Low intakes of energy, protein and other nutrients, together with high infection rates and poor socio-economic status may lead to linear growth retardation and impaired child development(Reference Martorell, Mendoza, Castillo and Waterlow1). Cross-sectional studies have linked stunting (short stature for age) and low weight-for-age to poor development and school achievement in infants and children(Reference Grantham-McGregor and Baker-Henningham2). The detrimental effects of undernutrition early in life ( < 2 years of age) on intellectual development seem irreversible and remain apparent during childhood and adolescence(Reference Chang, Walker and Grantham-McGregor3, Reference Walker, Chang and Powell4).
The n-3 fatty acid DHA and the n-6 fatty acid arachidonic acid are important structural components of the human central nervous system(Reference Martinez5) and play a role in brain functioning through their involvement in aspects of neuron function and of neurotransmitter synthesis(Reference Yehuda6). These two fatty acids can be synthesised by the human body from the α-linolenic acid (ALA) and linoleic acid. However, dietary intake of the n-3 fatty acid ALA in children is considered to be low(Reference Elmadfa, Weichselbaum and Konig7, Reference Kris-Etherton and Innis8), which possibly limits adequate cognitive functioning. In fact, high fish intake during pregnancy has been associated with better cognitive development of infants(Reference Daniels, Longnecker and Rowland9–Reference Hibbeln, Davis and Steer11) and maternal and infant DHA supplementation may benefit visual, motor and mental development of infants and young children(Reference Helland, Smith and Saarem12–Reference Agostoni, Zuccotti and Radaelli18). For healthy children >2 years of age such evidence is currently limited(Reference Eilander, Hundscheid and Osendarp19).
Among the micronutrients, Fe and iodine interventions have been shown to improve intelligence scores of children(Reference Grantham-McGregor and Ani20, Reference Sachdev, Gera and Nestel21). Fe is needed for the formation of Hb for adequate oxygen transport in the human body. In the brain, Fe is required for myelination and neurotransmitter synthesis(Reference Beard and Connor22). Iodine is an important component of the thyroid hormones, thyroxin and tri-iodothyronine, which plays a major role in the growth and development, function and maintenance of the central and peripheral nervous system(Reference Delange23). For the B-vitamins, however, little research has been conducted to investigate whether these vitamins are of influence on mental development in children. Vitamin B12 (cobalamin) deficiency has been associated with lower scores on cognitive tests in Guatemalan(Reference Louwman, van Dusseldorp and van de Vijver24) and Dutch(Reference Allen, Penland and Boy25) children. Folate is important for closure of the neural tube during fetal development(Reference Butterworth and Bendich26), but no studies have investigated the role of folate on cognitive functioning in children after birth. In the brain, folate is required for neurotransmitter production and myelination(Reference Sugden27, Reference Hutto28). Because of the interactions with folate metabolism, vitamin B12 is indirectly involved in neurotransmitter synthesis. Furthermore, the vitamin B12 cofactors adenosylcobalamin and methylcobalamin are involved in myelination of the spinal cord and the brain(Reference Dror and Allen29).
The primary objective of the present study is to investigate the associations between indicators of body size, fatty acid status, and Fe, iodine and B-vitamin status on overall cognitive performance in 598 Indian school-age children. Secondary, we will explore the relationships of the nutritional parameters with specific cognitive domains known to be sensitive to differences in nutritional status in children. We hypothesise that the indicators of body size, fatty acid and micronutrient status (Fe, iodine, folate and vitamin B12) will be positively related to overall cognitive performance and specific cognitive domains.
Experimental methods
The Children's Health And Mental Performance Influenced by Optimal Nutrition study was designed to investigate the efficacy of foods fortified with n-3 fatty acids and micronutrients on improving intellectual performance and growth in Indian school children(Reference Muthayya, Eilander and Transler30). The baseline data of the present study, collected in the period between November 2005 and February 2006, were used to assess the associations between height-for-age (HAZ) and weight-for-age z-scores (WAZ), Hb concentration and indicators of n-3 and n-6 fatty acid, Fe, iodine, folate and vitamin B12 status and cognitive performance. These nutritional parameters were selected based on their possible relationship with children's mental development.
Subjects
Two primary schools serving children from a poor socio-economic background in Bangalore city, India were selected for participation in the study. Almost all children living in the surrounding communities attended these schools, where they were taught in the local Kannada language. Before study start, parents or caretakers of all children aged 6–10 years, attending grades 2–5 of these schools were invited for a meeting during which the study procedures were explained to them. Informed, written consent from the parents and verbal assent from their children was obtained from 645 parent–child pairs. Children were included in the study if they were: (1) apparently healthy, without any chronic illness and physical/mental handicaps; (2) not severely anaemic (Hb < 80 g/l); (3) not severely undernourished ( < − 3 sd for WAZ and HAZ-scores of National Health Centre for Statistics/WHO standards(31)); (4) not intending to use micronutrients supplements during the study; (5) planning to reside in the study area during the next 12 months. Children who were frequently absent from school (>40 d during 6 months before start of the study) and children who took micronutrient supplements in the period of 3 months before the study start were excluded. A total of 598 children were enrolled in the study. Details on the enrolment, including a flow chart of children recruited in the study have been published elsewhere(Reference Muthayya, Eilander and Transler30).
Socio-demographic information
Socio-demographic information on household composition, parental education, income and use of fortified foods was collected by a structured questionnaire that was administered to the mother or primary caretaker of the subjects. The age of the children was verified by the school records.
Cognitive performance
Cognitive performance was evaluated using age-appropriate, validated psychometric tests that were administered by seven masters-level psychologists in Kannada language. The psychologists were trained extensively during 3 weeks before the study to ensure standardisation in the test administration and scoring procedures. The cognitive test battery was administered to each child on a single day over three sessions of which two took place in the morning and one in the afternoon. Care was taken to ensure all the children had breakfast before testing began in the morning since omitting breakfast is known to impair cognitive performance(Reference Grantham-McGregor32). The cognitive test battery consisted of eleven subtests, including six core tests of the Kaufman Assessment Battery for Children, second edition for children 3–18 years (pattern reasoning, triangles, rover, number recall, word order, atlantis)(Reference Kaufman and Kaufman33), two tests from Wechsler Intelligence Scale for Children-Revised and Wechsler Intelligence Scale for Children-4 (picture arrangement, coding) and three additional tests from Rey Auditory Verbal Learning Test (auditory-verbal learning test), NEPSY (neuropsychological assessment tool, verbal fluency) and number cancellation, which was specifically designed for the study. The eleven subtests covered four cognitive domains as specified in Carroll's model as described in the Kaufman Assessment Battery for Children, second edition manual(Reference Kaufman and Kaufman33), including fluid reasoning, short-term memory, retrieval ability and cognitive speediness (Fig. 1). These domains were chosen because they have shown to be influenced by previous nutritional interventions(Reference Hughes and Bryan34). The test battery underwent an extensive adaptation process to ensure its suitability in the local cultural context(Reference Malda, van de Vijver and Srinivasan35). For each subtest, a sum score was calculated and converted into a standardised z-score. The domain score was composed by taking the average of standardised z-scores for the tests constituting a domain. The average of the domain scores named the mental processing index (MPI) was a composite measure of overall cognitive performance based on the Kaufman Assessment Battery for Children, second edition manual(Reference Kaufman and Kaufman33). Our model of clustering of individual sum scores to form a composite score in four separate cognitive domains showed good validity assessed by structural equation modelling techniques(Reference Malda, van de Vijver and Srinivasan36).
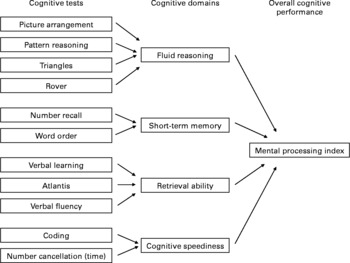
Fig. 1 Clustering of cognitive tests in domain scores. Fluid reasoning involves basic processes of reasoning and other mental activities that depend only minimally on learning and acculturation; short-term memory is an ability that requires apprehending and holding information in immediate awareness briefly and then using that information within a few seconds; retrieval ability comprises the capacity to store information in long-term memory and to retrieve that information fluently and efficiently; cognitive speediness measures the ability of rapid cognitive processing of information involving attention.
Anthropometry
Anthropometric measurements were conducted in duplicate according to standard techniques(Reference Lohman, Roche and Martorell37) by trained research assistants. Height was recorded to the nearest 0·1 cm using a locally made stadiometer (BioRad, Chennai, India) that was fixed to a wall. Body weight was recorded to the nearest 0·1 kg using a digital weighing scale (Breuer, Germany). During the measurements, children wore their school uniform and no shoes, caps or hats. HAZ and WAZ were computed by data on height, weight, age and sex using the National Health Centre for Statistics/WHO growth reference data(31). Children with HAZ and WAZ < − 2 sd of this reference median were classified as stunted and underweight, respectively. We did not include weight-for-height z-scores, because National Health Centre for Statistics/WHO reference data were lacking for children >10 years of age, which concerned 57 children aged 10–11 years in the present study.
Biochemical indicators
A whole blood sample (10 ml) was collected in the morning from non-fasted children by venepuncture in an EDTA vacutainer. A spot urine sample was also collected in a sterile plastic container, and the samples were transported to the laboratory on ice. Care was taken to limit the exposure of the samples to light. Hb concentrations were determined within 4 h of collection using an AcT Diff2 Counter (Beckman Coulter Inc., Fullerton, CA, USA). One aliquot of whole blood for erythrocyte folate estimation was immediately treated with freshly prepared 1 % ascorbic acid. The remaining blood was immediately centrifuged (3000 rpm, 10 min, 4°C), and the plasma was stored in 2 ml eppendorf tubes at − 80°C until analysis. One millilitre of erythrocytes was washed with 5 ml saline containing EDTA (1 litre normal saline+0·00 324 g disodium EDTA), flushed under nitrogen and stored at − 80°C until analysis for fatty acid content. Serum ferritin was measured by an enzyme immunoassay (Access® 2 Beckman Coulter autoanalyser, Brea, CA, USA)(Reference Lohman, Roche and Martorell37) against an external 3-level control material (WHO Standard 80/578; Ramco Laboratories Inc., Houston, TX, USA). Serum soluble transferrin receptor (sTfR) was measured by using an enzyme immunoassay (Ramco Laboratories Inc.) with two-level control materials provided by the manufacturer. C-reactive protein was analysed by a turbidimetric method (Roche Hitachi 902, Indianapolis, IN, USA)(Reference Burtis and Ashwood38). Plasma vitamin B12 and red blood cell folate were analysed using a chemiluminescence system (ACS:180, Bayer Diagnostics, Tarrytown, NY, USA)(Reference Miale39, Reference Chen, Sperling, Heminger, Pesce and Kaplan40). Fatty acid content of erythrocyte membrane phospholipids was analysed using GC with a flame ionization detector (Varian 3800, Palo Alto, CA, USA). The procedure involved the extraction of total lipids, isolation of phospholipid fraction by TLC and transmethylation of phospholipids(Reference Rose and Oaklander41–Reference Morrison and Smith43). The fatty acid methyl esters were separated by chain length and degree of saturation by injection onto a 50 m × 0·2 mm capillary column (Varian, Palo Alto, CA, USA) with nitrogen as the carrier gas. Urinary iodine was measured using the Sandell–Kolthoff reaction as modified by Pino et al. (Reference Pino, Fang and Braverman44). Satisfactory agreement in urinary iodine was obtained on urine samples at four different concentrations measured and the Ensuring the Quality of Urinary Iodine Procedures, Centers for Disease Control and Prevention (Atlanta, GA, USA). The following criteria were used to define micronutrient deficiencies: anaemia: Hb < 115 g/l(45); Fe deficiency: serum ferritin < 15 mg/l and/or sTfR >7·6 mg/l(Reference Zimmermann, Molinari and Staubli-Asobayire46); folate deficiency: erythrocyte folate < 305 nmol/l(47); vitamin B12 deficiency: plasma vitamin B12 < 148 pmol/l(Reference Jones, Ramirez-Zea and Zuleta48); iodine deficiency: urinary iodine < 100 μg/l(49).
Statistical analyses
Values for serum ferritin concentrations from the subjects with elevated C-reactive protein (>10 mg/l) were excluded from statistical analyses. Body Fe stores were calculated from serum ferritin and sTfR concentrations using the formula by Cook et al. (Reference Cook, Flowers and Skikne50). Differences in mean cognitive outcomes between boys and girls, schools and different levels of education of the mother were assessed by t tests. Distributions of parameters of fatty acid status, serum ferritin and sTfR, erythrocyte folate, plasma vitamin B12 and urinary iodine were normalised by natural logarithm (ln) transformation before analysis. Associations between the nutritional parameters and the cognitive scores were analysed using ANOVA (SAS General Linear Modelling procedure) taking into account age, sex, school, maternal education level and assessor of cognitive tests as covariates. All available data were analysed, missing values were not replaced. All analyses were performed using Statistical Analysis Software version 9.1 statistical software package (SAS Institute Inc., Cary, NC, USA).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committees at St John's National Academy of Health Sciences, Bangalore, India and Wageningen University, The Netherlands. Written informed consent was obtained from the parents of all subjects and verbal assent from all the subjects. Verbal consent was witnessed and formally recorded.
Results
Five hundred and ninety eight children completed the baseline measurements on cognitive performance, anthropometry and Hb concentrations. Data on biochemical indicators of micronutrient and fatty acid status were available for at least 529 and 541 children, respectively. The socio-demographic characteristics and nutritional status of the subjects are presented in Table 1. Mean age of the children was 8·7 (sd 1·2) years and 49 % of them were boys. Nearly, half of the mothers were uneducated and median family income was 2700 Indian rupees per month, which is close to the poverty line of US$2 per d. Twenty-two percent of the children were stunted and 30 % were underweight. The prevalence of anaemia was 9 %, while that of Fe, folate, vitamin B12 and iodine deficiencies were 31, 17, 23 and 47 %, respectively.
Table 1 Characteristics of the study population
(Mean values and standard deviations; median and percentile values)

Associations of covariates with cognitive performance
Age was significantly positively related with all cognitive outcomes (β = 0·31 (95 % CI 0·27, 0·35), P < 0·0001 for MPI). Mean cognitive scores for boys and girls are presented in Table 2. Scores on the domains of retrieval ability and cognitive speediness and MPI were significantly lower in boys compared with girls (P < 0·001). These findings did not change when scores were corrected for age (data not shown). There was a significant difference in performance on short-term memory and retrieval ability between the two schools (data not shown). Children of mothers with < 6 years of education had significantly lower MPI scores compared with children of mothers with ≥ 6 years of education (means were − 0·05 (sd 0·66) v. 0·07 (sd 0·63), P = 0·03, respectively).
Table 2 Cognitive domain scores for boys and girls*
(Mean values and standard deviations)
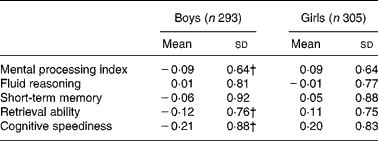
* Domain scores are expressed in z-scores.
† Scores between boys and girls were significantly different, t test (P < 0·001).
Associations of nutritional parameters with cognitive performance
Table 3 provides an overview of the associations between the nutritional parameters and the indicators of cognitive performance. Scatter plots of the correlations between the MPI and HAZ, WAZ, Hb and vitamin B12 concentrations are shown in Fig. 2. HAZ scores were significantly positively related to all cognitive domains and MPI. WAZ were significantly positively associated with all cognitive parameters, except cognitive speediness. The associations of HAZ and WAZ would in theory mean that an increase of 1 sd in HAZ and WAZ would correspond with 0·09 sd increase in MPI, P = 0·0006 and P = 0·002, respectively.
Table 3 Overview of associations of nutritional factors with cognitive performance*
(β coefficients and 95 % confidence intervals)
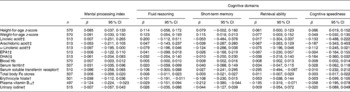
* Using a model adjusted for age, sex, school, maternal education and assessor of cognitive tests. The R 2 of the models with the different nutritional indicators ranged from 0·34 to 0·37 for MPI, 0·37 to 0·39 for fluid reasoning, 0·08 to 0·10 for short-term memory, 0·25 to 0·27 for retrieval ability and 0·34 to 0·36 for cognitive speediness.
† Variables were normalised by natural logarithm transformation.
‡ Fatty acids were measured in the erythrocyte membranes in the phospholipid fraction.

Fig. 2 Scatter plots of correlations between the mental processing index and height-for-age z-score (r 0·10, P = 0·012), weight-for-age z-score (r 0·11, P = 0·007), Hb concentration (r 0·21, P < 0·0001) and log-transformed vitamin B12 (r − 0·09, P = 0·046).
No significant relationships were detected between linoleic acid, arachidonic acid, EPA and DHA and any of the cognitive parameters. ALA was significantly inversely related to the MPI, but no significant associations were observed with the separate cognitive domains.
Hb concentrations were significantly positively related to all cognitive domains and MPI. Our findings suggest that an increase of 10 g/l in Hb concentration would translate into a 0·08 sd increase in MPI, P = 0·0008. There was a significant inverse association between sTfR concentrations and retrieval ability. Other indicators of Fe status were not significantly related to cognitive performance. Similarly, there were no significant associations between urinary iodine concentrations and cognitive parameters. In contrast, significantly inverse relationships were found between erythrocyte folate concentrations and fluid reasoning (β = − 0·10 (95 % CI − 0·19, − 0·01) and short-term memory ( − 0·11 (95 % CI − 0·23, 0·02)). However, when vitamin B12 status was added to the model, these inverse associations were not significant anymore for fluid reasoning ( − 0·07 (95 % CI − 0·17, 0·02) and for short-term memory ( − 0·08 (95 % CI − 0·21, 0·05)). Vitamin B12 concentrations were significantly inverse related to short-term memory and retrieval ability and the MPI. These associations remained significant after further adjusting for Hb and folate status and HAZ (β (95 % CI) were − 0·19 (95 % CI − 0·36, − 0·03) for short-term memory; − 0·20 (95 % CI − 0·33, − 0·08) for retrieval ability; − 0·12 (95 % CI − 0·22, − 0·02), P = 0·02 for MPI).
Discussion
The present study shows that indicators of body size, HAZ and WAZ and Hb concentrations were significantly positively related to various cognitive domain scores and MPI, while plasma vitamin B12 concentrations were significantly inversely associated with short-term memory and retrieval ability and MPI. Other indicators of Fe, folate, iodine and fatty acid status were not significantly related to cognitive performance.
Strengths of this cross-sectional study were the availability of biochemical parameters of micronutrient and fatty acid status in a relatively large sample of >500 children from a low socio-economic background. The sample is the representative for school children aged 6–10 years from poor socio-economic classes in Bangalore city and the surrounding peri-urban areas, based on a similar prevalence of anaemia measured and similar average heights and weights in our studies and other studies conducted in children in Bangalore(Reference Muthayya, Thankachan and Zimmermann51). The cognitive test battery was thoroughly adapted to local language and culture and showed good internal and external validity, which is essential to detect any associations between cognitive functioning and nutritional status(Reference Isaacs and Oates52). In addition, we chose to assess the cognitive abilities that have been shown to be influenced by nutritional interventions before(Reference Hughes and Bryan53).
A limitation of a cross-sectional study design is the inability for causal inference. Furthermore, the high number of comparisons made between nutritional and cognitive variables may have yielded false-positive findings (type I error). However, we tried to limit the number of comparisons by the use of composite scores for the cognitive tests. In addition, we aimed to look for patterns among our findings, such as the consistent positive association of HAZ with all cognitive parameters. Another limitation of the study was the finding that our overall model explained only 10–40 % of the variation in cognitive parameters. Genetic variation and environmental factors such as socio-emotional stimulation at home may account for this unexplained variation. In additional analyses we explored whether the interactions of age and sex with the nutritional indicators could explain any variation in cognitive test scores, but the results of these analyses did not yield further insights.
In agreement with our findings, lower HAZ, reflecting longer term undernutrition, has previously been associated with poorer cognitive performance in younger (1–3 years)(Reference Sigman, Neumann and Baksh54–Reference Whaley, Sigman and Espinosa56) and school children(Reference Powell and Grantham-McGregor57–Reference Johnston, Low and de Baessa59). Moreover, intervention studies have demonstrated that protein–energy supplementation in young children benefits cognitive development on the longer term(Reference Walker, Chang and Powell4, Reference Grantham-McGregor, Walker and Chang60, Reference Pollitt, Watkins and Husaini61) and therefore an adequate intake of energy and protein is required for optimal development.
Erythrocyte fatty acid status was unrelated to cognitive performance, which is in line with findings from a cohort study in children aged 7 years(Reference Bakker, Ghys and Kester62). Possibly, the range in fatty acid status among the children was too narrow to determine effects on cognition. It may also be that erythrocyte or plasma fatty acid status does not resemble brain fatty acid status at school age when most brain growth has been completed. A study in human subjects estimated that DHA requirements of the brain are rather low and the authors suggested that the liver may synthesise sufficient amounts of DHA to maintain brain DHA concentrations, provided that dietary intake of the precursor ALA is adequate(Reference Rapoport, Rao and Igarashi63). Moreover, animal studies indicated that synthesis of DHA in the liver is enhanced and the turnover of DHA in the brain is reduced when diets were low in ALA and free of DHA(Reference Rapoport, Rao and Igarashi63). Thus, intake and erythrocyte concentrations of n-3 fatty acids may not be related to brain function. Besides, there is some evidence that children with attention-deficit hyperactivity disorders have lower plasma–erythrocyte DHA and higher linoleic and arachidonic acid concentrations than control children(Reference Mitchell, Aman and Turbott64–Reference Colter, Cutler and Meckling67), which could be attributed to differences in fatty acid metabolism(Reference Burgess, Stevens and Zhang68). Therefore, more research is needed to investigate whether specific subgroups of children may be sensitive to fatty acid interventions and whether fatty acids may predominantly influence certain aspects of behaviour, such as attention.
For Hb, we showed a very small but significant positive relationship with mental performance. However, for the other parameters of Fe status, no such relationships could be detected. Possibly, this relationship becomes only apparent when Fe deficiency has caused anaemia, which was the case in only 6 % of the present study population. This has also been reported in a review of literature of observational studies showing that (Fe deficient) anaemic children have poorer cognitive development and school performance than non-anaemic children, and it was concluded that it is unclear whether Fe deficiency without anaemia impairs mental performance(Reference Nokes, van den Bosch and Bundy69). In contrast, Fe supplementation has been shown to improve mental performance in children >2 years of age in (Fe deficient) anaemic as well as in non-anaemic children(Reference Grantham-McGregor and Ani20, Reference Sachdev, Gera and Nestel21), indicating that extra Fe may also be beneficial for development of non-anaemic children. The higher cognitive scores with increasing Hb concentrations found in the present study, suggest that the Hb level for optimal mental performance may be higher than the current definition of anaemia ( < 115 g/l).
Against our expectations, both folate and vitamin B12 were inversely associated with some of the cognitive domain scores. For folate these inverse relationships disappeared after controlling for vitamin B12 status, while for vitamin B12 the inverse associations with memory remained significant even after controlling for folate, Hb and height-for-age. Our findings are in contrast with two earlier observational studies indicating that children with lower plasma vitamin B12 concentrations had poorer cognitive test scores(Reference Louwman, van Dusseldorp and van de Vijver24, Reference Allen, Penland and Boy25) and could be due to chance. In elderly, however, eight studies did not show significant associations between plasma vitamin B12 and cognitive test performance(Reference Raman, Tatsioni and Chung70) and one study showed an inverse relationship(Reference Durga, van Boxtel and Schouten71). Our finding and the observations in elderly contradict to the overt clinical signs of vitamin B12 deficiency of neurological damage. Therefore, it has been questioned whether plasma vitamin B12 is a suitable indicator to study effects on cognition(Reference Raman, Tatsioni and Chung70, Reference Miller72). It is of interest to investigate whether higher plasma homocysteine concentrations are related to poorer mental performance in children, as has been observed in elderly(Reference Raman, Tatsioni and Chung70, Reference Durga, van Boxtel and Schouten71). In both children and adults, plasma homocysteine concentrations are increasing when folate and vitamin B12 intake are low(Reference Bjorke Monsen and Ueland73) and elevated homocysteine may impair cognitive functioning through neurotoxic and vasotoxic effects(Reference Obeid and Herrmann74). Also other indicators of vitamin B12 status, such as holotranscobalamin and methylmalonic acid may be worth evaluating in future research(Reference Miller72).
In addition, we could speculate on other confounding factors that influence the relationship between higher plasma vitamin B12 concentrations and poorer cognitive performance. Possibly, consumption of animal products infected with pathogens or vegetables contaminated with vitamin B12-producing bacteria from manure may improve vitamin B12 status(Reference Allen75, Reference Antony76) and simultaneously increase the risk of disease, resulting in poor school attendance and impaired cognition. However, no literature is available to support this hypothesis.
Despite the evidence in literature that iodine deficiency is detrimental to cognitive development(Reference Bleichrodt, Born and Stanbury77) and that iodine supplementation improves cognitive functioning in children(Reference Zimmermann, Connolly and Bozo78), we failed to detect any association between urinary iodine concentrations and cognition, which may be due to day-to-day within subject variation in iodine excretion in urine(49).
In conclusion, findings of the present study are in agreement with other observational studies showing that undernutrition (lower HAZ and WAZ) and lower Hb concentrations adversely influence cognitive performance in school-age children, while serum ferritin and sTfR concentrations, and indicators of iodine, folate and fatty acid status were unrelated and an inverse association was found for vitamin B12 and memory. Future research is needed to elucidate the role of B-vitamins and homocysteine in cognitive development of children and to investigate whether fatty acid status at school age may be of influence on specific cognitive functions not measured in the present study, such as attention.
Acknowledgements
We are most grateful to the principals and teachers of the schools, the children and their parents for their participation in the study. We want to express our thanks to all our colleagues from St Johns Research Institute and Unilever who were involved in data collection and biochemical analyses, and to Maike Malda and Prof Fons van de Vijver, University of Tilburg, The Netherlands for the adaptation of the cognitive test battery and training of psychologists. Funding. The study was supported by Unilever Netherlands BV. Conflict of interest. A. E., H. v. d. K. and S. J. M. O. are employees of Unilever. All other authors do not have any conflict of interest. Author's responsibilities. All authors were involved in the design of the study and statistical analysis plan. A. E. and S. M. were responsible for data collection and overall study management. A. E. and H. v. d. K. performed the statistical analysis. A. E. wrote the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.









