Today, it is clearly acknowledged that environmental factors and especially dietary factors may largely contribute to the development of obesity(Reference Comuzzie and Allison1). To study the implication of nutrients at the onset of obesity, most nutritional experiments are done using unbalanced hyperenergetic diets. One of the most commonly studied diets is the cafeteria-type diet, consisting of palatable high-fat, high-energetic food, that clearly mimics the diet of humans in the Western world, which has been responsible for the increase in obesity during the last decade(Reference Astrup, Buemann, Western, Toubro, Raben and Christensen2–Reference Lichtenstein, Kennedy, Barrier, Danford, Ernst, Grundy, Leveille, Van Horn, Williams and Booth4). Many studies performed on rats with this type of experimental diet show a rapid increase in fat storage, as a result of a clear imbalance between energy input and energy expenditure(Reference Llado, Estrany, Rodriguez, Amengual, Roca and Palou5).
Under these conditions, the increase in fat mass observed is the result of two morphological changes in the white adipose deposits. First, cell sizes increase rapidly due to excessive lipogenesis activity that leads to increased TAG storage in the lipid droplet. The number of cells per tissue also increases by a recruitment of precursors that proliferate and differentiate to form ‘de novo’ mature adipocytes(Reference Faust, Johnson, Stern and Hirsch6–Reference Mandrup and Lane8). The relative contribution of each phase to the development of adipose tissue depends on the nature of the diet, the time of treatment and the localisation of the adipose deposit(Reference Sclafani and Gorman9). This differentiation capacity of adipocyte precursors to form mature adipocytes, called adipogenesis, is a result of a complex process and is characterised by changes in cell morphology, hormone sensitivity and gene expression(Reference Gregoire, Smas and Sul10). Many studies confirm that an excess of fatty acids, brought about by a high-fat diet, directly influences gene expression of the adipose tissue program through up-regulation of a key transcription factor: PPARγ(Reference Lowell11). However, fatty acids are not the only nutrient involved in the control of adipogenesis. Many other hormonal and nutritional factors could interact with this complex signalling pathway, and especially retinoic acid (RA), the active form of vitamin A(Reference Bonet, Ribot, Felipe and Palou12).
It is classically recognised that after the liver, adipose tissue is a major site of vitamin A storage and metabolism(Reference Tsutsumi, Okuno, Tannous, Piantedosi, Allan, Goodman and Blaner13, Reference Villarroya, Iglesias and Giralt14). At the nuclear level, the different isoforms of RA, all-trans-RA and 9-cis-RA are able to bind the retinoic acid receptor (RAR), but only the 9-cis-RA binds equally well to RAR and retinoid X receptor (RXR)(Reference Love, Gooch, Benko, Li, Nagy, Chatterjee, Evans and Schwabe15). These complexes are able to bind to specific sequences in the promoter region of their target genes. This regulation of transcriptional activity by retinoids plays a crucial role in the signalling pathway of many other factors and particularly in the fatty acid signalling pathway. Because PPAR and RAR need to form functional heterodimers with the same common partner, RXR, to bind to hormone response elements(Reference Chambon16, Reference Aranda and Pascual17), it appears that RA, via RXR, could interfere with the molecular action of fatty acids at the nuclear level in adipose tissue(Reference Ribot, Felipe, Bonet and Palou18–Reference Miyata, McCaw, Marcus, Rachubinski and Capone20).
Many studies, performed in vitro, have shown different actions of RA in the control of adipose tissue proliferation and differentiation(Reference Chawla and Lazar21, Reference Suryawan and Hu22). Classically, RA is considered as a powerful inhibitor of adipogenesis, when added at high doses during the early stage of adipocyte differentiation(Reference Stone and Bernlohr23, Reference Xue, Schwarz, Chawla and Lazar24). However, when lower doses are added before confluence, RA has been seen to activate adipocyte differentiation(Reference Safonova, Darimont, Amri, Grimaldi, Ailhaud, Reichert and Shroot25). These differences could be explained by different impacts of RA in the regulation of this cascade of transcriptions factors involved in adipogenesis(Reference Villarroya, Iglesias and Giralt14, Reference Schwarz, Reginato, Shao, Krakow and Lazar26, Reference Hida, Kawada, Kayahashi, Ishihara and Fushiki27).
Furthermore, few studies have been performed in vivo to observe retinoid effects on the control of adipose tissue development in an ‘adipogenic’ situation, such as cafeteria diet exposure. Based on a previous study, where male adult rats were exposed for 8 weeks to two cafeteria-type diets containing different levels of vitamin A, it was observed that, despite a high increase in fat mass induced by the two cafeteria regimens, no marked difference was seen in the mRNA nuclear profile measured in two different deposits between the two groups of cafeteria diets(Reference Bairras, Menard, Redonnet, Ferrand, Delage, Noel-Suberville, Atgie and Higueret28). Therefore, in the present study, we decided to expose younger rats to the same diets but for a shorter period of 1 week. Under these conditions, mRNA from subcutaneous white adipose tissue (Swat) and subcutaneous mature isolated adipocytes were extracted to measure the expression of nuclear receptor subtypes (PPARγ, RARα and γ, RXRα) and for a specific marker of adipocyte differentiation (aP2). In the same experiment, the consequences of exposure to these different diets were tested on the capacities of adipocyte precursors from Swat to proliferate and differentiate during 1 week of primary culture.
Experimental methods
Chemicals and reagents
All products added to the culture medium and Dulbecco's modified Eagle's medium (DMEM-F12) were purchased from Sigma-Aldrich (Lyon, France), except for insulin, which was obtained from Novo Nordisk (Paris, France). The TRIzol reagent was obtained from Invitrogen (Cergy Pontoise, France). Serum TAG and cholesterol were measured using a bioMérieux assay kit (Marcy l'Etoile, France). Serum glucose and lactate were also measured using Sigma glucose and lactate kits. TAG accumulation in preadipocytes in culture was measured using a Boehringer analysis kit (Mannheim, Germany).
Animals and methods
All studies were performed on male Wistar rats weighing 50–70 g (age 3 weeks), obtained from Janvier (Le Genest St Isle, France). The use of animals was in accordance with the Institute for Laboratory Animal Research (ILAR) Guide for Care and Use of Laboratory Animals. The rats were housed two per cage with a 12 h light–12 h dark cycle at 50 % humidity and 24 ± 1°C. The rats were fed for a minimum 5 d of adaptation with a standard pellet diet (UAR, Paris, France). The animals were then housed individually to receive for 1 week the same standard pellet diet defined as the control diet or the two cafeteria diets with different vitamin A levels (Caf+ and Caf). These diets were prepared from a variety of highly palatable human food (bacon, chocolate, potato chips, biscuits and a pellet diet) containing liver pâté (for Caf+) and ham pâté (for Caf). The Caf+ and Caf diets contained respectively 10·4 and 12 % proteins, 35·2 and 37·8 % carbohydrates and 54·4 and 50·1 % lipids. The main source of fat was bacon (20 g per 100 g food). The calculated ratios of the n-6 PUFA v. n-3 PUFA were no different in the two diets. The cafeteria diet with the upper level of vitamin A (Caf+) contained 820 μg retinol per g diet while the second cafeteria diet (Caf) contained 225 μg retinol per g diet, the same as the control diet (UAR, Paris, France).
Measurement of tissue samples and serum parameters
At the end of the diet period (8 d), the rats were killed by decapitation. Blood was collected and centrifuged for 5 min at 5000 g at 4°C. Serum were rapidly collected, and stored at − 80°C for measurement of biochemical parameters. The Swat, retroperitoneal, epididymal and visceral white adipose tissues (Vwat) were removed and weighed. Swat was washed in a cold saline solution (NaCl, 0·9 %; diethyl pyrocarbonate, 1 %). Portions of Swat (200 mg) were placed in TRIzol reagent (Invitrogen), and stored at − 80°C before nucleic acid extraction. Serum TAG and cholesterol were measured using a bioMérieux assay kit. Serum glucose and lactate were also measured using Sigma glucose and lactate kits. The serum retinol measurements were performed by an HPLC chromatographic method according to the NF EN 12823-1 standard.
Isolation of subcutaneous adipocytes and culture conditions
Swat were removed immediately after the animals were killed, washed several times with a DMEM-F12 medium and the majority of connective tissues and blood clots were removed. Mature adipocytes and the stroma vascular cells were then isolated according to the method previously described by Cousin et al. (Reference Cousin, Munoz, Andre, Fontanilles, Dani, Cousin, Laharrague, Casteilla and Penicaud29) with some modifications. Briefly, Swat was minced into small fragments, which were digested with collagenase (1 g/l) in a DMEM-F12 medium supplemented with serum bovine albumin (3·5 g/l), in a shaking water-bath at 37°C for 45 min in a polypropylene flask. Cells were then filtered through nylon filters (100 and 30 μm mesh). The suspension was then centrifuged at 1600 g for 10 min to separate the pelleted preadipocytes from the floating adipocyte fraction. The adipocyte fraction was counted using an automatic cell counter (Easycam Coulter; Beckman Coulter, Roissy, France), put into TRizol reagent and stored at − 80°C before nucleic acids extractions. The pellets of preadipocytes were then washed three times with DMEM-F12 culture medium and erythrocytes were discarded by contact for 3 min with a hypotonic haemolytic solution. Preadipocytes were suspended in DMEM-F12 medium, supplemented with 10 % calf bovine serum, with antibiotic, and a solution of biotin (17 μm), ascorbic acid (100 μm), pantothenic acid (17 μm) and ampothericin (5 μm). Stroma vascular cells were counted with the Easycam Coulter and then cultured (500 000 cells per well) in polypropylene culture plates stored for 8 d in a humidified incubator (at 37°C, under 5 % CO2 and 95 % O2). The following day (day 1), the culture medium was removed from each well of the plates, adherent cells rinsed with 2 ml sterile phosphate buffer and the preadipocytes cultured in fresh DMEM-F12 containing 10 % fetal calf serum and insulin (100 nm). Media were changed every 48 h.
Measurement of preadipocyte proliferation activity
Between day 1 and day 8, proliferation activity of preadipocytes in culture was measured by counting the number of cells in three different wells after a trypsinisation procedure. Briefly, at day 1, day 3, day 5 and day 8, the media were removed from three wells of each culture plate, and the adherent cells were rinsed with 2 ml sterile phosphate buffer. Next, 0·5 ml of a trypsin solution (1 %) was added for 3 min at 37°C. The trypsin action was then stopped by adding 0·5 ml of DMEM-F12 with 10 % fetal calf serum. After 10 min of centrifugation at 1600 g, the pellet was washed twice with phosphate buffer and the preadipocytes were counted.
Measurement of the differentiation level
On day 8 of the culture experiment, the differentiation levels of the preadipocytes were determined by measuring accumulated TAG in each well of the culture plates. Briefly, the culture media were removed; the cells rinsed twice with phosphate buffer and then scraped. After homogenisation in 1 ml of phosphate buffer, TAG were measured using a Boehringer analysis kit.
Quantification of messenger ribonucleic acid expression
Total ribonucleic acid preparation
Portions of the adipose tissue (200 mg) or adipocyte cells were homogenised in 1 ml TRIzol reagent (Invitrogen, Cergy Pontoise, France) and total RNA was extracted according to the manufacturer's recommended instructions. The quality of total RNA was assessed using an off-the-shelf kit (RNA LabChip kit; Agilent Technologies, Meyrin, Switzerland) and an Agilent 2100 bioanalyser. Quantification was achieved by measuring light absorbency at 260 nm. Average yield of total RNA extraction was not significantly different in tissues from Caf+, Caf and the control groups.
Reverse transcription
cDNA was synthesised with ImpromII RT (Promega, Charbonnières-les-Bains, France) according to the manufacturer's recommended protocol, with modifications. Briefly, an amount of 1 μg of total RNA was incubated with RNAsin Ribonuclease inhibitors (Promega) and with DNase-I (Roche Diagnostics, Meylan, France) 15 min at 37°C in order to degrade DNA and inhibit RNase. Then specific reverse primers (120 ng) were added and incubated at 70°C for 10 min. The RT reaction was performed at 42°C for 60 min. Each target mRNA was co-reverse transcribed with β2-microglobulin as housekeeping gene, relating to the level of expression of the quantified gene. Parallel reactions for each RNA samples were run in the absence of reverse-transcriptase to assess the degree of any contaminating genomic DNA.
Analysis of gene expression using real-time polymerase chain reaction
Analysis of gene expression using a real-time PCR (PCR assay employing a Light Cycler™ technology) was carried out as described by Redonnet et al. (Reference Redonnet, Bonilla, Noel-Suberville, Pallet, Dabadie, Gin and Higueret30) with minor modifications specific to the gene studied. The forward and reverse primer sequences of β2-microglobulin, RARα, RARγ, RXRα and PPARγ were described by Bairras et al. (Reference Bairras, Menard, Redonnet, Ferrand, Delage, Noel-Suberville, Atgie and Higueret28) and amplification of aP2 was performed with the following primers: forward 5′CCGAGATTTCCTTCAAACTGGG3′ and reverse 5′CCACCACCAGCTTGTCACCATC3′. Specificity of primers was validated through the verification of RT-PCR product specificity. RT-PCR products were subjected to analysis by electrophoresis on a 1·5 % agarose gel and resulted in a single product with the desired length. The identity of amplified products was verified by sequencing with the Dye Terminator Reaction Cycle Kit (Perkin-Elmer, Norwalk, CT, USA) and analysed on an ABI PRISM™ 377 automated DNA sequencer (Perkin-Elmer).
β2-Microglobulin cDNA was used as housekeeping for the relative quantification of cDNA of RARα, RARγ, RXRα, PPARγ and aP2. The results were normalised by the ratio of the relative concentration of target to those of β2-microglobulin sample. The real-time PCR method ensured that the expression level of the housekeeping gene was unaffected by the different diets.
Statistical analysis
All the results are presented as mean values with their standard errors. ANOVA and Fisher's positive least-significant difference post hoc test were performed using Statgraphic plus 5.1 software (StatPoint, Inc., Herndon, VA, USA) to assess the statistical significance. The correlation coefficient was determined by a linear model of regression analysis performed with the same statistical software. Statistical significance level was set at P < 0·05.
Results
Effects of 8 d of two cafeteria diets on body weight and energy intake
As shown in Table 1, all the animals in the three groups increased their body weight between the start (day 1) and end of treatment (day 8), but these increases were less significant in the two cafeteria groups (Caf and Caf+) as compared with the control group. The total body-weight gains for the Caf+ (47 (se 1·9) g) and Caf (37 (se 4·7) g) groups were significantly (P < 0·05) lower than those of the control group (72 (se 4·4) g). The daily energy intakes at the start of the diet period (day 1) were significantly (P < 0·05) higher in the two cafeteria groups as compared with the control group, but these differences disappeared on the last day of the diet period (day 8), where the daily energy intakes of the three groups of rats were not statistically different. The total energy intakes measured in the three groups of rats, for the 8 d of treatment, showed higher values in the two Caf groups compared with the control group, but this difference was much larger in the Caf+ group than in the Caf group (26·3 % higher than control in the Caf+ group; 18·6 % higher than control in the Caf group; P < 0·05).
Table 1 Body weight and energy intake of the three groups of rats during 8 d of exposure to the different diets*
(Mean values with their standard errors for ten to twelve animals per group)

Caf, cafeteria diet with normal vitamin A levels; Caf+, cafeteria diet with higher vitamin A levels; DEI, daily energy intake; TEI, total energy intake.
* For details of diets, see Experimental methods. Body weights were recorded at the beginning of the experiment (day 0) and at the end of the diet (day 8). Body-weight gains (Δ body weight) were calculated as the difference between day 8 and day 0. Daily energy intakes (DEI) were reported at the first day (day 1) and at the last day (day 8) of the treatment. Total energy intake (TEI) means the cumulated energy intake during the 8 d of the regimen.
† Mean value was significantly different from that of the control group (P < 0·05).
‡ Mean value was significantly different from that of the Caf group (P < 0·05).
Effects of 8 d of two cafeteria diets on serum triacylglycerol, cholesterol, glucose, lactate and retinol levels
As shown in Table 2, serum TAG levels were significantly higher (91 %) in the rats in the Caf+ group compared with the two other experimental groups (Caf and control). The effects of the three different diets on the serum cholesterol levels were slightly different. In fact, as shown in Table 2, serum cholesterol levels were statistically higher (71 and 73·9 % higher than control for Caf and Caf+, respectively) in both the Caf and Caf+ groups, without any significant difference between them. No significant effect of the three diets was observed on serum glucose and lactate levels (Table 2). After 8 d exposure to the cafeteria diet with the high level of vitamin A (Caf+), a slight increase in serum retinol level was induced (1·43 v. 1·29 and 1·24 for Caf and control respectively; see Table 2) but these differences did not reach statistical significance.
Table 2 Effect of 8 d exposure to the three diets on serum biochemical parameters*
(Mean values with their standard errors for ten to twelve animals per group)
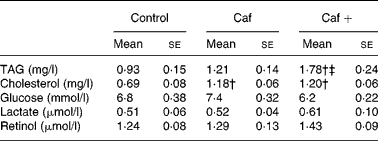
Caf, cafeteria diet with normal vitamin A levels; Caf+, cafeteria diet with higher vitamin A levels.
* For details of diets, see Experimental methods.
† Mean value was significantly different from that of the control group (P < 0·05).
‡ Mean value was significantly different from that of the Caf group (P < 0·05).
Effects of 8 d of two cafeteria diets on different white adipose deposit weights
Figure 1 shows that all the white adipose deposits were significantly higher in the two Caf groups than in the control group. However, these differences were greater in the subcutaneous and visceral adipose deposits of the Caf+ group than in the Caf group (P < 0·05). The total white adipose tissue, estimated by pooling all white adipose tissue removed, showed a significantly higher expansion in the Caf+ group than in the Caf group.

Fig. 1 Effect of 8 d exposure to the three diets (control diet (□), cafeteria diet with normal vitamin A levels (Caf; ![]() ), cafeteria diet with higher vitamin A levels (
), cafeteria diet with higher vitamin A levels (![]() )) on the subcutaneous (Swat), epididymal (Ewat), retroperitoneal (Rwat), visceral (Vwat) and total (Total wat) white adipose tissue weights. Values are the means of ten to twelve animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05). † Mean value was significantly different from that of the Caf group (P < 0·05).
)) on the subcutaneous (Swat), epididymal (Ewat), retroperitoneal (Rwat), visceral (Vwat) and total (Total wat) white adipose tissue weights. Values are the means of ten to twelve animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05). † Mean value was significantly different from that of the Caf group (P < 0·05).
Effects of 8 d of two cafeteria diets on subcutaneous adipocyte sizes and numbers and on stroma vascular cells numbers
Figure 2 (a) shows the total lipid content of isolated adipocytes from the Swat. This parameter was chosen as an index of cell size. We observed that adipocytes isolated from the Caf+ and Caf groups were larger than those isolated from the Swat of the control rats (320 and 430 % larger cell size for Caf and Caf+ compared with control, respectively; P < 0·001). However, the results in Fig. 2 (b) show that the two Caf diets caused no change in the number of subcutaneous mature adipocytes and stroma vascular cells compared with the number of these same cell populations in the control group.

Fig. 2 (a) Total lipid isolated from mature adipocytes (see Experimental methods) of the subcutaneous white adipose tissue (Swat) of young rats after 8 d exposure to the three different diets (control diet (□), cafeteria diet with normal vitamin A levels (![]() ), cafeteria diet with higher vitamin A levels (
), cafeteria diet with higher vitamin A levels (![]() )). Results are expressed as mg lipid/106 cells. (b) Numbers of total adipocytes and stroma vascular cells (adipocyte precursors) isolated from the Swat of rats. Results are expressed as × 106 cells per tissue. Values are the means of ten to twelve animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05).
)). Results are expressed as mg lipid/106 cells. (b) Numbers of total adipocytes and stroma vascular cells (adipocyte precursors) isolated from the Swat of rats. Results are expressed as × 106 cells per tissue. Values are the means of ten to twelve animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05).
Effects of 8 d of two cafeteria diets on nuclear receptor expression in subcutaneous white adipose tissue
Results of nuclear receptor mRNA levels are shown in Fig. 3. Compared with the control group, PPARγ mRNA levels were significantly higher in the Caf+ group only (+59·5 %; P < 0·05) and aP2 mRNA levels were significantly higher in the Caf group (+170; P < 0·05) and Caf+ group (+235 %; P < 0·0005). Thus, the Caf group rats had a lower aP2 mRNA level compared with the Caf+ groups. In addition, as shown in Fig. 4 (a), the PPARγ mRNA level and the weight of Swat were positively correlated for each rat (r 0·648; P < 0·005).

Fig. 3 Effect of 8 d exposure to the three diets (control diet, cafeteria diet with normal vitamin A levels (Caf; ![]() ), cafeteria diet with higher vitamin A levels (Caf+;
), cafeteria diet with higher vitamin A levels (Caf+; ![]() )) on retinoic acid receptor (RAR) α, RARγ, retinoid X receptor (RXR) α, PPARγ and aP2 mRNA levels in the rat subcutaneous white adipose tissue. Results are expressed as percentages of the control data. Values are means (n 6 per group) for the Caf and Caf+ groups, with standard errors represented by vertical bars. Mean value was significantly different from that of the control group: *P < 0·05, ***P < 0·0005. † Mean value was significantly different from that of the Caf group (P < 0·05).
)) on retinoic acid receptor (RAR) α, RARγ, retinoid X receptor (RXR) α, PPARγ and aP2 mRNA levels in the rat subcutaneous white adipose tissue. Results are expressed as percentages of the control data. Values are means (n 6 per group) for the Caf and Caf+ groups, with standard errors represented by vertical bars. Mean value was significantly different from that of the control group: *P < 0·05, ***P < 0·0005. † Mean value was significantly different from that of the Caf group (P < 0·05).

Fig. 4 Relationships between subcutaneous white adipose tissue (Swat) weights and nuclear receptor expression in control rats (♦; n 5), rats fed a cafeteria diet with normal vitamin A levels (△; n 5) and rats fed a cafeteria diet with higher vitamin A levels (○; n 5). (a) Correlation between PPARγ mRNA levels and Swat weight (r 0·648; P < 0·005). (b) Correlation between PPARγ mRNA levels and retinoid X receptor (RXR) α levels (r 0·676; P < 0·005). (c) Correlation between retinoic acid receptor (RAR) γ mRNA levels and Swat weight (r − 0·776; P < 0·001).
Determination of the expression of RXRα showed no significant change after 8 d of feeding. However, a positive correlation (r 0·676; P < 0·005) was found between RXRα and PPARγ mRNA levels, regardless of the group considered, in Swat of rats (Fig. 4 (b)).
RARα and RARγ mRNA levels were significantly lower ( − 34·6 and − 38·5 %, respectively; P < 0·05) in the Caf+ group than those of the control group. In the Caf group, only the RARα mRNA level was significantly lower ( − 52 %; P < 0·005) compared with the control rats. Compared with Caf+ and control groups, the Caf rats exhibited an intermediate value of RARα mRNA, but it did not reach significance. However, a significant negative correlation (r − 0·776; P < 0·001) was found between RARγ mRNA and the Swat weight in each rat (Fig. 4 (c)).
Effects of 8 d of two cafeteria diets on nuclear receptor expression in isolated adipocytes
Figure 5 shows that PPARγ mRNA level was lower in the Caf group as compared with the control group ( − 38·9 %; P < 0·05). No difference was found between the Caf+ and control groups, but the Caf group had a lower PPARγ mRNA level compared with the Caf+ group (P < 0·05). Thus, aP2 mRNA level was 46·5 % higher in the Caf+ group relative to the control group (P < 0·005). The relative abundance of the Caf group aP2 mRNA was not significantly different from that of the control group but was significantly lower than that of the Caf+ group.

Fig. 5 Effect of 8 d exposure to the three diets (control diet, cafeteria diet with normal vitamin A levels (Caf; ![]() ), cafeteria diet with higher vitamin A levels (Caf+;
), cafeteria diet with higher vitamin A levels (Caf+; ![]() )) on nuclear receptor and aP2 mRNA expression in subcutaneous isolated adipocytes. Levels of retinoic acid receptor (RAR) α, RARγ, retinoid X receptor (RXR) α, PPARγ and aP2 mRNA in rat mature adipocytes. Results are expressed as percentages of the control data. Values are means (n 7 per group) for the Caf and Caf+ groups, with standard errors represented by vertical bars. Mean value was significantly different from that of the control group: *P < 0·05, **P < 0·005, ***P < 0·0005. Mean value was significantly different from that of the Caf group: †P < 0·05, ††P < 0·005, †††P < 0·0005.
)) on nuclear receptor and aP2 mRNA expression in subcutaneous isolated adipocytes. Levels of retinoic acid receptor (RAR) α, RARγ, retinoid X receptor (RXR) α, PPARγ and aP2 mRNA in rat mature adipocytes. Results are expressed as percentages of the control data. Values are means (n 7 per group) for the Caf and Caf+ groups, with standard errors represented by vertical bars. Mean value was significantly different from that of the control group: *P < 0·05, **P < 0·005, ***P < 0·0005. Mean value was significantly different from that of the Caf group: †P < 0·05, ††P < 0·005, †††P < 0·0005.
The amount of RXRα mRNA was different between the Caf and control group rats ( − 42 %; P < 0·0005) and was lower in the Caf group v. the Caf+ group. As in the Swat, we found a positive correlation between mRNA levels of PPARγ and RXRα in isolated adipocytes of all rats (r 0·649; P < 0·01) (Fig. 6).
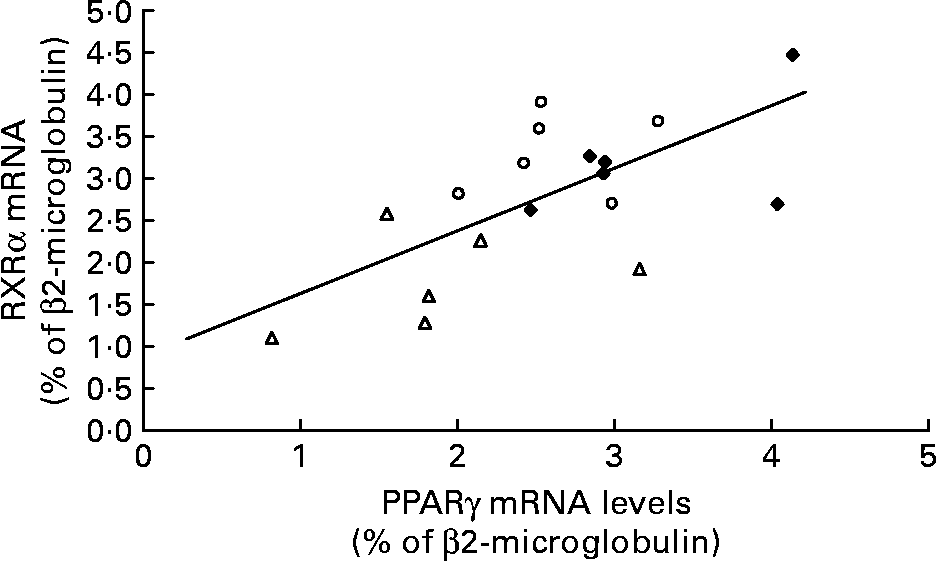
Fig. 6 Relationship between PPARγ and retinoid X receptor (RXR) α mRNA levels in isolated adipocytes in control rats (♦; n 5), rats fed a cafeteria diet with normal vitamin A levels (△; n 5) and rats fed a cafeteria diet with higher vitamin A levels (○; n 5) (r 0·649; P < 0·01).
We also observed significantly lower RARα mRNA levels in the Caf ( − 39·2 %; P < 0·005) and Caf+ groups ( − 30 %; P < 0·05) compared with the control group. However, the amount of RARγ was not affected in the isolated adipocytes.
Effects of 8 d of two cafeteria diets on the proliferation and differentiation capacities of subcutaneous adipocyte precursors in culture conditions
As shown in Fig. 7 (a), the number of preadipocytes derived from the precursors of each Swat was increased (approximately 2–2·5-fold) during the 8 d of primary culture under our in vitro conditions. However, the kinetics of this increase in the number of cultured cells was different depending on the experimental group considered. On day 1 of culture, the number of cells in the culture plates was significantly higher (P < 0·05) in the two Caf groups as compared with the control group (Fig. 7 (a)). On the third day of culture (day 3), this increase in proliferation activity was accentuated only in the Caf group, in which the number of cells doubled as compared with the Caf+ and control groups. On day 5 and day 8 of the primary culture experiments, corresponding to the submaximal and maximal proliferation activities, no difference was observed in the number of preadipocytes derived from the adipocyte precursors from the Swat of the three experimental groups.

Fig. 7 Effect of 8 d exposure to the three diets (control diet (□), cafeteria diet with normal vitamin A levels (Caf; ![]() ), cafeteria diet with higher vitamin A levels (
), cafeteria diet with higher vitamin A levels (![]() )) on the proliferating activity (a) and differentiation level (b) of subcutaneous adipocyte precursors in culture. Precursor adipocyte cells, isolated from the subcutaneous white adipose tissue of rats, were cultured in vitro (see Experimental methods). The numbers of cells at 1, 3, 5 and 8 d of culture are reported. (a) Results are expressed as × 105 cells per well of culture. (b) The levels of differentiation at 8 d of culture were estimated by the measurement of TAG accumulation in the preadipocyte (see Experimental methods). Results are expressed as mg TAG per well. Values are the means of six to eight animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05). † Mean value was significantly different from that of the Caf group (P < 0·05).
)) on the proliferating activity (a) and differentiation level (b) of subcutaneous adipocyte precursors in culture. Precursor adipocyte cells, isolated from the subcutaneous white adipose tissue of rats, were cultured in vitro (see Experimental methods). The numbers of cells at 1, 3, 5 and 8 d of culture are reported. (a) Results are expressed as × 105 cells per well of culture. (b) The levels of differentiation at 8 d of culture were estimated by the measurement of TAG accumulation in the preadipocyte (see Experimental methods). Results are expressed as mg TAG per well. Values are the means of six to eight animals per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05). † Mean value was significantly different from that of the Caf group (P < 0·05).
After 8 d of culture, the level of accumulated intracellular TAG (chosen as an index of the differentiation activity) was significantly higher (Fig. 7 (b)) in the Caf+ preadipocytes than in the Caf and control preadipocytes (80·3 % higher; P < 0·05), suggesting a higher degree of differentiation.
Discussion
The purpose of the present study was to investigate the change in adipose tissue nuclear profile of young rats exposed to a short period of cafeteria diets with normal or higher levels of vitamin A. Exposure during 8 d to these types of diet rapidly affected lipid status, since increased serum cholesterol was observed as already reported in our previous study(Reference Redonnet, Groubet, Noel-Suberville, Bonilla, Martinez and Higueret31). Moreover, we noticed an increase in TAG levels that seemed more pronounced in the Caf+ group. This observation could be attributed to an effect of vitamin A supplementation on liver lipid metabolism leading to a marked hyperlipidaemia. However, the retinol levels, measured in our conditions, did not change in the three groups. According to the studies of Périquet et al. (Reference Périquet, Bailly, Periquet, Ghisolfi and Thouvenot32), the lack of change could be normal, because serum retinol level is not a good index of change in vitamin A status, even after a high supplementation. Despite this marked increase of lipidaemia, neither blood glucose nor lactate levels were affected by the 8 d with Caf or Caf+ treatments. This observation led us to conclude that a short period of cafeteria exposure, with or without elevated levels of vitamin A, did not alter the insulin sensitivity, considering that a change in blood lactate level could indirectly underline a change in insulin action on glucose metabolism(Reference Lovejoy, Newby, Gebhart and Digirolamo33).
Exposure for 8 d to the cafeteria diets was sufficient to induce a significant hyperphagia. This increase in food intake was classically observed after a short period of hyperlipidaemic, hyperenergetic food consumption(Reference Prats, Monfar, Castella, Iglesias and Alemany34). However, it was interesting to note a more pronounced effect of the vitamin A-rich cafeteria diet. This may be due to the palatable aspect of this food made with liver pâté rather than to a direct action of vitamin A on the mechanism of food intake regulation, despite the fact that RA could act directly in the hypothalamus to inhibit the neuropeptide Y (NPY) action by downloading the NPY receptors(Reference Mannon and Kaiser35).
Despite this marked increase in total energy intake, all the animals in both Caf groups (either Caf+ or Caf) showed a 30–40 % deficit in normal body-weight gain, corresponding to 8 d of feeding in this growth period, during which control rats doubled their body weight.
However, all of the animals in both cafeteria groups increased their total fat mass, but the Caf+ diet had a more pronounced effect on the Swat and Vwat. These two deposits are classically considered to be more directly involved in the metabolic adaptation to nutritional changes(Reference Wajchenberg36), and are more susceptible to grow in response to an increase in energy intake. These results therefore suggest that a cafeteria diet could certainly influence the normal regulation of the energetic metabolism, inducing increased fat deposition rather than increased lean mass. It has been demonstrated that exposure of young rats during a growth period to a cafeteria diet could change body weight and tissue composition through an imbalance in N metabolism and amino acid metabolism(Reference Gianotti, Roca and Palou37, Reference Esteve, Rafecas, Remesar and Alemany38), and of course through a change in lean mass growth. A significant decrease observed in several muscles masses supports this hypothesis (data not shown).
It could be speculated that the positive action of the Caf+ diet in the Swat and Vwat growth may be explained as an indirect consequence of the increased energy intake. Diet-induced thermogenesis, which is the main adaptative mechanism to regulate this imbalance of the energetic metabolism, could be saturated in the Caf+-exposed animals. Indeed, higher interscapular brown adipose deposits, with a paler brown colour, were observed in the animals from the Caf+ group (data not shown), suggesting hypertrophied tissues, with deficient thermogenesis activities. Moreover, it has also recently been demonstrated that vitamin A, through its active form RA, is able to directly modulate leptin synthesis in adipose tissue, and that change in circulating leptin levels could directly influence the regulation of food intake and energy metabolism(Reference Kumar, Sunvold and Scarpace39).
However, it was surprising to note that, under our experimental conditions, a high level of vitamin A in the diet led to an increase in fat mass, whereas most works conclude that adipose tissue is increased in a situation of vitamin A deficiency in mice and that administration of RA leads to significant reduction of the adipose deposits(Reference Bonet, Ribot, Felipe and Palou12, Reference Mercader, Ribot, Murano, Felipe, Cinti, Bonet and Palou40). On the other hand, Rodriguez et al. (Reference Rodriguez, Ribot, Rodriguez and Palou41) reported in a recent study that 4-week-old rats exposed for 2 weeks to a cafeteria diet consisting of liver pâté (equivalent to our Caf+ group) markedly increased their Swat and Vwat adipose deposits.
When we considered only the change observed in the Swat, it appeared that the observed increase in mass could mainly be associated with cellular hypertrophy, with no change in stroma vascular cell population. These results confirm that 8 d of exposure to the two different cafeteria diets was too brief to increase the number of adipose cells in the Swat. This was classically observed upon the onset of obesity in rodents by exposure to a high-fat hyperenergetic diet(Reference Woods, Seeley, Rushing, D'Alessio and Tso42). Together with these changes in subcutaneous adipose cell size, induced by the two cafeteria diets, we found significant variations in the pattern of nuclear receptor expressions for the retinoic (RAR and RXR) and fatty acids (PPAR). These changes observed under our experimental conditions were more pronounced that those we previously reported with longer diet exposures of adult rats(Reference Bairras, Menard, Redonnet, Ferrand, Delage, Noel-Suberville, Atgie and Higueret28, Reference Redonnet, Groubet, Noel-Suberville, Bonilla, Martinez and Higueret31). Indeed, the previously characterised decreases in both subtypes (RARα and RARγ) involved in the signalling pathway of the all-trans-RA(Reference Redonnet, Groubet, Noel-Suberville, Bonilla, Martinez and Higueret31) were once again observed, concomitantly with an increase in PPARγ expression, with the highest effect observed in the Caf+ group. So, in this group, the results of RARα and RARγ reductions and PPARγ up-regulation were combined with a much higher global differentiation level of the tissue, as attested by a higher expression of measured aP2. These results are in accordance with the commonly recognised inhibitory action of the RAR pathway on the adipogenesis program(Reference Stone and Bernlohr23). Our correlation analysis clearly supported the hypothesis that the overall changes in the balance between RA and fatty acid pathways were strongly dependent on the differentiation level of the tissue, and that the RXRα receptor variation was also strongly dependent on those of the PPARγ receptors.
The observation of the same nuclear receptor expression profile in the isolated adipocytes from the Swat of the three experimental groups revealed more attenuated variations than in the whole tissue, with, in addition, specific differences induced by the two cafeteria diets. The effects observed on the RAR subtypes were less pronounced and only significant differences remained for the RARα subtype. We could try to explain these differences between the overall adipose tissue and the functional mature adipocytes by a possible selection of the largest adipocyte population by the enzymic dissociation procedure. These hypertrophied adipocytes were undoubtedly in a terminal differentiation state, and, as a result, it was not surprising to observe a marked down-regulation of PPARγ and aP2, which are classically considered as early differentiation markers. However, it was interesting to note that significant differences remained between the nuclear receptor expression profiles of isolated adipocytes from Caf+ and Caf groups.
Our tests performed on primary cultures were very interesting because they confirmed that a change in nutrient availability surrounding the adipose precursors in situ could induce profound changes in their capacity to proliferate and differentiate under in vitro conditions, and of course in situ in a longer exposure to cafeteria diets. These early changes revealed by culture conditions could be directly induced by a direct retinoic action on these cells in situ or by indirect action after a change in paracrine signals that could influence their genetic program. It was logical to postulate, according to recent observations by Ribot et al. (Reference Ribot, Oliver, Serra and Palou43), that a slight change in the RA signalling pathway could directly influence the normal regulation of the cell cycle program. Moreover, it was also possible that very early action of RA on the stroma vascular cells could increase the number of stem cells engaged in the adipoblast cell line(Reference Bost, Caron, Marchetti, Dani, Le Marchand-Brustel and Binétruy44).
In conclusion, in young growing rats, brief exposure to a cafeteria diet can lead to a marked imbalance in the pattern of retinoid and fatty acid nuclear receptors of subcutaneous adipose deposits. These changes seem to be directly or indirectly dependent on the level of vitamin A in the diet and are strongly correlated to the adipose differentiation level. These results underline that the adipocyte precursors in the tissue are very sensitive to changes in their environment, and that differences in nutrient availability could influence their development capacities. More experiments need, of course, to be performed to better understand the molecular mechanism involved in these adaptations.
Acknowledgements
C. B. was the recipient of a doctoral fellowship from the MENRT (French Ministry of Education, Research and Technology). The study was supported by the CTP Network (Communauté de Travail des Pyrénées).
All the authors have nothing to declare and contributed equally to the manuscript. There are no conflicts of interest.













