, Ibrahim and Zaim79), further research regarding the use of vitamin C might be needed, especially evaluation of lower doses and therapy duration.
Vitamin B
Folate (Vitamin B9)
Folate or B9 is a naturally occurring water-soluble B vitamin that consists of pteridine ring, para-aminobenzoic acid and glutamate residues. RDA for folate is 400 μg for both adult males and females; however, pregnant women require 600 μg of folate daily. Although the deficiency is rare due to the food fortification program, supplementation is advised to women of childbearing age, especially during pregnancy and lactation(52,80) .
The role of folate in immune system regulation
Folate acts as a coenzyme or co-substrate in methylation reactions for nucleic acids and protein synthesis and amino acids metabolism. Additionally, folate is required for ATP synthesis and the proper function of the immune system. Purine molecules, inosine and adenosine modulate the proliferation and cytotoxic activity of NK cells in response to pathogens, as well as adaptive responses by secreting cytokines such as IL-1β and IFN-γ (Reference Bayer and Fraker81). Folate is a survival factor for regulatory T (Treg) cells that express high levels of folate receptor 4(Reference Kunisawa, Hashimoto and Ishikawa82).
Implications for the use of folate in the therapy of coronavirus disease 2019
Based on molecular docking studies, two recent pre-prints hypothesised that folic acid might be beneficial in the early stages of COVID-19 due to the potential inhibition of furin, which enables entry to the host cells or 3CLpro (Mpro) protease of SARS-CoV-2 virus(Reference Sheybani, Dokoohaki and Negahdaripour83,Reference Serseg, Benarous and Yousfi84) . However, SARS-CoV-2 requires host folate and one-carbon metabolism to support nucleotide synthesis and viral replication, which can be inhibited by folate inhibitors such as methotrexate(Reference Zhang, Guo and Kim85). Although low serum folate levels are present among hospitalised patients with COVID-19, there is no association between serum folate levels and incidence of hypoxemia, invasive ventilation, length of hospital stay and mortality(Reference Meisel, Efros and Bleier86). At this moment, further studies are needed to assess the potential benefit of folate supplementation in COVID-19 patients, especially regarding the evidence that suggests that folic acid supplementation might reduce the hospitalisation rate in pregnant women with SARS-CoV-2 infection(Reference Acosta-Elias and Espinosa-Tanguma87).
Vitamin B6
Vitamin B6 is a water-soluble vitamin whose active form pyridoxal 5′-phosphate acts as a coenzyme in various enzymatic reactions such as synthesis and/or degradation of amino acids, neurotransmitters, sphingolipids, haemoglobin and glycogen. RDA for vitamin B6 is 1·3 mg/d, but is higher in pregnancy, lactation and the elderly. Deficiency is uncommon, but lower plasma levels are commonly found in individuals with renal function impairment, autoimmune disease and alcoholics(52,88) .
The roles of vitamin B6 in the immune system regulation implicated in coronavirus disease 2019
It has been proposed that vitamin B6 supplementation improves immune functions in humans and animals, possibly by affecting metabolic pathways that result in metabolites with immunomodulating effects such as kynurenine and transsulfuration pathway and sphingosine 1-phosphate metabolism(Reference Ueland, McCann and Midttun89). Sphingosine 1-phosphate metabolism regulates lymphocyte trafficking, endothelial barrier permeability and cytokine synthesis(Reference Spiegel and Milstien90). In B6 deficiency, amplified kynurenine signalling through the aryl hydrocarbon receptor (Ahr) may stimulate an IL-6 positive feedback cycle, which could amplify inflammation(Reference Guarnieri, Abruzzo and Bolotta91,Reference Kaiser, Parker and Hamrick92) . Additionally, vitamin B6 prevents IL-1β synthesis by inhibiting NLRP3 inflammasome activation(Reference Zhang, Tsuchiya and Kinoshita93). Therefore, it was recently proposed that vitamin B6 might suppress hyper-inflammation and cytokine storm syndrome in COVID-19, but this hypothesis should be tested in clinical trials(Reference Kumrungsee, Zhang and Chartkul94).
Vitamin B12
Vitamin B12 (cobalamin) is a water-soluble vitamin with a complex molecular structure that contains a cobalt atom in the centre of the corrin ring bound to various side groups. The active forms of vitamin B12 (hydroxo-, adenosyl- and methyl-cobalamin) play an important role in methylation, DNA, protein and lipid synthesis. RDA for vitamin B12 is 2·4 μg, and this amount is commonly acquired through food. The absorption is dependent on the presence of intrinsic factor, and deficiency is found in pernicious anaemia, gastric resections and in some cases might be related to the use of certain medications(52,95) .
The roles of vitamin B12 in the immune system regulation implicated in coronavirus disease 2019 and vitamin B12 in the therapy of coronavirus disease 2019
When it comes to the immune system, vitamin B12 appears to affect lymphocyte count and the activity of NK cells. Vitamin B12 deficiency leads to a decrease in absolute numbers of CD4+ and CD8+ lymphocytes, increases CD4/CD8 ratio and reduces the activity of NK cells(Reference Tamura, Kubota and Murakami96). Besides, the changes in a number of the lymphocytes and NK cells activity observed in vitamin B12 deficiency can be reversed by vitamin B12 replacement therapy(Reference Erkurt, Aydogdu and Dikilitaş97). Interestingly, COVID 19 is characterised by significant decreases in both CD4+ and CD8 + T cell counts(Reference Tavakolpour, Rakhshandehroo and Wei98). Also, it was shown recently that vitamin B12 supplementation in combination with vitamin D and Mg was associated with a significant reduction in the proportion of patients requiring oxygen support, intensive care support, or both, in older COVID-19 patients(Reference Tan, Ho and Kalimuddin33). The promising results emphasise the need for further investigation to assess the ameliorating effect of vitamin B12 on the severity of COVID-19.
Folate/Vitamin B6/Vitamin B12 and hyperhomocysteinemia: implications in coronavirus disease 2019
Plasma homocysteine levels are elevated in vitamin B12 deficiency, vitamin B6 deficiency and the folic acid deficiency(Reference Pirouzpanah, Taleban and Mehdipour99). Hyperhomocysteinemia leads to a reduction in endothelial nitric oxide bioavailability and induces inhibition of nitric oxide production in platelets, which might result in vasoconstriction and platelet aggregation(Reference Signorello, Segantin and Passalacqua100,Reference Djuric, Jakovljevic and Zivkovic101) . Similar endothelial dysfunction, along with impairment in nitric oxide biosynthetic pathway and massive platelet activation, has been observed in COVID-19 patients(Reference Canzano, Brambilla and Porro102). Interestingly, levels of citrulline that is produced from arginine along with nitric oxide were negatively correlated with IL-6 levels in compromised COVID-19 patients, suggesting the mechanism by which cytokine storm potentiates endothelial dysfunction, especially in folate/B6/B12 deficiency(Reference Canzano, Brambilla and Porro102). Recent studies have identified homocysteine as a potential predictive biomarker in COVID-19 patients with multiple comorbidities, in addition to already established biochemical and hematological biomarkers of progression, severity and mortality in COVID-19; however, further testing is needed to determine if this finding relates to all COVID-19 patients(Reference Ponti, Roli and Oliva103,Reference Ponti, Maccaferri and Ruini104) . Although it seems likely that COVID-19 patients might benefit from folate/B6/B12 supplementation, randomised controlled trials are certainly needed to answer.
Microelements against coronavirus disease 2019
Selenium
Se is an essential trace element, well known for its requirements for optimal immune and thyroid functions. The RDA for Se is 55 µg (0·7 µmol), while the tolerable UL for adults is set at 400 µg (5·1 µmol). Se is contained in amino acid selenocysteine, positioned at the active sites of selenoproteins. Selenoprotein P is a major form of Se in plasma and, together with glutathione peroxidases, comprise nearly 90 % of the circulating Se pool, of around 85 µg/l(69,Reference Avery and Hoffmann105) .
Selenoprotein P, containing ten selenocysteine residues, exerts several functions, such as Se transport and storage, potent extracellular antioxidant effects, protection of endothelium by peroxynitrite scavenging and regeneration of ascorbic acid. Selenoprotein K is a cofactor in the process of post-translational protein modifications(69,Reference Avery and Hoffmann105,Reference Broome, McArdle and Kyle106) . Interestingly, selenoprotein K knockout mice showed up to a 50 % reduction in most immune cell functions(Reference Avery and Hoffmann105).
A rapid fall in serum selenoproteins characterises acute phase response due to a block in hepatic mRNA translation. This reaction may disrupt a Se supply to the peripheral tissues(Reference Renko, Hofmann and Stoedter107). However, it does not reflect a global Se deficiency but a redistribution related to inflammation.
The roles of selenium in the regulation of immune system functions
Many reactions in the immune system depend on Se-containing enzymes. Se thus may influence leukocyte interactions, TLR signalling and microbicidal processes, expression and availability of inflammatory mediators and endothelial and platelet functions. Se was shown to switch pro-inflammatory macrophage phenotype (M1) towards anti-inflammatory (M2), stimulate T cell proliferation and differentiation of CD4 + Th cells, induce a stronger response to some vaccines (polio) and indirectly enhance NK cell and cytotoxic T-cell activities(Reference Avery and Hoffmann105,Reference Hawkes, Kelley and Taylor108–Reference Nelson, Lei and Prabhu112) . It is an established inhibitor of NF-kB, and thus an important transcription modulator for a variety of pro-inflammatory cytokines and chemokines(Reference Hawkes, Kelley and Taylor108,Reference Hiffler and Rakotoambinina113) .
Generally, a poor Se status reduced adaptive immunity and exacerbated inflammation. Lower plasma Se concentrations were found in the elderly, in ICU patients, with sepsis, organ failure or polytrauma, and were associated with increased mortality. In severe sepsis, the minimal plasma Se levels inversely correlated to the maximum leucocyte count, CRP and IL-6, while directly to the minimum platelet count and antithrombin activity(Reference Hiffler and Rakotoambinina113–Reference Zhang, Taylor and Bennett115).
Importantly, Se deficiency was found to be a risk factor for viral infections, higher virulence, mutations and higher susceptibility to RNA viruses. Besides, a low Se status is implicated in HIV disease progression(Reference Avery and Hoffmann105,Reference Hiffler and Rakotoambinina113,Reference Moghaddam, Heller and Sun114) .
The effects of selenium supplementation in critical illness and coronavirus disease 2019
Se supplementation is proposed to restore immune balance after a Se deficit. For example, a prolonged high-dose Se intake (297 μg for 99 d) provoked significant changes in the leukocyte counts and responses, compared with the low Se intake (13 μg). High Se-mediated immune-enhancing properties encompassed a transient increase in lymphocyte count by 17 %, stimulated lymphocyte proliferation to a mitogen in vitro, improved B-lymphocyte activation/proliferation, decreased granulocyte count by 5 %, while serum immunoglobulins concentrations were largely unaffected(Reference Hawkes, Kelley and Taylor108). The beneficial effects are thought to reside in the restoration of cellular redox control. Both organic and inorganic Se supplementation (50 μg for 28 d) induced an increase in phospholipid and cytosolic glutathione peroxidases activities in lymphocytes, granulocytes and platelets(Reference Brown, Pickard and Nicol116). Se supplementation also restored the antioxidant capacity of the lungs and improved respiratory mechanics(Reference Mahmoodpoor, Hamishehkar and Shadvar117).
However, there is no clear suggestion over Se supplementation during critical illnesses(Reference Allingstrup and Afshari109). In a meta-analysis of Heyland et al. (Reference Heyland, Dhaliwal and Suchner118), Se supplementation, alone or in combination with other antioxidants, was associated with reduced mortality, while other antioxidants were not. There is an increasing number of studies reporting a trend towards better outcomes in Se supplemented COVID-19 patients(Reference Hiffler and Rakotoambinina113–Reference Zhang, Taylor and Bennett115,Reference Sakr, Reinhart and Bloos119) .
Also, several of them determined a higher risk for severe COVID-19 and related mortality in Se-deficient patients(Reference Hiffler and Rakotoambinina113–Reference Zhang, Taylor and Bennett115,Reference Sakr, Reinhart and Bloos119) . Low Se serum levels were associated with the severity of COVID-19 in a South Korean study. Se deficiency (< 95 ng/ml) was present in 44·4 % of cases with a mild disease without pneumonia, opposed to 100 % of those with severe pneumonia requiring mechanical ventilation(Reference Im, Je and Baek12). A study from July 2020 showed an insufficient Se status (< 2·5th percentile of a reference European population or < 45·7 µg/l) and Se availability for the optimal selenoproteins expression in COVID-19 patients. Additionally, Se status was significantly higher in surviving COVID-19 patients when compared to non-survivors(Reference Moghaddam, Heller and Sun114).
Population-based, retrospective analysis of Zhang et al. reported an association between Se status and cure/death rate from COVID-19 in the Chinese population. Despite the limitations, this study points towards the need for further research, particularly in light of associations between Se status and other viral disease outcomes(Reference Zhang, Taylor and Bennett115).
There may be a specific role for Se in disease caused by RNA viruses. Through the increase in oxidative stress, Se deficiency possibly increases the mutation rate of the viral genome and virulence of the viral agent(Reference Guillin, Vindry and Ohlmann120). RNA viruses synthesise their selenoproteins and thus are likely to exploit cellular Se for replication and survival, causing Se deficiency in the host cell. In this situation, Se supplementation would compensate for the lost quantities and restore redox balance and immune defense(Reference Hiffler and Rakotoambinina113,Reference Guillin, Vindry and Ohlmann120) .
It is also important to note that SE deficit may predispose to endothelial dysfunction and platelet activation, both leading to coagulopathy, an established complication of COVID-19. Preclinical studies have demonstrated that sodium selenite has a reversal effect on platelet aggregation, acting through the reduction of lipid peroxidation and thromboxane A2 formation(Reference Ersöz, Yakaryilmaz and Turan121,Reference Vavougios, Ntoskas and Doskas122) .
To counteract the SARS-CoV-2 infection, there is a suggestion for Se supplementation in doses well above RDA (200–400 µg/d), based on the previous studies on severe viral infections and good tolerance of the high Se doses given in a short period (2–3 weeks)(Reference Hiffler and Rakotoambinina113).
Zinc, copper and iron
Nutritional immunity
Trace elements such as Zn and Cu are required for the optimal function of the immune system, but are considered essential for the growth and survival of various pathogens as well. The competition between the host and the pathogen leads to defensive sequestration of trace minerals during infection and uses the toxicity of these metals against the pathogen in a process called nutritional immunity(Reference Rahman and Karim123). Metallothioneins are a group of cysteine-rich low molecular weight proteins, with a metal-binding ability that sequestrates Zn and Cu in unstressed cells and regulates Zn/Cu signalling during immune cells activation(Reference Lynes, Hidalgo and Manso124,Reference Rice, Zweifach and Lynes125) . However, they might also sequestrate heavy metals, scavenge reactive oxygen and nitrogen species and regulate cellular redox potential(Reference Lynes, Hidalgo and Manso124,Reference Lynes, Zaffuto and Unfricht126) . Extracellular metallothioneins act as danger signals because they could induce chemotaxis and influence the immune response(Reference Lynes, Hidalgo and Manso124).
Zinc
Zn is an essential micro-nutrient that catalyses enzyme activity, contributes to protein structure and regulates gene expression. RDA for Zn is 11 mg for males and 8 mg for females, respectively. Deficiency is not common; however, vegetarians, pregnant and lactating women, alcoholics and individuals with chronic diseases might be at risk of inadequacy(52,127) .
Zn homoeostasis is controlled through the action of Zn transporters and Zn-binding proteins and is essential for maintaining the membrane barrier structure, cell differentiation, cell division and the proper function of immune cells(Reference Haase and Rink128,Reference Gammoh and Rink129) .
Implications for the use of zinc in coronavirus disease 2019
Zn contributes to the host defense by maintaining the membrane barrier structure and function(Reference Gammoh and Rink129).
During infection or inflammation, plasma Zn levels are reduced due to the effect of IL-6 that upregulates the expression of Zn transporters in hepatocytes and the accumulation of metallothionein-bound Zn in the liver(Reference Beker Aydemir, Chang and Guthrie130). Prophylactic Zn supplementation demonstrated a survival advantage and reduced neutrophil infiltration in the lungs of mice in a murine model of sepsis and acute lung injury(Reference Nowak, Harmon and Caldwell131,Reference Wessels, Pupke and Von Trotha132) . Additionally, low Zn levels result in increased release of NET and enhanced neutrophils degranulation, whereas Zn supplementation inhibits NET release and degranulation in both human and murine neutrophils by inhibiting citrullination of histone H3(Reference Wessels, Pupke and Von Trotha132,Reference Kuźmicka, Manda-Handzlik and Cieloch133) . Although the physiologic concentration of Zn did not affect cytokine expression or apoptosis of peripheral blood mononuclear cells, pharmacologic concentrations stimulated both cytokine expression and apoptosis in vitro (Reference Chang, Hung and Hsieh134).
It was shown previously that an increase in the intracellular Zn concentration impairs the replication of a variety of RNA viruses, including poliovirus, influenza and SARS-CoV virus(Reference te Velthuis, van den Worm and Sims135). Therefore, due to the potential effect on SARS-CoV-2 replication and the effect on the NET formation, Zn supplementation might exert highly protective effects in COVID-19 patients and especially in the pathogenesis of multiorgan failure. However, supraphysiological doses of Zn might bust cytokine production and aggravate cytokine storm.
Zinc in the therapy of coronavirus disease 2019
Zn supplementation was previously associated with reductions in the rates of pneumonia in children aged up to 5 years in low-income areas(Reference Lassi, Moin and Bhutta136). The addition of Zn to the standard treatment and care was proven beneficial in infants with a severe bacterial infection in a randomised controlled trial(Reference Bhatnagar, Wadhwa and Aneja137). Additionally, Zn deficiency was associated with more complications, prolonged hospital stay and increased mortality in COVID-19 patients(Reference Jothimani, Kailasam and Danielraj138). Observational and cohort studies regarding the use of Zn in COVID-19 patients showed promising results, reporting reduced mortality among hospitalised patients, especially when administered together with Zn ionophore(Reference Carlucci, Ahuja and Petrilli139,Reference Frontera, Rahimian and Yaghi140) . However, the study involving ambulatory COVID-19 patients recently showed that 50 mg of Zn gluconate for 10 days did not affect the severity or duration of symptoms compared with usual care(Reference Thomas, Patel and Bittel77). Additionally, supplementation with 80 mg of Zn did not reduce the risk of infection with the SARS-CoV-2 virus(Reference Seet, Quek and Ooi141).
Still, patients with COVID-19 that require mechanical ventilation might benefit from Zn supplementation, and there is a need for further studies. Low serum values of Zn are found in critically ill patients with COVID-19, especially in those who require invasive mechanical ventilation and correlate with clinical presentation and worse outcomes(Reference Jothimani, Kailasam and Danielraj138,Reference Gonçalves, Gonçalves and Guarnieri142,Reference Vogel-González, Talló-Parra and Herrera-Fernández143) . Mechanical ventilation is often necessary to support patients with the severe presentation of COVID-19. However, it also might exacerbate lung injury through mechanical stress-activated signalling pathways(Reference Oeckler and Hubmayr144). It was shown that stretch applied to cultured human cells and mouse lungs in vivo induces expression of metallothionein, which requires Zn to limit lung injury(Reference Boudreault, Pinilla-Vera and Englert145). Additionally, Zn deficiency potentiated the development of ventilator-induced lung injury in mice(Reference Boudreault, Pinilla-Vera and Englert145). On this subject, further clinical research is needed since ventilator-induced lung injury is commonly present among ICU patients with acute respiratory distress syndrome. Currently, there are published protocols for registered RCT that are to test the effects of intravenous Zn administration in critically ill patients(Reference Perera, El Khoury and Chinni146).
Copper
Cu is required for the proper function of both innate and adaptive immune responses.
Physiologically, in acute phase response, Cu plasma concentrations increase as a result of increased ceruloplasmin synthesis and are thought to provide an antioxidant defense(Reference Galloway, McMillan and Sattar147). Cu deficiency is reflected in reduced neutrophil count, as well as in impaired neutrophil and macrophage function reduced cytokine synthesis and T cell proliferation, but is rare in humans(Reference Lazarchick148,Reference Wahab, Mushtaq and Borak149) . Cu, being transitional metal, increases reactive oxygen and nitrogen species generation during the respiratory burst, thus potentiating the antimicrobial effect excreted by neutrophils and macrophages(Reference Djoko, Cheryl-lynn and Walker150). A recent preprint suggested that Cu might inhibit coronavirus main protease (Mpro) by docking cysteine (Cys145) in the Mpro active-site region(Reference Garza-Lopez, Kozak and Gray151).
Iron
In addition to Cu, Fe, as well causes nicotinamide adenine dinucleotide phosphate oxidase-dependent respiratory burst in macrophages and neutrophils, which can be inhibited by Fe chelation(Reference Collins, Kaufmann and Schaible152). Both increase and decrease in the intracellular Fe level act as danger signals and activate inflammatory and anti-microbial pathways through NF-κB and HIF-1, respectively(Reference Cherayil153). Cytokines such as IL-6 and the acute phase protein hepcidin lead to the retention of Fe within macrophages in the form of ferritin and a sequential decrease in serum Fe concentration(Reference Nairz, Haschka and Demetz154).
Fe metabolism might play an important role in COVID-19. Severe COVID-19 cases had lower haemoglobin, red blood cell count and higher ferritin levels when compared with moderate cases, whereas ferritin levels were higher in non-survivors when compared with survivors(Reference Taneri, Gómez-Ochoa and Llanaj155). Fe overload has been proposed as a contributor to the pathogenesis of COVID-19 due to its role in the generation of reactive oxygen species, but possibly more important due to induction of nonapoptotic cell death ferroptosis(Reference Habib, Ibrahim and Zaim79,Reference Riley, Hicks and Irvine156) . The process of ferroptosis is characterised by the Fe-dependent accumulation of lethal lipid reactive oxygen species that promotes inflammation, but can be inhibited by Fe chelators and lipophilic antioxidants such as tocopherols and carotenoids(Reference Habib, Ibrahim and Zaim79,Reference Song and Long157) .
Interactions of zinc, selenium, copper and iron: implications for the supplementation in coronavirus disease 2019 patients
Regarding supplementation, interactions of Zn with other trace metals and vitamins should be considered. Absorption and bioavailability of Zn, Cu and Fe are dependent on competition among elements and, when given together in equal ratio, absorption of Fe and Cu is reduced approximately 40 %(Reference Arredondo, Martínez and Núñez158). Therefore, excessive Zn supplementation might result in Cu or Fe inadequacy. Both Cu and Zn bind to metallothioneins, but Cu binds with higher affinity that results in the formation of mixed metal–metallothionein complexes and the release of Zn(Reference Calvo, Jung and Meloni159). However, Se compounds catalytically couple with the glutathione/glutathione disulphide and metallothionein/thionein redox pairs to either release or bind Zn, thereby expressing antioxidant or prooxidant effects through redox catalysis in Zn metabolism(Reference Maret160,Reference Yildiz, Kaya and Tanriverdi161) .
Conclusion
One year after the COVID-19 pandemic was proclaimed by WHO, we are still learning about the disease. Therapeutic protocols have been evolving and improving ever since. Finally, since the beginning of 2021, vaccines have become available, but the roll-out has not been as fast as expected in some countries. Meanwhile, vitamins and microelements have been widely used as supplements in prophylaxis and the fight against COVID-19. Even though several studies reported a promising association of these micronutrients on the reduction of severity and improved survival, up to date there is not enough scientific evidence neither for nor against their use in the treatment of COVID-19. Some of them, i.e., vitamin D, vitamin C and Zn, have been widely prescribed by medical professionals, sometimes in supraphysiological or bolus doses, disregarding potential side effects and interactions. Although the use of some micronutrients in the COVID-19 therapy might be scientifically sound, the potential beneficial effects should be evaluated in RCT first. From the current standpoint, vitamin and microelement supplementation in COVID-19 should not exceed the doses recommended for the general population and age group, except for clinical trials. Inevitably, vitamin D, Zn and Se show a significant place in the management of COVID-19, especially in populations prone to deficiencies. Besides, regular intake of recommended doses of vitamin D should be encouraged in healthy people to maintain a well-balanced immune system. Given the importance of providing optimal resistance to SARS-CoV-2, maintaining a balanced diet, in line with necessary vitamin and microelement substitutions, represents a feasible, safe and readily available therapeutic option.
Acknowledgements
We thank Jelena Slavkovic for language editing. We wish to attribute to Vecteezy (<a href=“https://www.vecteezy.com/free-vector/corona-virus”>Corona Virus Vectors by Vecteezy) for creating vector used in the graphical abstract and Servier Medical Art by Servier (available on https://smart.servier.com/) licensed under a Creative Commons Attribution 3.0 Unported License, for creating vectors used in graphical abstract and Fig. 1 preparation.

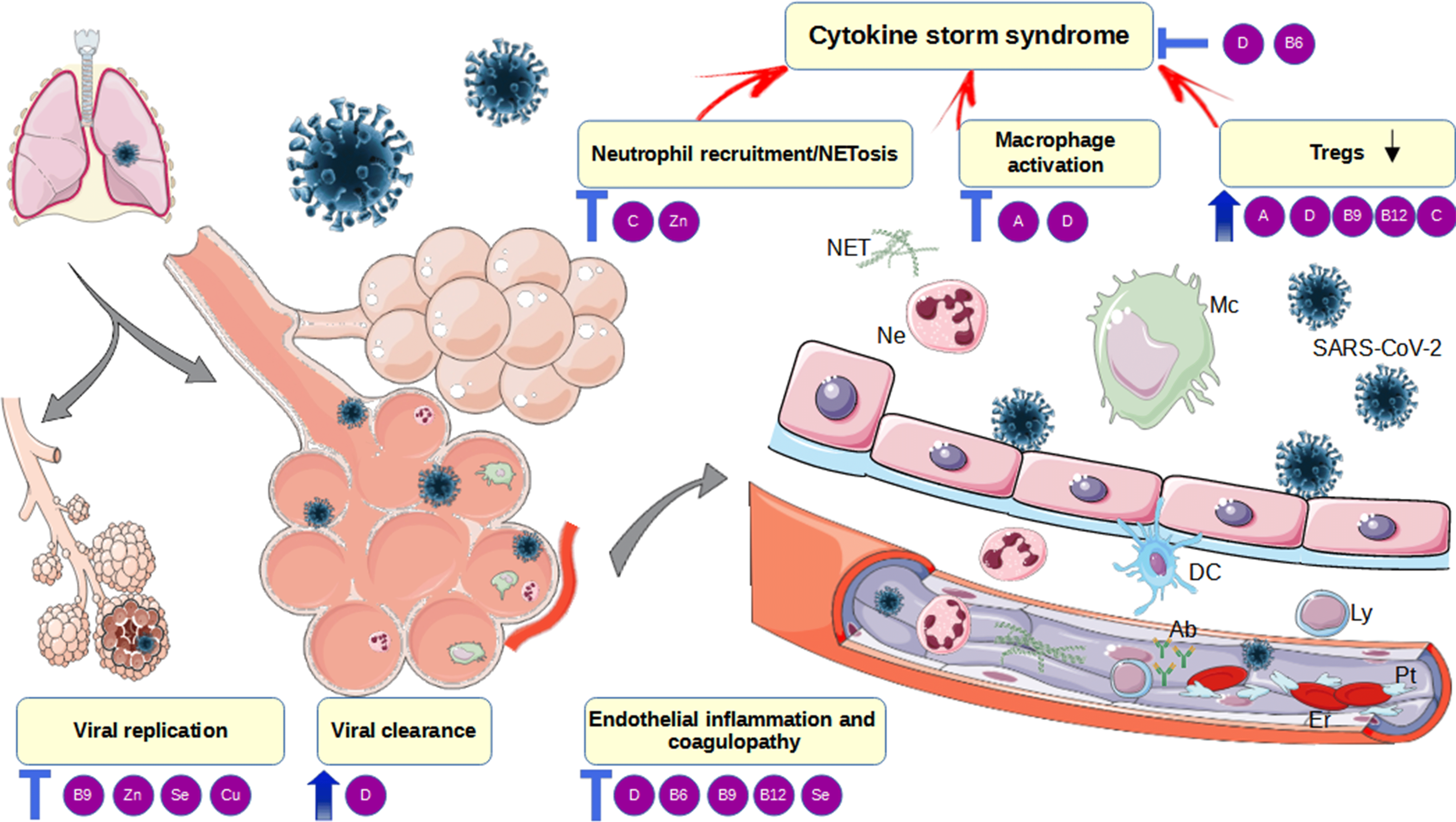
Fig. 1. Mechanisms of vitamins beneficial effects in the pathogenesis of COVID-19. Mc, macrophages; Ly, lymphocytes; Tregs, T regulatory lymphocytes; TLR ½, toll-like receptor 1/2; VDR, vitamin D receptor; 25OHD, calcidiol; 25(OH)2D, calcitriol; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; INF-γ, interferon γ; M1, pro-inflammatory macrophage phenotype; M2, anti-inflammatory macrophage phenotype; TGF-β, transforming growth factor-beta; NET, neutrophil extracellular traps; DC, dendritic cell; Ab-antibodies; SAM-S-adenosyl methionine; SAH-S, adenosyl-L-homocysteine; 5-MTHF, 5-methyltetrahydrofolate; THF, tetrahydrofolate; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; NK cells, natural killer cells. Red/blue arrows – stimulation. This figure was drawn using the vector image bank of Servier Medical Art (http://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3·0 Unported License (·https://creativecommons.org/licenses/by/3·0/).

Fig. 2. Mechanisms of microelements sequestration (iron, copper, zinc and selenium). Red arrows, stimulation; block arrow, inhibition; Hpc, hepcidin; Hph, hephestin; Fpn, ferroportin, Tf, transferrin; Crlp, Ceruloplasmin; SeP, selenoprotein P; ZIP, Zrt/Irt-like protein (mediate Zn influx); ZnT, zinc transporters (mediate Zn efflux); MT, metallothioneins.
This work was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia [grant number 451-03-9/2021-14/200113].
B. D., J. M., B. D. and T. J. S. conceptualised the paper. All the authors were involved in the literature search, extraction, analysis and interpretation of data. B. D., J. M., D. S. and A. V. wrote an original draft. B. D. and T. J. S. reviewed and edited the manuscript. B. D., J. M. and A. V. contributed to visualisation. All the authors approved the version to be submitted. B. D. and J. M. contributed equally.
There are no conflicts of interest.