Thiamin (vitamin B1) is a water-soluble vitamin. It plays a substantial role in carbohydrate metabolism since it serves as a coenzyme in the pyruvate dehydrogenase complex for the transformation of pyruvate to acetyl-CoA. The prevalence of thiamin deficiency is regionally different(Reference Sechi and Serra1). In developed countries the prevalence of histopathologically confirmed thiamin deficiency in adults ranges from 0·8 to 2·8 %(Reference Sechi and Serra1). In emergency-affected populations and countries relying on polished rice as a dietary staple, thiamin deficiency often manifests as the classic thiamin deficiency disease beriberi. However, symptoms of thiamin deficiency vary greatly and there are further circumstances which increase the risk of thiamin deficiency; for example, alcohol misuse and malnutrition, gastrointestinal surgery, recurrent vomiting and chronic diarrhoea, cancer and chemotherapeutic treatments, systemic diseases such as AIDS, Mg depletion and unbalanced nutrition(Reference Sechi and Serra1). Numerous cases of thiamin deficiency have been reported in patients receiving parenteral nutrition which had not been supplemented with thiamin at all(Reference Oguz, Ergenekon and Tümer2–Reference Thauvin-Robinet, Faivre and Barbier6). Thiamin deficiency leads to a reduction of tissue glucose utilisation(Reference Hakim and Pappius7). Absolute deficiency leads to severe lactic acidosis which is fatal if untreated but usually resolves within hours after administration of thiamin(Reference Thauvin-Robinet, Faivre and Barbier6).
Serious thiamin deficiency can also be found in critically ill children(Reference Seear, Lockitch and Jacobson8). Thiamin deficiency might impair brainstem function and has been associated with sudden infant death syndrome(Reference Lonsdale9). It causes intra-uterine growth retardation (IUGR) in rats(Reference Butterworth10) and is also regarded as a risk factor for IUGR in humans(Reference Heinze and Weber11). Children with a history of infantile thiamin deficiency have an increased risk for epilepsy(Reference Fattal-Valevski, Bloch-Mimouni and Kivity12) and delayed language development(Reference Fattal-Valevski, Azouri-Fattal and Greenstein13).
Premature infants are at risk for thiamin deficiency because they require a relatively high intake of carbohydrates for the maintenance of an adequate growth rate. This results in an increased need for thiamin(Reference Sauberlich14). Although premature infants represent a risk group, their thiamin status has been investigated only marginally. A well-established way to assess thiamin status is the determination of its metabolic active form thiamin diphosphate (TDP) in whole blood(Reference Talwar, Davidson and Cooney15). The present study intends to investigate TDP concentrations in a group of premature infants, to establish reference values, and to detect risk factors for low TDP concentrations.
Subjects and methods
Study design and subjects
In a prospective, explorative study whole blood TDP concentrations were determined at birth or in the first days of life and approximately every 2 weeks until discharge. The days of assessment were not standardised to avoid distress caused by additional blood collections. Thus, TDP was determined only when blood had to be collected because of clinical indications. The study was conducted at the Department of Neonatology of the Children's Hospital of the University of Cologne, Germany. The only inclusion criterion was prematurity and the only exclusion criterion was a missing parental informed consent.
Date of birth, weeks of gestation, birth weight, reasons for premature delivery, history of IUGR, and age at the time of assessment were recorded. The Nursery Neurobiologic Risk Score (NBRS) was assessed for each patient(Reference Brazy, Eckerman and Oehler16). The Clinical Risk Index for Babies (CRIB) was applied for patients weighing < 1500 g at birth and the Pediatric Risk of Mortality III score (PRISM III) was applied for all others(17, Reference Pollack, Patel and Ruttimann18). To monitor the infants’ health status at the time of assessment we instructed the responsible neonatologists to specify whether the infant had an infection and whether it was severely ill based on their own judgement. Additionally, C-reactive protein (CRP) was recorded when available. Blood Hb concentration was recorded to take into account that TDP mainly occurs in erythrocytes. We also determined blood glucose, pH and lactate as parameters of carbohydrate metabolism.
Current weight, weight gain (g/d per kg), and the type and amount of nutrition (enteral and parenteral) were recorded. Weight gain was calculated using the following equation:
where W c is current weight and W n is weight before n d.
A time span of 5 d was applied but if the child was younger than 5 d the difference from current weight to birth weight was used. From the nutritional data daily intakes of thiamin, energy and carbohydrates were estimated. Calculations were based on the declared nutritional specifications. Thiamin and carbohydrate contents of breast milk were estimated at 100 ng/ml(19, Reference Ford, Zechalko and Murphy20) and 70 g/l(Reference Cockburn21), respectively. As a breast milk fortifier FM85 (Nestlé) was used. As formula nutrition Beba FG and Beba HA Start (Nestlé) and Aptamil Pre and Aptamil Primergen (Milupa) were used. Parenteral nutrition was prepared individually and water-soluble vitamins were supplemented with Soluvit N (Baxter).
In summary, the following variables were introduced in the statistical analyses as covariates: whole blood TDP, sex, weeks of gestation, postnatal age, birth weight, current weight, weight gain, NBRS, CRIB, PRISM III, presence of infection, presence of severe illness, history of IUGR, CRP, blood Hb, blood glucose, blood pH and blood lactate, type of nutrition, intake of thiamin, energy and carbohydrates.
Sample collection and determination of thiamin diphosphate
EDTA whole blood samples of approximately 120 μl were collected during clinically indicated blood samplings. Specimens were labelled with unique numbers only to avoid bias in further laboratory analyses. The blood was frozen at − 20°C directly after collection and transferred to the laboratory using dry ice. It was frozen at − 80°C until determination of TDP using a validated HPLC method which was developed previously(Reference Körner, Vierzig and Roth22).
Data collection
Data were collected by contemporary data entry into spreadsheets created with Microsoft Excel 2003 (version 11, SP 2; Microsoft Corp.). Functions implemented into the spreadsheet automatically calculated intake of thiamin, energy and carbohydrates by the kind and amount of nutrition. Weight gain and age at the time of assessment were also calculated automatically.
Statistics
SPSS (version 11.0 for OS X; SPSS Inc.) was used for statistical analyses and GraphPad Prism (version 5; GraphPad Software) was used for graphics and curve fitting. Since the distribution of TDP concentrations did not follow a normal distribution the Mann–Whitney U test was used to compare TDP concentrations between groups. When more than two groups were involved in the analysis, Kruskal–Wallis one-way ANOVA was used. In the case of a significant difference, post hoc analysis by making pairwise comparisons using the Mann–Whitney U test was carried out. The relationships between TDP concentrations and other variables were assessed by simple linear regression analyses. Missing data were excluded from analyses. Statistical significance was assigned for P< 0·05. In subgroup analysis with more than two groups a Bonferroni adjustment of significance was applied.
Ethics
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the local ethics committee of the University Hospital of Cologne and written informed consent was obtained from both parents.
Results
A total of 111 premature infants were included from May 2009 until December 2010. None of the patients was withdrawn from the study. Mean gestational age was 30·6 (sd 3·4) weeks and mean birth weight was 1478 (sd 580) g. Reasons for preterm delivery consisted mainly of premature rupture of membranes, pre-eclampsia, eclampsia, HELLP syndrome (haemolysis, elevated liver enzymes, low platelet count), cervical insufficiency, placental abruption, placental insufficiency, amniotic infection syndrome, or a combination of different factors. A history of IUGR was present in seven infants.
There were 241 blood collections. From all collected blood samples 222 were analysed for TDP. The remaining nineteen samples could not be analysed due to insufficient sample volume. Of the measurements, two were below the lower limit of quantification (4 ng/ml) and two measurements were above the previously tested range of linearity (4–400 ng/ml). These samples were not excluded from statistical analyses because extended linearity of the HPLC method of up to 800 ng/ml was demonstrated and the two low values were near the lower limit of quantification and clearly above the lower limit of detection (0·2 ng/ml). A mean of two samples per patient was determined (range 1–7). Median observation span was 19 d from the day of birth (lower quartile 3·5, upper quartile 39·5, range 0–359 d). The broad range was due to one infant whose treatment was prolonged due to severe illness.
The study population was generally healthy. Only six (3 %) assessments were made during an infection and ten (5 %) assessments were made during severe illness. Considering all valid TDP assessments CRP was available in 181 cases. It was below the detection limit (3 mg/l) in 162 cases (90 %). Mean CRP of the elevated cases (n 19) was 9·9 (sd 8·2; range 3–33·7) mg/l. Median CRIB score was 2 (n 40; range: 0–9) and median PRISM III score was 6 (n 68; range 0–14). No score could be calculated for three patients because of missing laboratory data. The median NBRS was 1 (n 108; range 0–10). It was also not available for three patients because of missing data.
Table 1 displays the characteristics of the sample population and gives an overview of the essential results. Infants were grouped according to sex, gestational age and birth weight. The range of all measured TDP concentrations was remarkably wide and the distribution was skewed towards lower concentrations. Mean TDP concentration was higher in female patients but the difference was not statistically significant.
Table 1 Whole blood thiamin diphosphate (TDP) concentrations in relation to characteristics of the sample population

* No significant difference.
† Significant pairwise comparisons: very early preterm and early preterm; very early preterm and late preterm (P< 0·017).
‡ Significant pairwise comparisons: extremely low and low; extremely low and normal; very low and low; very low and normal (P< 0·008).
Most of the included children were late preterm infants (32 < 37 weeks of gestation). TDP concentrations were generally lower in early (28 < 32 weeks of gestation) and very early preterm infants ( < 28 weeks of gestation). Considering only the samples taken immediately after birth there was no correlation between TDP and gestational age (R 2 0·002; P= 0·788; n 32). Low birth weight was also associated with low TDP concentrations. In contrast to this observation, TDP did not correlate with current weight at the time of assessment (R 2 < 0·001; P= 0·8).
Thiamin diphosphate concentrations and age
Figure 1 shows TDP concentrations related to postnatal age. In the first days of life very high TDP concentrations were observed with a wide range of values. With increasing age the range narrowed towards lower concentrations. This trend was fitted to a model of exponential one-phase decay ending in a plateau:

Fig. 1 Whole blood thiamin diphosphate (TDP) concentrations show an age-dependent decline. Data were fitted to an exponential decay ending in a plateau. The middle curve represents the best-fit curve; the outer curves represent 95 % CI. Of the data points, three are outside the axis limits.
In this model Y 0 is the Y intercept at X= 0, Plateau is the Y value at infinite times, and K is the rate constant. Best-fit analysis yielded Y 0 = 120·8 ng/ml, Plateau= 20·7 ng/ml and K= 0·0419/d (R 2 = 0·171). The decrease in TDP concentrations is also displayed in Table 2, which shows TDP concentrations according to different age groups. Mean TDP concentrations decreased slightly after the the first 10 d of life. After the age of 20 d, there was a considerable drop and thereafter values stabilised. TDP values in the age group 21–103 d were significantly different from those of the age groups 0–10 and 11–20 d.
Table 2 Whole blood thiamin diphosphate (TDP) concentrations in relation to postnatal age
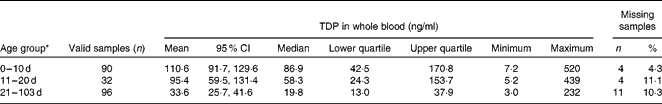
* Significant pairwise comparisons: 0–10 d and 21–103 d; 11–20 d and 21–103 d (P< 0·017).
Thiamin intake and nutrition
In Table 3, samples were grouped according to thiamin intake and to the type of nutrition. Thiamin intake was divided into three categories: low (0–20 μg/kg per d); intermediate (60–120 μg/kg per d); and high (250–270 μg/kg per d). Category boundaries were chosen with regard to the distribution of overall observed thiamin intake which peaked within these limits. This grouping included 85 % of all valid samples. The mean postnatal age of each group is also given and reveals that the low and high intake groups correspond to younger infants whereas the intermediate intake group corresponds to older infants. Thiamin intake was dependent on the kind of nutrition: in the low intake group the main thiamin source was breast milk in 85 %, in the intermediate group it was breast milk fortified with FM85 in 84 %, and in the high group it was parenteral nutrition in 97 %. These findings are consistent with the mean postnatal age of each group since pure breast milk or parenteral nutrition was usually used at the beginning and fortified breast milk was usually used for older infants. Moreover, the previously described age-dependent decline in TDP conforms to the fact that low thiamin intake did not lead to low TDP concentrations and that the lowest thiamin concentrations were observed in the group of intermediate intake. In the group of high thiamin intake TDP concentrations were highest. Considering all measurements, there was no correlation between TDP and current thiamin intake (R 2 0·008; P= 0·198).
Table 3 Whole blood thiamin diphosphate (TDP) concentrations in relation to thiamin intake and nutrition

* Significant pairwise comparisons: 0–20 and 60–120; 60–120 and 250–270 (P< 0·017).
† Significant pairwise comparisons: parenteral nutrition and breast milk + FM85 (P< 0·005).
‡ Nestlé.
Another grouping was established regarding the type of nutrition. As in the previous grouping the mean postnatal age is given for each group. It becomes evident that pure breast milk and parenteral nutrition were used in younger infants, fortified breast milk and formula in older infants, and glucose or no nutrition only on the first day of life. Older infants who were fed fortified breast milk showed lower mean TDP concentrations than younger infants who were fed pure breast milk. Infants fed formula nutrition had higher mean TDP concentrations than children fed fortified milk. However, their mean postnatal age was lower. Infants who received parenteral nutrition had the highest TDP concentrations. Finally, there was no statistically significant difference between groups except for the parenteral nutrition and fortified milk group.
Correlation of thiamin diphosphate with other variables
TDP moderately correlated with Hb (log TDP = 2·215 × log Hb – 0·931; R 2 0·313; P< 0·05). Very weak correlations were found for intake of energy (R 2 0·127; P< 0·05) and carbohydrates (R 2 0·119; P< 0·05). There was no correlation with weight gain (R 2 0·027; P= 0·019), blood pH (R 2 0·003; P= 0·491), blood glucose (R 2 0·003; P= 0·445) and blood lactate (R 2 0·003; P= 0·533). During the study no lactic acidosis due to thiamin deficiency occurred. There were no correlations with NBRS (R 2 0·004; P= 0·384), PRISM III score (R 2 < 0·001; P= 0·857) or CRIB score (R 2 0·011; P= 0·265). No regression analysis of CRP was performed due to insufficient measurements of elevated CRP (n 19). Median TDP concentration of measurements associated with elevated CRP values was 53·4 (range 5·2–328·0) ng/ml and not significantly different from median TDP concentration of all other assessments (42·2 ng/ml). Infants with a history of IUGR had a median TDP concentration of 48·2 ng/ml (n 16) which did not differ significantly from median TDP concentration of all other assessments (37·8 ng/ml).
Extreme values
In the present study, six samples were below percentile 3 (5·3 ng/ml), and twenty-two samples were below percentile 10 (9·2 ng/ml). However, none of these patients showed symptoms of thiamin deficiency. Of the patients, 50 % with TDP concentrations lower than the third percentile and 32 % with TDP concentrations lower than the tenth percentile were anaemic at the time of assessment. Only one of the patients with a TDP concentration lower than the tenth percentile had a normal Hb level and a significantly increased lactate concentration (3·6 mmol/l) on the 18th day of life. The patient was a female infant with a gestational age of 32 weeks. Since she was without a medical problem, clinically unremarkable, and lactic acidosis was not severe, thiamin deficiency was not suspected.
Of the samples, six were above percentile 97 (280·6 ng/ml) and twenty-two were above percentile 90 (194·0 ng/ml). Of these twenty-two samples, 18 % were related to polyglobulia. None of these samples was accompanied by anaemia. No further abnormalities could be detected in these patients.
Discussion
In the present study thiamin status was evaluated in a larger group of premature infants by measuring whole blood TDP. To our knowledge, no studies on TDP concentrations in premature infants have been conducted so far. In one study thiamin status was assessed by measuring total thiamin in the blood of premature infants(Reference Navarro, Causse and Desquilbet23). However, since total thiamin includes free thiamin and thus is strongly influenced by recent thiamin intake it is not regarded as an appropriate measure of thiamin status. Other previous studies of premature infants were based upon fewer subjects and relied mainly on functional thiamin assays measuring the thiamin pyrophosphate effect(Reference Moore, Greene and Phillips24–Reference Friel, Bessie and Belkhode27). This test allows us to indirectly conclude on the saturation of erythrocyte transketolase with TDP. In the conducted studies no thiamin deficiencies were diagnosed in premature infants and, apart from a few exceptions, the thiamin pyrophosphate effect was within reference range in all infants at all times(Reference Moore, Greene and Phillips24–Reference Friel, Bessie and Belkhode27). However, a disadvantage of the thiamin pyrophosphate effect is that it can be influenced by factors other than thiamin deficiency and can only indicate deficient states but not high TDP concentrations. Moreover, it was shown that whole blood TDP is more specific and sensitive than the thiamin pyrophosphate effect(Reference Talwar, Davidson and Cooney15).
TDP concentrations in premature infants are different from adult concentrations since TDP concentrations do not depend on age in adults(Reference Körner, Vierzig and Roth22). In the present study we observed a wide range of TDP concentrations in the first days of life and a decrease with postnatal age. A similar decline was found in a previous study in thiamin concentrations of term infants(Reference Wyatt, Nelson and Hillman28). In that study, concentrations of phosphorylated thiamin in whole blood were calculated by measuring total and non-phosphorylated thiamin. Since TDP makes up approximately 93 % of phosphorylated thiamin in whole blood(Reference Tallaksen, Bohmer and Bell29) the results can be compared. In term infants the decline of phosphorylated thiamin occurred over a longer period of time. Phosphorylated thiamin concentrations decreased considerably after 0–3 months of age and further between 3–12 months of age. A plateau was reached in children older than 12 months. It was suggested that high thiamin concentrations in infants probably reflect higher metabolic needs of the rapidly growing brain and that the decline might be due to metabolic and neurological maturation(Reference Wyatt, Nelson and Hillman28).
We found that TDP concentrations were generally lower in early and very early preterm infants (Table 1). During pregnancy there might be a physiological increase of TDP concentrations to protect the newborn from thiamin deficiency. An important timepoint of measuring whole blood TDP seems to be immediately after birth since this determination is not influenced by nutritional thiamin intake of the newborn. However, in the present study there was no influence of gestational age on TDP concentrations measured immediately after birth. Due to the limited number of available samples further research is needed.
Cellular thiamin uptake is mediated by transporter proteins of the SLC19 gene family of solute carriers and by diffusion(Reference Ganapathy, Smith and Prasad30). In the cytosol TDP is synthesised from thiamin and ATP by thiamin diphosphokinase (EC 2.7.6.2)(Reference Bettendorff and Wins31). Enzymes which degrade TDP play an important role in maintaining the TDP concentration but have barely been characterised. In summary, thiamin metabolism is not well understood and the pathways which regulate TDP concentrations in humans are unknown. It is of interest to identify factors which influence TDP concentrations. In the present study, Hb concentration moderately correlated with TDP concentration. This can be explained by the fact that erythrocytes contain almost all the TDP found in whole blood. The relationship was observed before and attempts were made to correct whole blood TDP concentrations for erythrocyte parameters(Reference Talwar, Davidson and Cooney15, Reference Wyatt, Nelson and Hillman28). However, we did not correct TDP concentrations but we suggest that TDP concentrations might be falsely low in anaemia.
Recommended thiamin supply in infants fed by parenteral nutrition is 350–500 μg/kg per d(Reference Koletzko, Goulet and Hunt32). This range was derived from a study which demonstrated the absence of thiamin deficiency in premature infants receiving 510 (sd 280) μg/kg per d(Reference Friel, Bessie and Belkhode27). However, recommendations vary and the optimal dosage is unknown. In the present study, infants received relatively low doses of thiamin and also showed no signs of deficiency. Interestingly, TDP concentrations did not correlate with daily thiamin intake.
TDP concentrations varied in relation to thiamin intake and to the kind of nutrition (Table 3). However, thiamin intake and kind of nutrition depended on postnatal age and differences should be interpreted by taking into account the age-dependent decline of TDP as described above. For example, children who were given fortified milk were generally older than infants who were fed pure breast milk.
Breastfed infants of mothers who have inadequate intake of thiamin can develop infantile thiamin deficiency(Reference McGready, Simpson and Cho33, Reference Ortega, Martínez and Andrés34). However, in the study cohort TDP concentrations were not remarkably lower in breast-fed infants than in infants receiving other forms of nutrition. This might be due to the fact that the studied infants were of well-nourished mothers who were not at risk for insufficient thiamin intake. Another factor might be that pure breast milk was fed during the first days of life when TDP concentrations were generally higher.
A drawback of the present study is that thiamin intake was estimated and not determined. It is known that thiamin degrades over time when given as a parenteral infusion, although not substantially(Reference Ribeiro, Pinto and Lima35). A further problem poses the estimation of thiamin in breast milk. The normal range of breast milk thiamin as proposed by the WHO is 100–200 ng/ml(19) and is based on mean concentrations of various populations. However, there are interindividual deviations and thiamin content increases with duration of lactation(Reference Ford, Zechalko and Murphy20). Breast milk of mothers of premature infants was reported to contain 24 ng/ml in colostrum, 54 ng/ml in transitional milk and 89 ng/ml in mature breast milk(Reference Ford, Zechalko and Murphy20). The estimated value of 100 ng/ml used in the present study was a compromise.
Neither CRIB, nor PRISM III, nor NBRS were able to predict low TDP concentrations. In a recent study on critically ill children it was shown that high CRP concentrations (>200 mg/l) were independently associated with low whole blood thiamin concentrations(Reference Lima, Leite and Taddei36). Since the infants included in the present study were generally healthy and CRP elevations were rare and rather mild a similar association was not apparent. It is unknown which lower limit of whole blood TDP defines the border to thiamin deficiency in premature infants. Moreover, it is uncertain for how long TDP concentration needs to be below that limit until a thiamin deficiency becomes clinically manifest. In a report on eleven neonates who developed thiamin deficiency due to unsupplemented total parenteral nutrition the onset of symptoms was 11–29 d after initiation of thiamin-deficient nutrition(Reference Thauvin-Robinet, Faivre and Barbier6). Symptoms of thiamin deficiency depend on age(Reference Lonsdale9). In infants they include unspecific symptoms such as lethargy, irritability, vomiting, anorexia, abdominal distension, diarrhoea, failure to thrive, developmental delay, respiratory symptoms, infection, and more specific symptoms like cardiomyopathy, ophthalmoplegia, bilateral abduction deficit, upbeat nystagmus and seizures(Reference Thauvin-Robinet, Faivre and Barbier6, Reference Fattal-Valevski, Kesler and Sela37). Since there was no clinical suspicion of thiamin deficiency in any of our patients we cannot report a threshold value which allows the exclusion of thiamin deficiency.
Conclusions
None of the studied infants showed symptoms of thiamin deficiency. TDP concentrations showed an age-dependent decline, and gestational age and birth weight were associated with low TDP concentrations. There was a moderate correlation between TDP and Hb. Thus, low TDP concentrations which go along with anaemia should be interpreted with caution. TDP concentrations were not dependent on thiamin intake and infants receiving pure breast milk were not at risk for low TDP concentrations.
A cut-off TDP concentration to diagnose thiamin deficiency could not be determined. Further research is needed to evaluate the effect of low and high TDP concentrations on premature infants regarding development, clinical course and outcome, and to identify factors which influence these concentrations.
Acknowledgements
We thank Sarah Börner, Boris De Carolis, Miriam Jackels, Shino Junghänel, Inga Gartz, Titus Keller, Angela Kribs, Kyriakos Martakis, Andrea Mikolajczak, Guido Weißhaar, the nurses who were responsible for the care of the infants and parents for their help in carrying out the study, and Oliver Fricke for support in statistics. R. W. K. received a grant from the Maria Pesch Foundation, Cologne, Germany. All authors designed the research, conducted the research and edited the paper. R. W. K. analysed the data and wrote the first draft of the manuscript. Each author read and approved the final version of the submitted manuscript and takes full responsibility for the paper. The authors declare no conflict of interest.








