In February 2018, the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) released the Third Expert Report, Diet, Nutrition, Physical Activity and cancer: a Global Perspective (1). Based on comprehensive evaluations of the global body of scientific evidence(2), the Report provides the latest cancer prevention recommendations with an emphasis on a more holistic approach of maintaining a healthy body weight, being physically active and eating a healthy diet(1). It has been estimated that nearly one-third of all cancers can be linked to factors that are modifiable, including the consumption of red and processed meats(3).
Red meat refers to all types of unprocessed mammalian muscle meat, such as beef, veal, pork, lamb, mutton, horse and goat(Reference McAfee, McSorley and Cuskelly4,5) . Processed meat (e.g. ham, salami, bacon, pastrami and some sausages) refers to meat that is transformed through salting, curing, smoking, drying, fermentation or other processes to improve the flavour or the quality(5) and may contain poultry, offal or meat by-products(Reference Bouvard, Loomis and Guyton6). Evaluation of the evidence on red and processed meat consumption suggests that red meat is a probable human carcinogen, while processed meat is convincingly carcinogenic(1,Reference Bouvard, Loomis and Guyton6) ; when it comes to cancer risk, there is no safe level of processed meat intake(1,Reference Bouvard, Loomis and Guyton6) .
Potential mechanisms underlying the carcinogenesis of red and processed meat have been identified in the IARC Monograph(2) and include N-nitroso compounds, heterocyclic amines and polycyclic aromatic hydrocarbons, which are mutagenic compounds that form during cooking of meat at high temperatures and processing of meats(Reference Lauber and Gooderham7–Reference Alomirah, Al-Zenki and Al-Hooti9). Pro-oxidants, including haem Fe and N-glycolyneuraminic acid, are also hypothesised to induce inflammation which may lead to tumorigenesis(Reference Cascella, Bimonte and Barbieri10,Reference Turesky11) . Epidemiological studies on dietary carcinogen intake have been challenging, due in part to difficulties in capturing levels of exposure to heterocyclic amines or polycyclic aromatic hydrocarbons by dietary assessment questionnaires(Reference Sinha, Peters and Cross12).
For those who eat meat, the WCRF/AICR recommendations are to limit red meat consumption to moderate amounts (<500 g/week) and to eat little, if any, processed meat(1). Published findings from the Alberta’s Tomorrow Project (ATP) cohort participants have found that 35 % of men and 11 % of women reported consuming more than 500 g/week of red meat, exceeding WCRF/AICR Cancer Prevention recommendations(Reference Whelan, Xu and Vaseghi13). This has important public health and policy implications and represents an opportunity to help those who exceed consumption recommendations to make informed choices to reduce their cancer risk. The WCRF/AICR recommendations for red and processed meat consumption are largely based on convincing and probable evidence of elevated colorectal cancer (CRC) risk(1,2,Reference Bouvard, Loomis and Guyton6,Reference Diallo, Deschasaux and Latino-Martel14) ; however, these recommendations intended to reduce overall cancer risk. Limited suggestive evidence of increased cancer risk has been identified in a variety of other subsites, including nasopharynx, oesophagus, lung, stomach and pancreas(1,2) .
Cancer prevention recommendations are meant to work as whole and to be adopted as a lifestyle package to promote an overall healthy lifestyle for cancer prevention. Our previous work has shown that greater adherence to all six selected WCRF/AICR lifestyle recommendations for cancer prevention was associated with lower risk of cancer in this cohort(Reference Xu, Vena and Whelan15). The WCRF/AICR recommendations for red and processed meat consumption were not developed using evidence of a well-defined threshold exposure, but are intended to provide a balance between the advantages of consuming meat, which are sources of essential macronutrients and micronutrients, with the disadvantages of potential risk of carcinogenesis(1). In effort to explore these relationships in greater detail, many epidemiological studies have examined dose–response relationships(Reference Crippa, Larsson and Discacciati16) and compared highest v. lowest (tertiles, quartiles or quintiles) intakes(Reference Islam, Akter and Kashino17–Reference Keszei, Schouten and Goldbohm19), adjusting for a varying range of known risk factors for cancer(Reference Zhao, Yin and Zhao20,Reference Zhao, Yin and Pu21) , yet inconsistent associations across cancer subsites and between studies have prevented the refinement of current intake recommendations. Moreover, current evidence has yet to determine whether the carcinogenic effect of processed meat varies as a result of its origin. As a result, the current WCRF/AICR recommendations have not differentiated processed meat based on its source: from red meat v. non-red meat. By analysing processed meat from all origins combined, true carcinogenic associations with processed meat intake may be obscured. This knowledge gap limits our understanding on processed meat carcinogenicity, particularly how the independent carcinogenic effects of processed meat production methods and meat redness interact.
Understanding cancer risk related to varying intakes of red meat and different sources of processed meat will provide useful information concerning the potential role of different dietary patterns with respect to cancer prevention and will likewise provide valuable evidence towards the refinement of cancer prevention recommendations. Thus, the aim of the current analysis was (i) to evaluate whether all processed meats confer equitable cancer risk and (ii) to explore the association between red meat and cancer risk, while adjusting for other known risk factors for cancer.
Methods
Cohort design and data collection
ATP is a longitudinal prospective cohort of about 55 000 Albertans established in 2000 to facilitate studies into the aetiology of cancer and other chronic diseases. A full description of study feasibility, design and enrolment is presented elsewhere(Reference Bryant, Robson and Ullman22,Reference Robson, Solbak and Haig23) . Briefly, Albertans aged 35–69 years, with no history of cancer except non-melanoma skin cancer, were recruited throughout the province. Participants enrolled between 2000 and 2008 completed the Health and Lifestyle Questionnaire, the Canadian Diet History Questionnaire (CDHQ-I) and the Past-Year Total Physical Activity Questionnaire. The Health and Lifestyle Questionnaire collected information on personal and family health history, reproductive history, smoking habits, anthropometric variables and sociodemographic characteristics. CDHQ-I is a 257-item past-year FFQ of foods, beverages and dietary supplements, based on the US National Cancer Institute’s Diet History Questionnaire, modified for use in Canada(Reference Csizmadi, Kahle and Ullman24,25) . The validated Past-Year Total Physical Activity Questionnaire assessed the frequency, duration and intensity of physical activities performed over the previous year(Reference Friedenreich, Courneya and Neilson26). As part of the informed consent process, participants consented to ongoing data linkage with administrative health data including the Alberta Cancer Registry (ACR) and provided valid Personal Health Numbers to facilitate linkage.
Inclusion in the current study was restricted to participants who completed all three self-report baseline questionnaires (Health and Lifestyle Questionnaire, CDHQ-I and Past-Year Total Physical Activity Questionnaire). Participants were excluded from this analysis if they were: deemed as residing outside of Alberta at enrolment (n 29), recruited as second individual from the same household (n 342), had a prior cancer diagnosis, except for non-melanoma skin cancer, assessed via ACR linkage (n 71), outside of the age range of 35–69 years at enrolment (n 46), reported indeterminate sex (n 3) or did not consent for linkage to administrative health data (n 180). The final sample size was 26 218 adults (median age, 50·0 (interquartile range (IQR) 14·0) years, 37·5 % men). Ethical approval for baseline data collection in ATP was obtained from the former Alberta Cancer Board’s Research Ethics Committee and the University of Calgary Conjoint Health Research Ethics Board, Certification file number HREBA.CC-17-0461 (baseline data collection), while ethics approval for the current study was obtained from the Health Research Ethics Board of Alberta – Cancer Committee, Certification file number HREBA.CC-17-0099.
Dietary intake assessment
Past-year dietary intake data were collected using CDHQ-I(Reference Csizmadi, Kahle and Ullman24,25) . CDHQ-I data were analysed using Diet*Calc software for Windows (version 1.4.2; National Cancer Institute). The CDHQ-I nutrient database was used to estimate average daily intakes of energy, nutrients, foods, beverages and dietary supplements. Red meat and processed meat were defined following the WCRF/AICR criteria (Supplementary Material A: Table SA1).
We focused on selected food items recommended for cancer prevention (red meat, processed meat, non-starchy vegetables and fruits (excluding juices), pulses and wholegrains)(1) and further differentiated the source of processed meat (derived from red v. non-red meats) (Supplementary Material A: Table SA1). Adherence to the WCRF/AICR recommendations for red meat and processed meat consumption was based on 500 g/week(1) and 50 g/week, respectively. We used 50 g/week as the cut-off for processed meat intake since it is considered the standard serving size equivalent to approximately one hot dog or four strips of bacon(Reference Chan, Lau and Aune27,Reference Nomura, Inoue-Choi and Lazovich28) . In order to explore whether a dose–response relationship exists between processed meat derived from red v. non-red meat sources and cancer risk, we also categorised processed meat intake into quartiles.
Sociodemographic, health characteristics and assessment of physical activity
Age, sex, educational attainment, annual household income, family (father, mother, brothers and sisters) history of cancer, personal history of health conditions (high blood pressure, high blood cholesterol, angina, heart attack, stroke, emphysema, chronic bronchitis, diabetes, polyps in colon or rectum, inflammatory bowel diseases (which includes ulcerative colitis and Crohn’s disease), hepatitis and liver cirrhosis), personal history of bowel condition which includes inflammatory bowel diseases and/or a history of polyps in colon or rectum, smoking status (current smoker, former smoker, never smoked), body weight, standing height and geographical location of residence were obtained. The above were self-reported at enrolment using the Health and Lifestyle Questionnaire. Each participant’s past-year physical activity was also self-reported at enrolment using the Past-Year Total Physical Activity Questionnaire.
Assessment of cancer incidence
All participants included in this study were cancer-free at enrolment, as confirmed by linkage with the ACR. Primary incident cancer cases following enrolment were ascertained through data linkage with the ACR in June 2018. Primary malignant cancers, excluding non-melanoma skin cancer, were grouped into four outcomes of cancer incidence, based on cancer type:
-
1. All cancers combined.
-
2. Fifteen cancers combined – previously linked with red and/or processed meat intakes as identified in the IARC Monographs(2,3,Reference Bouvard, Loomis and Guyton6,Reference Grunst, Fateh and Lamerz29) : colorectal (colon, rectum and rectosigmoid junction), stomach, pancreas, prostate, breast, bronchus and lung, oesophagus, kidney, bladder, ovary, endometrium, non-Hodgkin lymphoma, liver and intrahepatic bile ducts, leukaemia and others (thyroid, gallbladder, testis, brain).
-
3. Gastrointestinal (GI) cancers, based on the WHO classification of digestive system cancers(Reference Bosman, Carneiro and Hruban30): oesophagus, stomach, small intestine, colorectal (colon, rectum and rectosigmoid junction), anus, anal canal and anorectum, liver and intrahepatic bile ducts, gallbladder and extrahepatic bile ducts and exocrine pancreas.
-
4. CRC: colon, rectum and rectosigmoid junction.
Statistical analyses
To investigate the association between red meat and processed meat intakes with cancer incidence (all cancers combined, fifteen cancers combined, GI cancers and CRC), Cox proportional hazard (PH) models were used. Person-years of follow-up were calculated from the date of enrolment to the date of cancer diagnosis or date of case ascertainment through the ACR linkage, whichever came first. To account for the effect of participant who passed away during the study on person-years follow-up, we conducted a sensitivity analysis using vital statistics data obtained from administrative databases. In these participants, follow-up time was calculated from age at enrolment to age at death. Competing risk analysis was performed, with the standard multivariable Cox PH regression model applied to the cause-specific hazard of interest and competing events treated as censored observations(Reference Prentice, Kalbfleisch and Peterson31).
For all cancers combined and fifteen cancer combined incidences, the PH assumption (e.g. constant relative hazard) was not met. Thus, adjusted hazard ratios (AHR) and 95 % CI were estimated separately for men and women using multivariable Cox PH models and stratified on age at enrolment in 5-year age categories.
For the outcomes of GI cancers and CRC incidence, the PH assumption was met. Thus, AHR and 95 % CI were estimated for men and women separately without any age-stratified adjustment. However, for GI cancer and CRC, the Firth penalised estimation method(Reference Firth32) was used in the multivariable Cox regression to account for the small number of cancer cases in these subgroups.
In all models, red and processed meat intakes were the exposure variables of interest and were modelled using two categorisation schemes. The first scheme was based on categories created using quartiles, and the second scheme was based on the WCRF/AICR recommendations, evaluated separately for men and women. Two models were run for each cancer outcome with different covariate adjustments. Covariates were chosen based on personal recommendations for cancer prevention published by the WCRF/AICR for cancer research (2018)(5) and univariate analysis to determine significant sociodemographic variables. AHR were estimated in comparison with the association of the lowest category of red or processed meat consumption with cancer outcomes. Analyses were conducted using SAS Enterprise Guide version 9.4 (SAS Institute Inc.), and statistical significance was set as alpha ≤ 0·05 (two-tailed).
Results
Participants’ characteristics
Participant characteristics at enrolment and cancer incidence during follow-up are presented in Table 1. The median consumption (g/week) of red meat, processed meat from red meat and processed meat from non-red meat was 267·9 (IQR 269·9), 53·6 (IQR 83·3), and 11·9 (IQR 31·8), respectively. Having a family history of all cancers combined, fifteen cancers combined, GI cancers, and CRC was reported by 52·5 %, 42·1 %, 14·6 % and 8·5 % of participants, respectively, whereas 46·8 % and 6·1 % of participants reported personal history of at least one health condition and bowel condition, respectively. At enrolment, most participants lived in urban regions (76·5 %), had attained or completed post-secondary education (72 %), were non-smokers (82·4 %) and were overweight or obese (BMI ≥ 25 kg/m2) (65·7 %). Greater proportions of women than men had normal BMI (18·5 ≥ BMI < 25 kg/m2; 40·5 % v. 23·1 %), reported consuming <500 g/week of red meat (90·4 % v. 66·8 %), <50 g/week of processed meat derived from red meat (59·2 % v. 28·3 %), processed meat from non-red meat (86·8 % v. 76·1 %) and processed meat from red and non-red meat combined (44·6 % v. 17·8 %). Lower proportions of women than men were diagnosed with all cancers combined (8·9 % v. 11 %), fifteen cancers combined (7·6 % v. 9·4 %), GI cancers (1·4 % v. 2·1 %) and CRC (0·9 % v. 1·2 %).
Table 1 Participants’ characteristics at enrolment to Alberta’s Tomorrow Project and cancer incidence at follow-up, stratified by sex*
(Numbers and percentages; median and interquartile range (IQR))
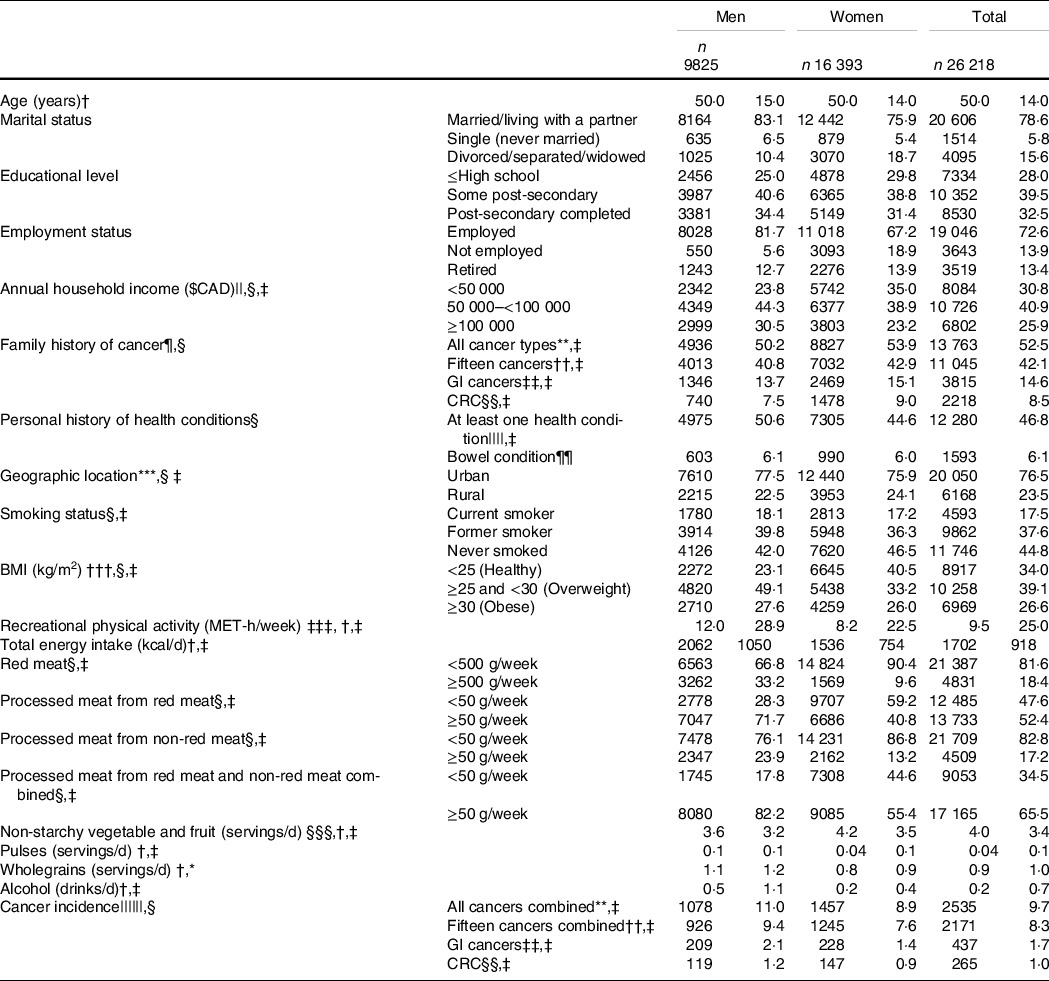
GI, gastrointestinal; CRC, colorectal cancer.
* A total of 711 participants (169 men, 542 women) had missing sociodemographic data.
† Values are presented as median (IQR).
‡ P-value < 0·05 men v. women (Pearson χ 2 tests for categorical variables; Wilcoxon rank-sum tests for continuous variables).
§ Values are presented as frequency (column percentage).
|| Education attainment and annual household income were treated as continuous variables.
¶ Family includes father, mother, brothers and sisters.
** Primary malignant cancers, excluding non-melanoma skin cancer.
†† Fifteen cancers combined previously linked to red and processed meat intakes: colorectal (colon, rectum and rectosigmoid junction), stomach, pancreas, prostate, breast, bronchus and lung, oesophagus, kidney, bladder, ovary, endometrium, non-Hodgkin lymphoma, liver and intrahepatic bile ducts, leukaemia and others (thyroid, gallbladder, testis, brain).
‡‡ Oesophagus, stomach, small intestine, colorectal (colon, rectum and rectosigmoid junction), anus, anal canal and anorectum, liver and intrahepatic bile ducts, gallbladder and extrahepatic bile ducts and exocrine pancreas.
§§ Colon, rectum and rectosigmoid junction.
||||High blood pressure, high blood cholesterol, angina, heart attack, stroke, emphysema, chronic bronchitis, diabetes, polyps in colon or rectum, inflammatory bowel diseases (IBD, which includes ulcerative colitis and Crohn’s disease), hepatitis and liver cirrhosis.
¶¶ Personal history of bowel condition which includes inflammatory bowel disease (IBD; includes ulcerative colitis and Crohn’s disease) and/or a history of polyps in colon or rectum.
*** Geographic location was determined using postal codes, where ‘0’ as the second digit corresponded to rural regions.
††† Calculated from self-reported height and weight.
‡‡‡ Total metabolic equivalent of task (MET)-h/week spent performing recreational physical activities at moderate (>3 to ≤6 MET) or vigorous (>6 MET) intensity.
§§§ Excluding juices.
|||||| Primary incident cancer cases were ascertained on November 2017 through data linkage with the Alberta Cancer Registry (ACR).
Associations of high red and processed meat intakes with incidence of all cancers combined
For incidence of all cancers combined, the median follow-up time was 13·4 (IQR 5·1) and 13·3 (IQR 5·1) years (total of 129 105·7 and 214 164·8 person-years follow-up) for men and women, respectively.
Women with a mild intake (i.e. 1st quartile) of processed meat derived from red meats had an increased risk of all cancers combined (AHR: 1·22 (95 % CI 1·05, 1·42). No significant associations were observed in men (Table 2 – model 2).
Table 2 Multivariable Cox proportional hazard (PH) models for the association of red and processed meat intake with incidence cases of all cancers combined*, separated by sex
(Adjusted hazard ratios (AHR) and 95 % confidence intervals)
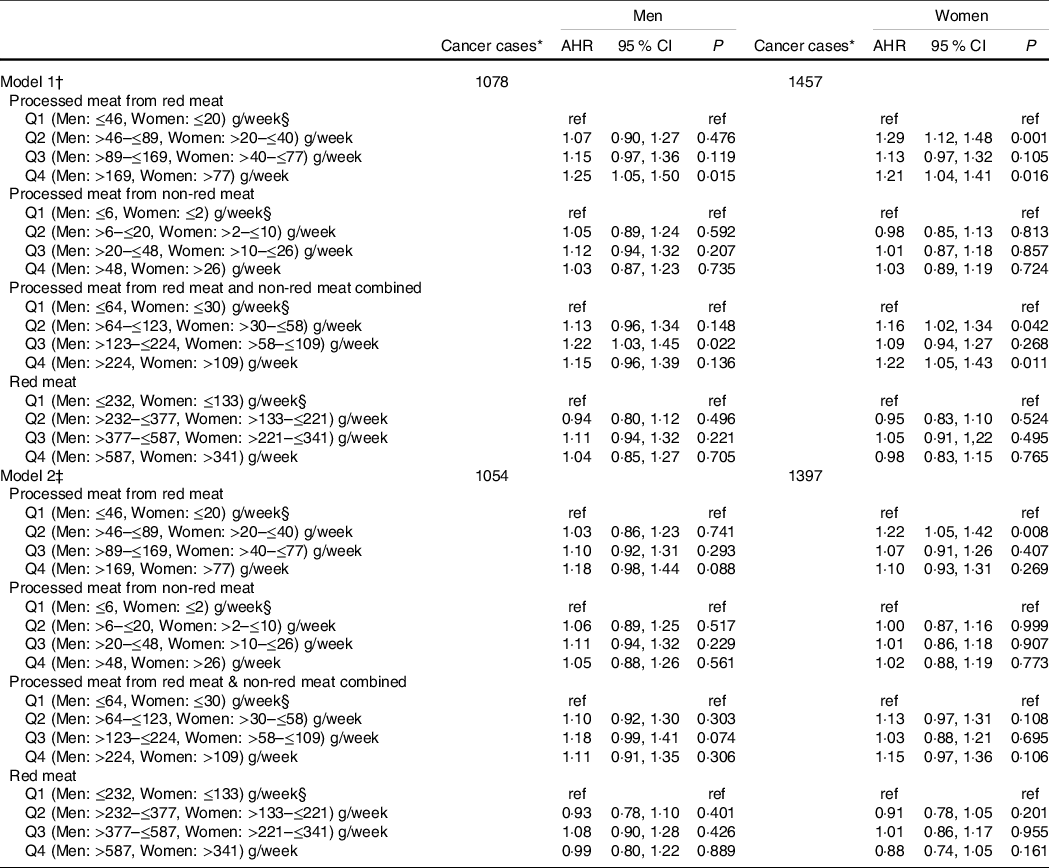
* Incidence primary malignant cancers, excluding non-melanoma skin cancer.
† Models 1: AHR were estimated using a Cox PH model stratified on age at enrolment in 5-year categories and adjusted only for total daily energy intake, separated by sex.
‡ Models 2: AHR were estimated using a Cox PH model stratified on age at enrolment in 5-year categories and adjusted for smoking status, BMI, recreational physical activity, total daily energy intake, non-starchy vegetables and fruits, pulses, whole grains, family history of all cancer types, alcohol consumption (drinks/d), annual household income, marital status, employment status, education level, geographic location, personal history of at least one health condition, red meat and all processed meat, separated by sex.
§ Reference category.
Association of high red and processed meat intakes with incidence of fifteen cancers combined
Women with a mild intake (i.e. 1st quartile) of processed meat derived from red meats had an increased risk of fifteen cancers combined (AHR: 1·20 (95 % CI 1·02, 1·41). No significant associations were found in men (Table 3, model 2).
Table 3 Multivariable Cox proportional hazard (PH) models for the association of red and processed meat intake with incidence cases of fifteen cancers combined*, separated by sex
(Adjusted hazard ratios (AHR) and 95 % confidence intervals)
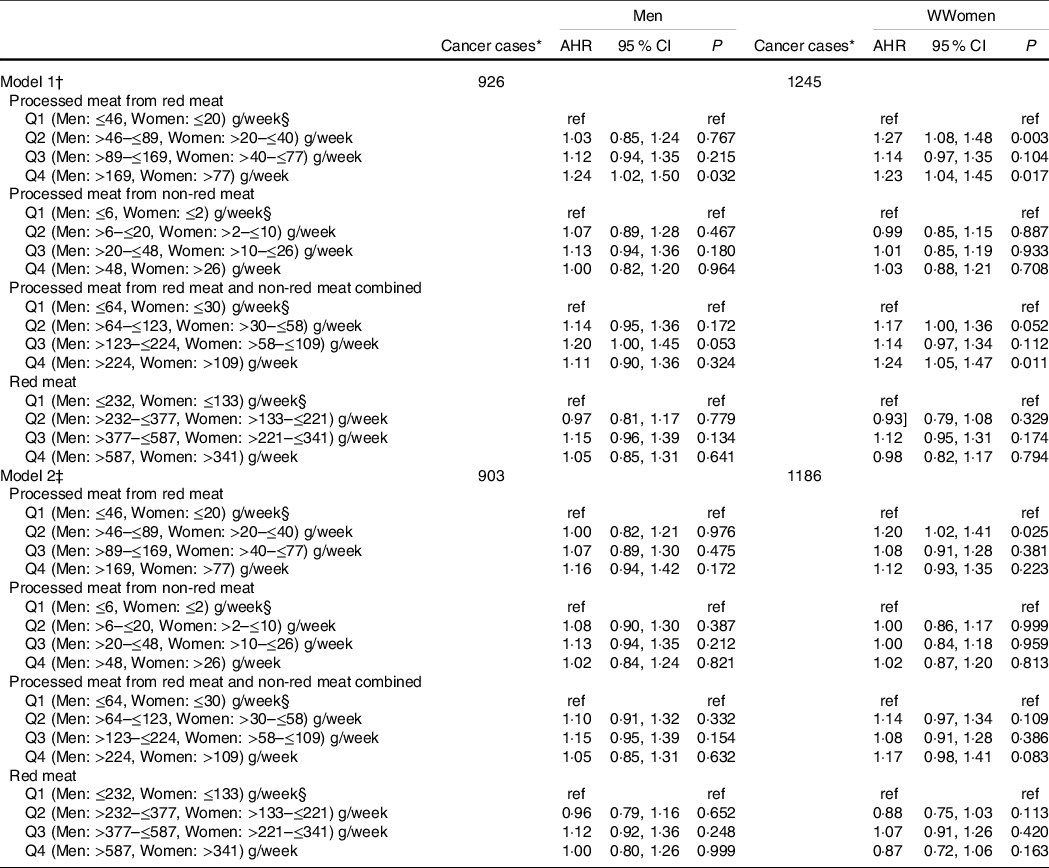
* Incidence primary malignant fifteen cancers combined previously linked to red and processed meat intakes: colorectal (colon, rectum and rectosigmoid junction), stomach, pancreas, prostate, breast, bronchus and lung, oesophagus, kidney, bladder, ovary, endometrium, non-Hodgkin lymphoma, liver and intrahepatic bile ducts, leukaemia and others (thyroid, gallbladder, testis, brain).
† Models 1: AHR were estimated using a Cox PH model stratified on age at enrolment in 5-year categories and adjusted only for total daily energy intake, separated by sex.
‡ Models 2: AHR were estimated using a Cox PH model stratified on age at enrolment in 5-year categories and adjusted for smoking status, BMI, recreational physical activity, total daily energy intake, non-starchy vegetables and fruits, pulses, whole grains, family history of all cancer types, alcohol consumption (drinks/d), annual household income, marital status, employment status, education level, geographic location, personal history of at least one health condition, red meat and all processed meat, separated by sex.
§ Reference category.
Associations of high red and processed meat intakes with incidence of gastrointestinal cancers
Women with a high intake (i.e. 4th quartile) of processed meat derived from red meat had an increased risk of GI cancer (AHR: 1·68 (95 % CI 1·09, 2·57). Mild intakes (2nd Quartile) of processed meat from red and non-red meat combined were also associated with increased risk of GI cancers in women AHR: 1·45 (95 % CI 1·01, 2·11). (Table 4, model 2).
Table 4 Multivariable cox proportional hazard (PH) models for the association of red and processed meat intake with incidence cases of gastrointestinal cancers*, separated by sex
(Adjusted hazard ratios (AHR) and 95 % confidence intervals)
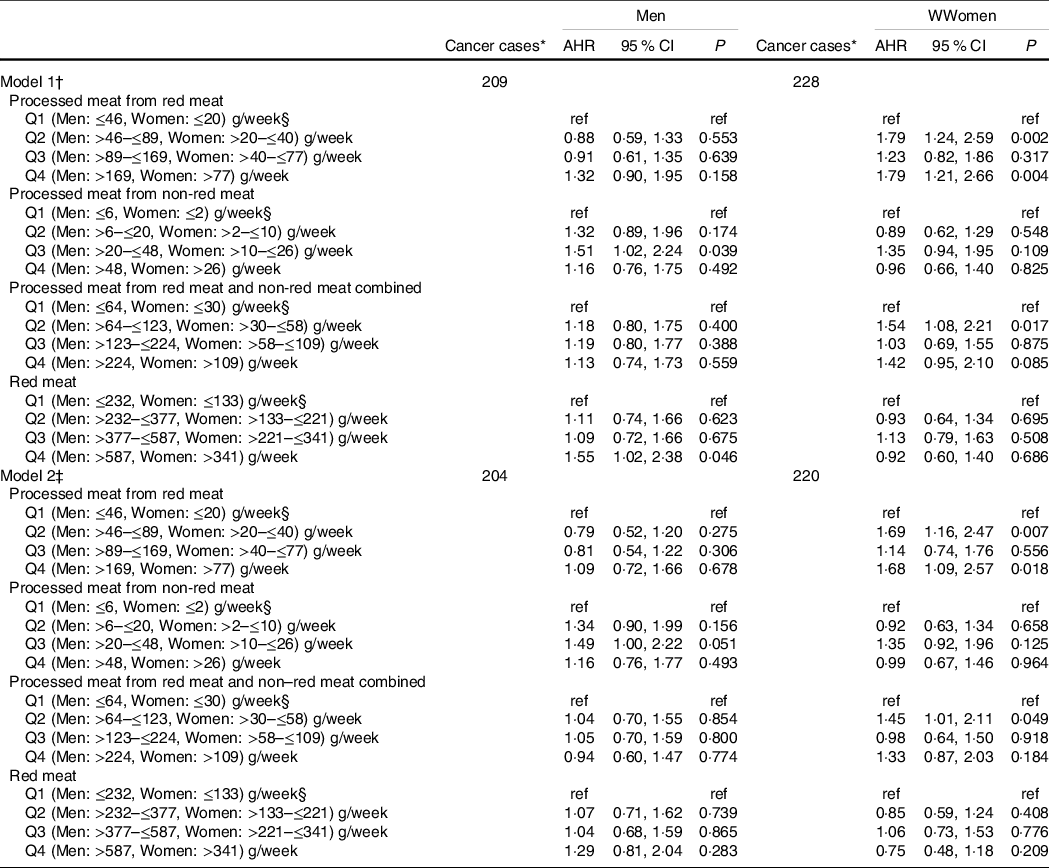
* Incidence primary malignant gastrointestinal cancers: cancers oesophagus, stomach, small intestine, colorectal (colon, rectum and rectosigmoid junction), anus, anal canal and anorectum, liver and intrahepatic bile ducts, gallbladder and extrahepatic bile ducts and exocrine pancreas.
† Models 1: AHR were estimated using a Cox PH model and adjusted only for total daily energy intake, separated by sex.
‡ Models 2: AHR were estimated using a Cox PH model and adjusted for smoking status, BMI, recreational physical activity, total daily energy intake, non-starchy vegetables and fruits, pulses, wholegrains, family history of all cancer types, alcohol consumption (drinks/d), annual household income, marital status, employment status, education level, geographic location, personal history of at least one health condition, red meat and all processed meat, separated by sex.
§ Reference category.
Association of high red and processed meat intakes with incidence of colorectal cancer
Women with high intake (i.e. 4th quartile) of processed meat derived from red meat had an increased risk of CRC AHR: 1·90 (95 % CI 1·12, 3·22). This association persisted even after adjustment for covariates (Table 5 – model 2).
Table 5 Multivariable cox proportional hazard (PH) models for the association of red and processed meat intake with incidence cases of colorectal cancers*, separated by sex
(Adjusted hazard ratios (AHR) and 95 % confidence intervals)

* Incidence primary malignant colorectal cancers: colon, rectum and rectosigmoid junction.
† Models 1: AHR were estimated using a Cox PH model and adjusted only for total daily energy intake, separated by sex.
‡ Models 2: AHR were estimated using a Cox PH model and adjusted for smoking status, BMI, recreational physical activity, total daily energy intake, non-starchy vegetables and fruits, pulses, wholegrains, family history of all cancer types, alcohol consumption (drinks/d), annual household income, marital status, employment status, education level, geographic location, personal history of at least one health condition, red meat and all processed meat, separated by sex.
§ Reference category.
When the analysis was repeated using adherence v. non-adherence to the WCRF/AICR recommendations, there were no significant associations observed after covariate adjustment (Supplementary Material B: Table SB1, SB2, SB3, SB4). Interaction terms between BMI and red and processed meat intakes were not significant, indicating that BMI does not modify the association between meat intake and cancer risk. Thus, these interaction terms were excluded from all the models.
Competing risk analysis to account for deaths before ACR linkage date in participants who were cancer-free during follow-up did not significantly change the observed hazard ratios (data not shown).
Discussion
In the present study, we evaluated associations between reported meat intake and cancer risk using two methods: quartiles to explore potential dose–response relationships, comparing high v. low intakes, and also a secondary analysis using the current WCRF/AICR recommendations for red meat intake cut-offs (500 g/week) and a 50 g/week intake cut-off for processed meat. Although no dose–response relationships were observed, we identified considerable differences in cancer risk conferred from the source of processed meat intake. Processed meat from red meat resulted in stronger associations with GI and CRC cancer outcomes compared with both red meat and processed meat from non-red meat, but these were not observed in all cancers and fifteen cancers combined. This may be the first time that a differential risk related to the source of processed meat has been identified.
Our findings build on an extensive body of research on the association between red and processed meat intake and cancer risk. In 2018, the WCRF/AICR Continuous Update Project report stated that there was strong evidence linking high red and processed meat consumption with an increased risk of cancer(1) and other cohort studies, systematic reviews and meta-analyses provide evidence for a positive association, especially for CRC(Reference Keszei, Schouten and Goldbohm19,Reference Chan, Lau and Aune27,Reference Huxley, Ansary-Moghaddam and Clifton33–Reference English, MacInnis and Hodge44) . Compared with other studies using different study designs and methodology, a Japanese cohort of men and women aged 35 years and older, which used an FFQ, examined associations of total meat consumption and intake of red meat and processed meat with risk of colorectal, colon and rectal cancer(Reference Wada, Oba and Tsuji45).The authors reported that the highest intake (4th Quartile) of processed meat was significantly associated with colon cancer among men(Reference Wada, Oba and Tsuji45). Moreover, the highest intake (4th Quartile) of red meat was significantly associated with colorectal and rectal cancers among men. No significant associations were observed among women(Reference Wada, Oba and Tsuji45). Similarly, an Australian cohort study of men and women aged 27–75 years, which also used an FFQ, examined the effect of red meat, processed meat, chicken and fish consumption on risk of colorectal cancer(Reference English, MacInnis and Hodge44). The authors reported that intakes of processed meat were significantly associated with colorectal cancers (2nd and 4th Quartiles) and rectal cancers (2nd, 3rd and 4th Quartiles)(Reference English, MacInnis and Hodge44). High intake of fresh red meat (4th Quartile) was also significantly associated with colorectal cancer(Reference English, MacInnis and Hodge44). Despite both the Japanese and Australian studies using similar prospective cohort study designs, dietary assessment tools and analysis methodology, neither differentiated the carcinogenic effects of processed meat based on the source (i.e. processed from red meat v. non-red meat). We identified similar significant positive associations between intake of processed meat, particularly from red meat sources, and risk of GI cancers and CRC, even after adjustment for covariates (model 1 v. model 2). We also observed that intake of red meat was associated with risk of GI cancer, but only in men. This could be attributable to the fact that on average, men consume more red meat than women(46). However, this effect was attenuated after covariate adjustment.
The WCRF/AICR indicates that the type of meat consumed is important and may influence exposure to certain known carcinogens (including nitrites and nitrates), especially with respect to processed meat(1,Reference Cross and Sinha47,Reference Cross, Pollock and Bingham48) . In studies that have examined the effects of red meat and processed meat separately, associations between cancer risk and processed meat were often stronger than associations with red meat and were more consistent across various studies, particularly for CRC risk(Reference Larsson and Wolk34,Reference Norat, Lukanova and Ferrari43,Reference Chao, Thun and Connell49) . However, differences in the intake amounts of processed meat and red meat reported by participants may affect these outcomes. Similar to current findings, many studies have observed no association or only a weak association between red meat intake and cancer risk, despite finding significant associations between processed meat consumption and cancer risk(Reference Wie, Cho and Kang50–Reference Anderson, Darwis and Mackay53).
While other studies have separated out type of meat (processed meat or red meat), few have explored the effect of the source of processed meat (i.e. processed from red meat or processed from other sources) on cancer risk. A recent pooled analysis of six cohort studies in Japan explored differences in CRC risk across red meat and processed meat from red meat sources and chicken(Reference Islam, Akter and Kashino17). The authors did not identify significant associations with high intakes of red meat and risk of CRC; however, processed meat from red meat sources was associated with an increased risk of CRC and colon cancer in women but not in men. Many studies evaluating processed meat have provided limited definitions of the source of processed meat, making it difficult to ascertain whether the observed associations are a result of intake of only processed red meat or of processed meat from any source. In our study, there was an overall association with cancer risk for processed meat from red meat and from non-red meat combined, but this association was stronger for processed meat derived from red meats. This finding provides reasonable support for potential biological mechanisms, in which the combined carcinogenic effects attributable to red meats and also to production methods involved in processing meat have together resulted in more significant cancer associations(Reference Lauber and Gooderham7–Reference Turesky11). Processed meat from non-red meat sources was only borderline significantly associated with risk of GI cancers in men providing some evidence that processed meat production methods may have a carcinogenic effect that is independent of the source of meat; however, this was not a dose–response relationship, as only the 3rd Quartile was borderline significantly associated with GI cancer risk(1).
There is a lot of variability in the way studies performing high v. low intake risk analyses categorise intakes (i.e. by tertiles, quartiles or quintiles), and as a result, associations with cancer may vary depending on the range of processed and red meat intake within the sample and the size of the study sample(Reference Islam, Akter and Kashino17–Reference Keszei, Schouten and Goldbohm19). Other studies performing dose–response analyses have applied different increments of exposure which may also contribute to variability in cancer associations(Reference Crippa, Larsson and Discacciati16). Studies utilising larger intake cut-offs, such as adherence to the WCRF/AICR cut-off for red meat consumption (500 g/week)(5,Reference Hastert and White54) , may lack the sensitivity to identify associations, particularly with smaller numbers of events(Reference Biau, Kernéis and Porcher55). This was noted in the current analysis; using the current WCRF/AICR recommendations as cut-offs resulted in few statistically significant associations, compared with the dose–response approach. Additionally, methodological differences in the assessment of the red and processed meat intake assessment, covariate adjustment and limited statistical power to examine certain cancers may, at least in part, explain some of the inconsistencies in the significance of cancer associations observed in different studies. We observed that adjustment for a greater range of known risk factors for cancer (model 2) attenuated many significant associations which had been identified in the unadjusted model (model 1), particularly for all cancers combined and fifteen cancers combined. These differences in associations demonstrate the importance of adjustment for all known risk factors to ensure more meaningful interpretations. Moreover, differences observed between cancer outcome categories may be due to site-specific carcinogenic effects which are not evenly shared across all cancers included in these outcome categories. Studies which use different dietary assessment tools may capture data on red and processed meat intakes with different levels of sensitivity. For example, the classifications used to define red meat and processed meat food groups and dishes and definitions of portion sizes may influence the calculations of total dietary intake(2). Compared with an FFQ, 24-h dietary recalls have been found to provide more comprehensive data including details on eating occasions and foods consumed in combination; however, 24-h recalls are infrequently used as primary dietary assessment tools in large cohort studies(Reference Subar, Freedman and Tooze56). Technological advances have made 24-h recalls increasingly feasible in these settings, and future research on the carcinogenic effects of red and processed meat may benefit from these tools(Reference Shim, Oh and Kim57).
This study made use of an existing cohort with a large sample size and a long median follow-up time of 13·3 (IQR 5·1) years, compared with that of other studies which have reported shorter follow-up periods(Reference Norat, Bingham and Ferrari38,Reference Cross, Leitzmann and Gail39,Reference Wie, Cho and Kang50,Reference Oba, Shimizu and Nagata58–Reference Kim, Park and Nam68) . Short follow-up periods may result in issues with sub-clinical disease or insufficient numbers of incident cancer cases resulting in low or inadequate statistical power to identify the associations of interest. Additionally, this study utilised a large data set which included a wide range of lifestyle, environmental and dietary components and risk factors and adjusted for a wide range of baseline covariates and well-known risk factors for cancer. To assess dietary habits, we used an FFQ tool which has been validated in other large studies to assess meat intake and captures a comprehensive list of foods enabling the separation of type and source of meat(Reference Norat, Vierira and Chan69). However, processed meat production methods and sources of processed meat differ largely worldwide, and the FFQ tool used in this analysis may not have captured all meat intakes. A limitation of our study is the possibility of measurement error due to misreporting of dietary intake data, which could result in attenuated risk estimates for cancer(Reference Kaaks, Riboli and van Staveren70). To partially deal with the influence of misreporting, we adjusted for total energy intake in all of the statistical models(Reference Willett71), a method which has been utilised in other large cohort studies(Reference Norat, Bingham and Ferrari38). As with all observational studies, there is potential for residual confounding by unknown risk factors. For example, the WCRF/AICR Third Expert Report mentions that certain cooking methods confer carcinogenic risk(1). We were unable to adjust for this aspect due to insufficient data on cooking methods. We were likewise unable to adjust for menopausal status and hormone replacement therapy as these variables were characterised by a high degree of missingness in ATP data. We also did not adjust for race because the ATP cohort consists of >90 % Caucasian ethnicity; thus, any adjustment would have negligible impact on the cancer outcomes.
Despite these limitations, in this large informative cohort study, we considered both red meat and processed meat separately, captured a variety of associated and well-known cancers and cancer groups. This was made possible by linkage with Alberta Cancer Registry, which is Gold Certified by the North American Association of Central Cancer Registries(72). Linkage was facilitated using validated Personal Health Numbers to determine cancer incidence. We employed the totality of quantitative dietary data and lifestyle components obtained from validated questionnaires and adjusted for well-known cancer risk factors and confounders, which is a notable advantage compared with other existing studies which utilised only aspects or single components of diet and lifestyle factors.
Future directions
This analysis did not inquire about the variability in the co-current consumption of other foods, such as (specific) vegetables, fruit and fibre intake, which may modify the effects of processed and red meat consumption on cancer risk at various sites. It is recognised in the field that individuals who consume large amounts of red and processed meat also tend to consume less fish, poultry and vegetables(5). A previous study conducted using ATP data reported that low vegetables and fruit intake with high processed meat intake was associated with higher cancer incidence, compared with high vegetables and fruit intake with low processed meat intake(Reference Maximova, Moez and Dabravolskaj73). However, more large studies are required to understand the potential synergies of food co-occurrence which may result in a combination of influences on several pathways involved in carcinogenesis. Thus, future work of this nature could allow researchers to better capture the attributable cancer risk associated with specific dietary habits. Moreover, the prevalence of modifiable risk factors is also thought to be strongly socio-economically patterned. Future studies would do well to explore whether socio-economic disparities exist in the associations between dietary intake and cancer. Additionally, we found that existing data on the percentage of Canadians whose red meat consumption exceeds cancer prevention recommendation limits is scarce and more accurate and available estimates of these indicators are needed. Finally, more research is needed to evaluate whether all processed meats confer equitable risk and to determine what are the attributable risks by source and dose of processed meat.
Conclusion
In this study, we observed that cancer risk differs according to the source of processed meat consumed. Specifically, the carcinogenic effect associated with red and processed meat intake may be limited to processed meat derived from red meats. The finding that not all processed meats confer equitable risk and type of processed meat (i.e. processed from red meat v. processed from other) is meaningful aspects to consider when evaluating cancer risk is novel. These findings provide initial evidence towards developing and refining cancer prevention recommendations for red and processed meat intake.
Acknowledgements
Alberta’s Tomorrow Project is only possible because of the commitment of its research participants, its staff and its funders: Alberta Health, Alberta Cancer Foundation, Canadian Partnership Against Cancer and Health Canada, and substantial in kind funding from Alberta Health Services. Cancer registry data was obtained through linkage with Surveillance and Reporting, Cancer Research & Analytics, Cancer Care Alberta. The views expressed herein represent the views of the author(s) and not of Alberta’s Tomorrow Project or any of its funders. This work was partially supported by the Canadian Institutes of Health Research (Funding Reference Number: 151568). Although funding has been provided by several organisations, the analyses and interpretation of the data presented in this paper are those of the authors alone.
Formulating the research question: A. A. R., T. R. P., G. L. S., P. J. R., J. E. V. and K. M.; designing the study: A. A. R., G. L. S., T. R. H., P. J. R. and K. M.; analysing the data: G. L. S. and A. K. A.; writing and/or revising the manuscript: A. K. A., K. L. M., G. L. S., T. R. H., A. A. R., K. M., P. J. R. and J. E. V.
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the former Alberta Cancer Board’s Research Ethics Committee and the Health Research Ethics Board of Alberta Cancer Committee (ID: HREBA.CC-17-0099). Written informed consent was obtained from all participants.










