Globally, 370 million people are covered under the umbrella of indigenous tribal communities spread across seventy countries(1). They are a vulnerable population with sub-optimal socio-developmental, health and nutrition indicators. Their poor nutritional status with co-existence of the double burden of malnutrition, across different regions, is well documented(Reference Wong, Zalilah and Chua2,Reference Sheikh, Egeland and Johnson-Down3) . Nevertheless, these communities are custodians of diverse, rich and rooted knowledge, and of their cultural heritage, traditions, values and natural habitat. This knowledge and their insights on approaches that can foster a harmonious co-existence with their ecosystems can be instructive for a sustainable guide map for our future(Reference Kuhnlein and Receveur4,Reference Kuhnlein5) . The United Nations Declaration on the Rights of Indigenous Peoples affirmed their right to the highest attainable standard of health(6), but many Indigenous Peoples globally continue to experience unacceptable health and social inequities(Reference Browne, Lock and Walker7,Reference Anderson, Robson and Connolly8) .
The 705 notified tribes of India, clubbed under the broad umbrella of Scheduled Tribes, comprise 10·43 crore people, constituting 8·6 % of the total population, and predominantly inhabit rural (89·97 %) areas(9). The tribes are spread across thirty states and comprise communities with unique cultures inhabiting diverse geographies, and often difficult to access, remote areas. The tribal communities in general are resource poor with inconsistent access to essential services related to health, education and nutrition resulting in sub-optimal nutrient intakes which is further compromised due to concomitant infections. Though data on dietary intake and anthropometric profile demonstrating chronic energy deficiency in women is documented for some of the tribal communities, there is very limited information on nutritional biomarkers(Reference Kamath, Majeed and Chandrasekaran10–Reference Menon, Skeaff and Thomson13).
As is the case globally, the nutritional status of women and children of India’s indigenous communities is worse than their non-tribal counterparts(Reference Narain14,15) . A detailed nutritional assessment including comprehensive anthropometric, biochemical, clinical and dietary information would be a valuable resource for policy and programme planning and provide an impetus for identifying key interventions that can alleviate prevalent deficiencies and help in providing early warning towards health risks. Biomarkers provide quantitative estimates of micronutrient status and can offer accurate data on intake patterns, insights into the success or failure of supplementation programmes and compliance thereof. However, nutritional assessment using biomarkers especially in these communities can be challenging due to multiple reasons. These include their geographical isolation, logistic challenges like difficulty in sample procurement, transport and inadequate local infrastructure for testing. Finally, most biomarkers require sophisticated methodology (ELISA/chemiluminescence assays) and substantial sample volumes (in ml) requiring venous blood draw which the community is hesitant to provide.
Jharkhand is a state in the central eastern part of India that is home to thirty-two tribes, comprising 8·3 % of the national tribal population(9). Over the past decade, our group has been involved in exploring the indigenous food (IF) environment and food systems and its role in addressing food security of the major tribes of the state of Jharkhand including the Oraon, Munda, Santhal, Ho and Sauria Paharia(Reference Ghosh-Jerath, Singh and Kamboj16–Reference Ghosh-Jerath, Kapoor and Singh19).
The present paper reports development of the methodology and data on nutritional biomarkers among Santhals and Sauria Paharia tribes of Jharkhand. The Santhals constitute the largest tribe in Jharkhand(9), while the Sauria Paharias are particularly vulnerable tribal groups, characterised by pre-agricultural system of existence, extreme poverty and poor health status compared with other tribal communities(Reference Ghosh-Jerath, Kapoor and Singh20). While both the tribes reside in Santhal Parganas division of Jharkhand, the Sauria Paharias are located in remote villages situated across hill tops, whereas the Santhals reside in villages situated along plain lands. Previous studies have reported poor nutrition outcomes in these communities, with relatively higher nutritional vulnerability among the Sauria Paharia community(Reference Ghosh-Jerath, Kapoor and Singh19).
The goal of this paper was to develop an assay that could measure these biomarkers in small volumes and estimate biomarkers using the assay in our study participants. In order to economise on costs and minimise the sample requirement volumes for the routinely available assays, we attempted to develop the assay in a multiplex format that would be sufficient to measure these biomarkers in the small volumes obtained from a finger prick, that is, in capillary blood samples.
We selected biomarkers to assess iron (Fe), vitamin A and vitamin D status of the tribal communities given the significance of these micronutrients on reproductive and fetal health and their public health significance. Ferritin and soluble transferrin receptor (sTfR) were selected as markers of Fe status, and retinol binding protein (RBP) and hydroxycholecalciferol for vitamin A and vitamin D status, respectively. In order to adjust for the effects of inflammation on the levels of proteins like ferritin and sTfR due to acute phase response in areas that have high prevalence of malnutrition and where hookworm infestation and malaria are endemic(21), we measured C-reactive protein (CRP) and α-1-acid glycoprotein (AGP), concurrently to confirm the presence of an acute phase response(22), through these two inflammatory markers since they reflect different stages of the acute phase response(Reference Raiten, Sakr Ashour and Ross23).
Apart from assessing the micronutrient levels of women belonging to Santhal and Sauria Paharia tribes, we also explored their association with socio-demographic, economic and food environment factors.
Methodology
Study area
Selected Santhal and Sauria Paharia villages in two blocks of Godda district of Jharkhand were selected (Sauria Paharia population in Godda district = 13 688; Santhals = 22 4068(24)).
Study details
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the ethics committee of Indian Institute of Public Health-Delhi and the All India Institute of Medical Sciences, New Delhi (IEC-121/09·03·2018, RP-42/2018). Written informed consents were obtained at the cluster level, from head of the household (HH) and the participating women. In case of illiterate participants, witnessed verbal consent was witnessed and formally recorded and received. The present paper is part of an ongoing larger project documenting the role of IF consumed by tribal groups of Jharkhand and assessing their contribution to food security and dietary diversity among women and children. The details of the larger project and exhaustive methodological approaches are described elsewhere(Reference Ghosh-Jerath, Downs and Singh25). The blood sampling was done during the exploratory cross-sectional HH survey conducted in July–August 2018, and January 2019 in villages inhabited by Sauria Paharia and Santhal tribes, respectively.
Sampling framework and study population
A two-stage cluster sampling design was followed for the sampling strategy. Villages were randomly selected (from purposively chosen blocks based on the population density of tribal groups under investigation) using probability proportional to size sampling, with size being the number of HH in the village as per census 2011. From the purposively chosen administrative sub-divisions, that is, blocks, namely Sundarpahari, Boarijor, Pathargama and Poriyahat, eighteen and seventeen villages each were selected for the surveys among Sauria Paharia and Santhal and tribes, respectively. Subsequently, a house-listing exercise was carried out for all tribal HH in these villages to construct the sampling frame of all eligible HH. The eligibility included presence of at least one non-pregnant woman in the reproductive age group (15–49 years) and one child (6 months to 54 months) in the HH. In case of more than one eligible woman in a HH, one woman was randomly selected using Kish table(26).
Sample size calculation
The sample size calculation was based on the larger study objectives, that is, to assess differences in micronutrient intakes between those participants consuming IF and those who were not. Requisite sample size was calculated based on data from our previous study(Reference Ghosh-Jerath, Singh and Magsumbol17) to detect a difference in mean dietary intake of Fe of 4 mg/d (35 % increase) with a sd of 7 mg/d between consumers and non-consumers of IF. A minimum of ninety-seven women in each group were required to be sampled to detect the suggested minimum difference in Fe intake across the two groups with 80 % power and a 5 % level of significance. A design effect of 2·0 was considered to account for the loss in precision due to cluster sampling and deviation from normality. The above calculation suggested a sample size of 194 women.
Study procedures
Any adult member, especially women were interviewed during the HH survey to elicit the information on socio-economic and demographic characteristics, traditional food intake patterns and access to food sources. Special efforts were made to identify an adult woman member of the family for eliciting information since women have a predominant role in actual procurement as well as decision making regarding HH food access and preparation. Dietary survey was conducted on the eligible woman and index child. The sampling for obtaining capillary blood samples was done on the same eligible woman who responded to the dietary survey. An overview of the HH survey and sample collection, processing and estimation protocol is given in Fig. 1.
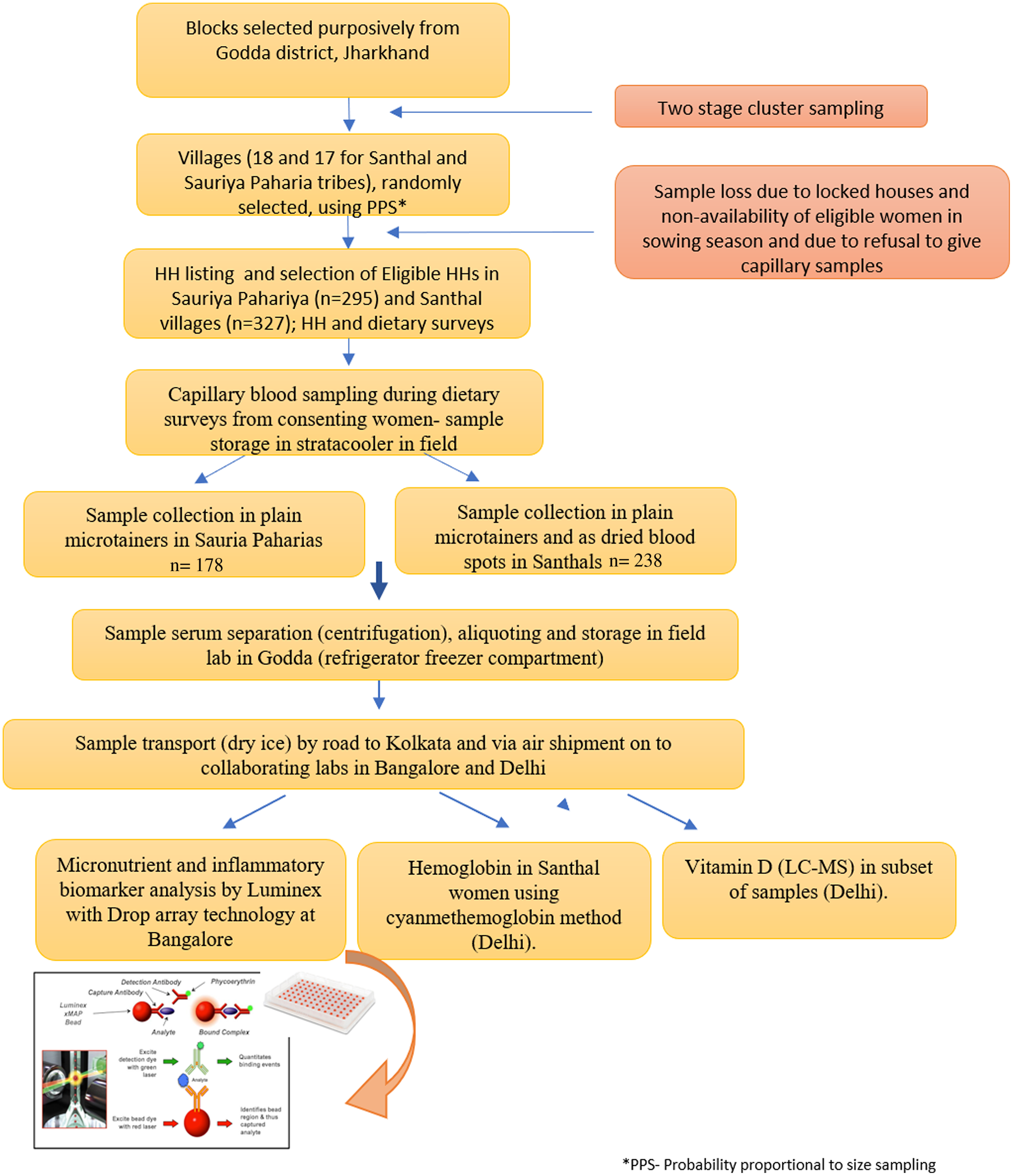
Fig. 1. Flow diagram depicting data and sample collection.
Blood sample collection and analysis
Sample collection, storage and transport
Prior to start of the survey, two teams comprising a field worker and a laboratory technician were trained by a clinical biochemist in sampling and processing procedures. Capillary sampling was done using BD Microtainer® contact-activated lancets through a finger prick on the ring finger after cleaning the finger with an alcohol swab. The blood was collected in labelled microtainers and stored in a cool box during the daily surveys. During the survey among Santhal women, blood was also spotted onto a labelled Whatman paper for making dried blood spot (DBS). At the end of each day’s survey, serum was separated by centrifugation, aliquots made and stored in cryo boxes in the freezer of a field site refrigerator till completion of the HH survey. Samples and blood spots were then transported in dry ice via road to Kolkata (370 km from Godda) and subsequently via air shipment to the partner laboratory at Bangalore (Certificate of incorporation no (CIN): U74900KA2015PTC079960; Start-up registration number: KBITS/SK-REGN/2016/165) and New Delhi. Cold chain was maintained throughout using a portable temperature data logger, the tempmate ®-S1 V2.
Laboratory analysis
Capillary blood was analysed for ferritin, sTfR, RBP4, AGP, CRP, Hb and vitamin D (in a subset).
Assay development for estimation of ferritin, soluble transferrin receptor, retinol binding protein 4, α-1-acid glycoprotein and C-reactive protein
The assay for estimation of micronutrients and inflammatory markers in capillary samples was developed in collaboration with the partner laboratory at Bangalore. The assay was developed on a Luminex xMAP ® platform along with the drop array technology (hereafter referred to as the in-house Luminex method). The Luminex system utilises magnetic microspheres that are internally fluorescently labelled to create distinct sets of microspheres. These microspheres act as both the identifier for a specific target and the solid surface to covalently couple the antigen-specific monoclonal antibodies, enabling the simultaneous capture and detection of ferritin, sTfR, RBP4, AGP and CRP in a single sample using analyte-specific detection antibodies. The beads are read in a Bio-Plex fluorescent suspension array reader, which consists of Luminex xMAP instrumentation supplied with Bio-Rad proprietary software enabling higher throughput and greater accuracy as opposed to conventional immunoassays. The biomarkers were quantified using the median fluorescent intensity available for each bead set of the biomarker generated by the Bio-Plex reader based on in-built instrument algorithms. Combining the Luminex platform with drop array technology(Reference Versteeg, Le Guezennec and Zhan27) allows quantification of these analytes even in low sample volumes in the range of 10–20 μl.
Method validation
The microsphere beads, standards and detection antibodies were commercially available for CRP, RBP4, sTfR and ferritin (R&D biosystems). The assay system for AGP was built in-house by conjugating a monoclonal antibody against AGP (R&D) to a microsphere of defined bead region (Bio-Rad) as per the manufacturers’ protocol. The conjugation of antibody to beads was tested by probing the conjugated beads with anti-mouse IgG-PE as compared with unconjugated beads. Subsequently, the beads were tested for its efficacy to detect AGP(Sigma) with different dilutions of a biotinylated detection antibody (R&D).
Before mixing the AGP assay system with CRP, RBP4, sTfR and ferritin, all analytes were tested individually and in multiplexed format to ensure lack of cross-reactivity. Assay Performance characteristics were assessed before testing samples of the participants from the tribal surveys. In order to ensure linearity of the standard curve over its detection range, multiple dilutions were run for all analytes.
Hb estimation
All DBS samples were processed for Hb estimation in a single batch. The estimation of Hb from DBS was done using a colorimetric method based on the formation of cyanmethaemoglobin (Benesphera™), as per manufacturers’ protocol. Each DBS was cut out and transferred to a labelled test tube containing 5 ml of Drabkin’s solution. The tubes were kept at room temperature (20°–25°) for 6 h and stirred on a vortex mixer twice during the incubation. Then the Drabkin’s solution containing the eluted blood spot was transferred to a cuvette and measured at 540 nm (OD540) on a spectrophotometer. Hb concentrations were calculated using a standard curve constructed from Hb standards provided with the kit.
Estimation of vitamin D in samples
The vitamin D methodology was developed at a Delhi-based laboratory for low sample volumes, that is, 25–30 µl to estimate the 25-hydroxy derivatives of both vitamin D3 and D2. The SRM LC-MS-based method was performed on a Thermo Scientific™ TSQ Fortis™ LCMS/MS system equipped with Thermo LC QUAN software. The internal standard used was D6 25-OH vitamin D3. The samples were run on a Hypersil Gold C18 (100 mm × 2·1 um) column (flow rate of 0·3 ml/min). The lowest limit of quantitation was 6·25 ng/ml.
Data analysis
Socio-economic status
Factor analysis with principal component extraction method was used to obtain a score for socio-economic status (SES) using the correlated variables from data on a HH’s ownership of selected assets, such as televisions and bicycles; materials used for housing construction; and types of water access and sanitation facilities. The SES score was divided into three quantiles such as low, middle and high SES.
Water, sanitation and hygiene indicators were defined according to UNICEF-WHO guidelines by classifying the HH drinking water source as being safe or unsafe. The HH sanitation facility was classified as being improved or unimproved, and open defecation was used as a third category of HH sanitation for HH with no sanitation facility or those reporting defecation being conducted in the bush or fields.
Access to food
In order to assess the access to different sources of food, we included a variable, the Food Access Diversity Index (FADI)(Reference Ghosh-Jerath, Kapoor and Singh19) adapted from the crop diversity index(Reference Michler and Josephson28). The FADI was calculated using the information obtained through the HH questionnaire (a questionnaire used during the HH surveys that collected information on socio-economic, demographic characteristics and availability of and access to different food sources), and an agricultural diversity tool(Reference Ghosh-Jerath, Kapoor and Singh19), by dividing the total number of foods accessed from natural food sources (farms, kitchen gardens, livestock, forests, pastures, roadsides, wastelands, rivers, ponds and/or lakes) with the maximum possible number of the foods accessed in the village. The FADI was expressed as:
Foods that were accessed from markets were not included in this index. Lower diversity in production and access to foods is indicated by lower values of FADI and vice versa.
Cut-offs and case identification based on biomarkers
Indicators for Fe stores and Fe deficient erythropoiesis used in our study were ferritin and sTfR, respectively. The ferritin concentrations reflecting depleted Fe stores were defined as concentrations of ferritin < 15 μg/l. The sTfR cut-offs for Fe deficient erythropoiesis were set at sTfR > 2·07(Reference Marković, Majkić-Singh and Subota29). The ferritin and sTfR concentrations were adjusted for inflammation as suggested in the WHO guidelines. Ferritin adjustment approaches were based on the BRINDA methods article(Reference Namaste, Aaron and Varadhan30) and included using a higher cut-off (> 30 μg/l), using a correction factor approach and a regression correction(24,Reference Marković, Majkić-Singh and Subota29) . Correction for sTfR was done using a similar approach. The WHO definitions of anaemia status were used to classify anaemia in non-pregnant women(31) (any anaemia: Hb concentration < 120 g/l; severe, moderate and mild anaemia: Hb concentrations < 80, 80–109, and 110–119 g/l, respectively). The magnitude of public health problem was based on WHO classification(32) (anaemia prevalence of 5·0–19·9 %, 20·0– 39·9 % and > 40 % denotes a mild, moderate and severe public health problem, respectively). Vitamin A deficiency and vitamin A insufficiency were defined as RBP4 concentrations less than 0·7 and 1·05 mmol/l, respectively. No correction factors were applied to RBP as studies demonstrate that the correlations between RBP4 and CRP and AGP do not require a correction factor(Reference Larson, Namaste and Williams33,Reference Engle-Stone, Haskell and Ndjebayi34) . Cut-offs for CRP and AGP were 4 and 840 mg/dl, respectively.
Statistical analysis
Data were entered and managed using Microsoft Excel. Categorical variables were presented as frequency and percentages, and continuous variables were presented as mean values and standard deviation or as median and inter-quartile range. All the quantitative variables were tested for the normality assumption using Shapiro–Wilks’s test. Continuous variables that followed normal distribution between the tribal groups were compared using unpaired t test and remaining variables which did not follow normal distribution were compared using Wilcoxon rank sum test. The ferritin and sTfR values were adjusted for inflammatory markers using correction factor method(Reference Namaste, Rohner and Huang35) and linear regression model. Linear regression model was fitted to predict the log (ferritin) using log (CRP) and log (AGP). The estimated regression coefficients were used in the following equation to obtain the adjusted ferritin values: log(ferritin)adj = log(ferritin) – 0·209 × (log (CRP) −1·00841) – ((–0·01936) × (log (AGP)-6·21 063)). Antilog of log(ferritin)adj values was taken to obtain the ferritin values in ng/ml. In the same way, linear regression model was fitted to predict log (sTfR) using log (AGP) and the estimated regression coefficients was used in the following equation to obtain sTfR values: log(sTfR)adj = log(sTfR)–0·241 × log (AGP)-6·211) and then antilog of log(sTfR)adj values was taken to obtain the sTfR values in µg/ml. The prevalence of anaemia and erythropoiesis along with 95 % CI was reported for both the adjusted ferritin values (if these values were ≥ 15 ng/ml) and sTfR values (if these values were > 2·07 µg/ml), respectively.
Three HH-level factors that were related to water, sanitation, hygiene and socio- SES were examined as potential covariates of women’s anaemia status. We also considered age, education and tribal group as covariates. The education categories were collapsed into three groups for analysis viz. Category 1–3 as group 1, 4 and 5 as group 2 and 6–8 as group 3. The FADI score was also used as potential covariate. We used both univariate and multivariate, logistic regression analysis to find the factors associated with anaemia. The results were reported as OR (95 % CI). The two-tailed P-value less than 0·05 was considered statistically significant. Statistical analysis was carried out using Stata 15·0(36) and SPSS 24(37).
Results
Socio-demographic description of the study population
Out of total of 295 and 327 eligible HH for Sauria Paharia and Santhal tribal groups, respectively, HH surveys could be completed in 247 and 275 Sauria Paharia and Santhals HH, respectively. Of these, the blood sampling was completed in 178 and 235 Sauria Paharia and Santhal women who consented to provide capillary blood sample. The participants from the two tribal groups did not differ significantly in age and source of drinking water. There were significant differences in educational status, occupation and place of defecation between the two groups. A higher number of Sauria Paharia women were involved in cultivation compared with their Santhal counterparts. A greater number of Santhal women had secondary or higher education level and had own toilet facility. The demographic characteristics of the participants from both tribal groups, and key HH variables are provided in Table 1.
Table 1. Socio-demographic characteristics of Sauria Paharia and Santhal tribal women
(Numbers and percentages)
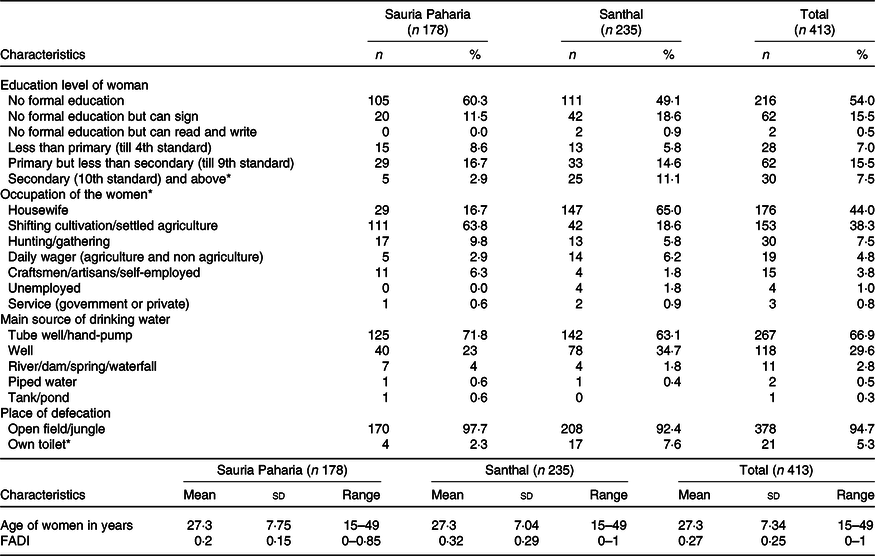
FADI, Food Accessed Diversity Index.
* Significant differences between tribal groups.
Development and validation of a Luminex-drop array assay for estimation of biomarkers ferritin, soluble transferrin receptor, retinol binding protein 4, C-reactive protein and α-1 acid glycoprotein
Development of α-1 acid glycoprotein assay system
The covalent conjugation of capture antibody for AGP to the microsphere was checked and the conjugation was found successful (Fig. 2(a)). The detection of AGP using the beads was tested using the AGP standards and different dilutions of the detection antibody for AGP as shown in Fig. 2(b).
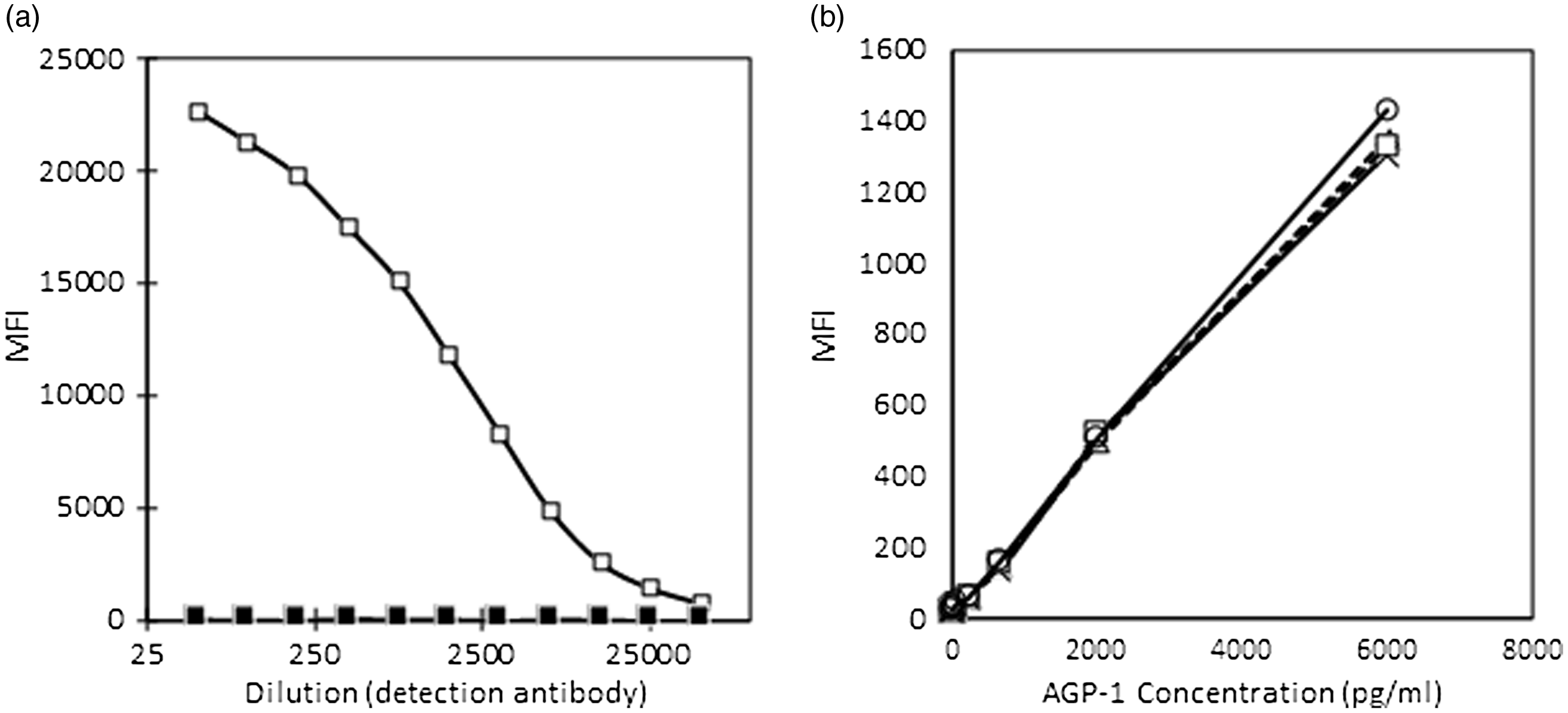
Fig. 2. (a) and (b) Conjugation of AGP antibodies to microbeads (a) and (b) validation of conjugation. AGP, α-1-acid glycoprotein; MFI, median fluorescent intensity. (a) ![]() , conjugated;
, conjugated; ![]() , unconjugated. (b)
, unconjugated. (b) ![]() , 1:40;
, 1:40; ![]() , 1:20;
, 1:20; ![]() , 1:10;
, 1:10; ![]() , 1:05.
, 1:05.
Cross-reactivity
AGP did not show cross-reactivity with the assay system used for ferritin, sTfR, RBP4 and CRP. Similarly, the ferritin, sTfR, RBP4 and CRP did not show any cross-reactivity between themselves or with AGP detection system (Fig. 3(a) and (b) and data not shown).
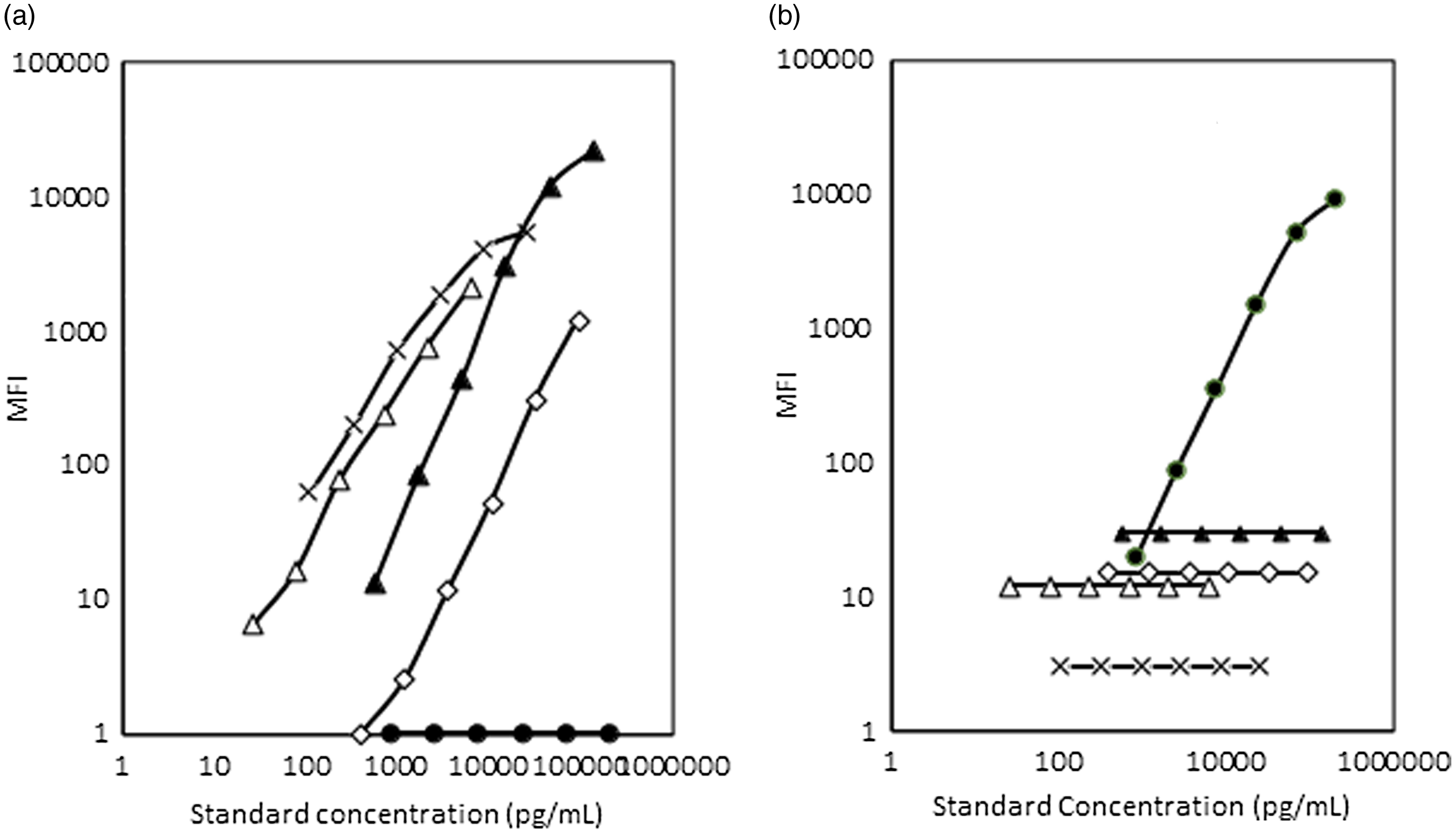
Fig. 3. (a) and (b) Cross-reactivity studies between the AGP detection system and CRP, RBP4, ferritin and sTfR systems and vice versa. MFI, median fluorescent intensity; AGP, α-1-acid glycoprotein; CRP, C-reactive protein; RBP4, retinol binding protein 4; sTfR, soluble transferrin receptor. ![]() , AGP-1a;
, AGP-1a; ![]() , CRP;
, CRP; ![]() , RBP4;
, RBP4; ![]() , ferritin;
, ferritin; ![]() , TfR.
, TfR.
Subsequently, the AGP beads were mixed with ferritin, sTfR, RBP4 and CRP beads, and the performance of the assay for ferritin, sTfR, RBP4, CRP and AGP was satisfactory (Fig. 4).
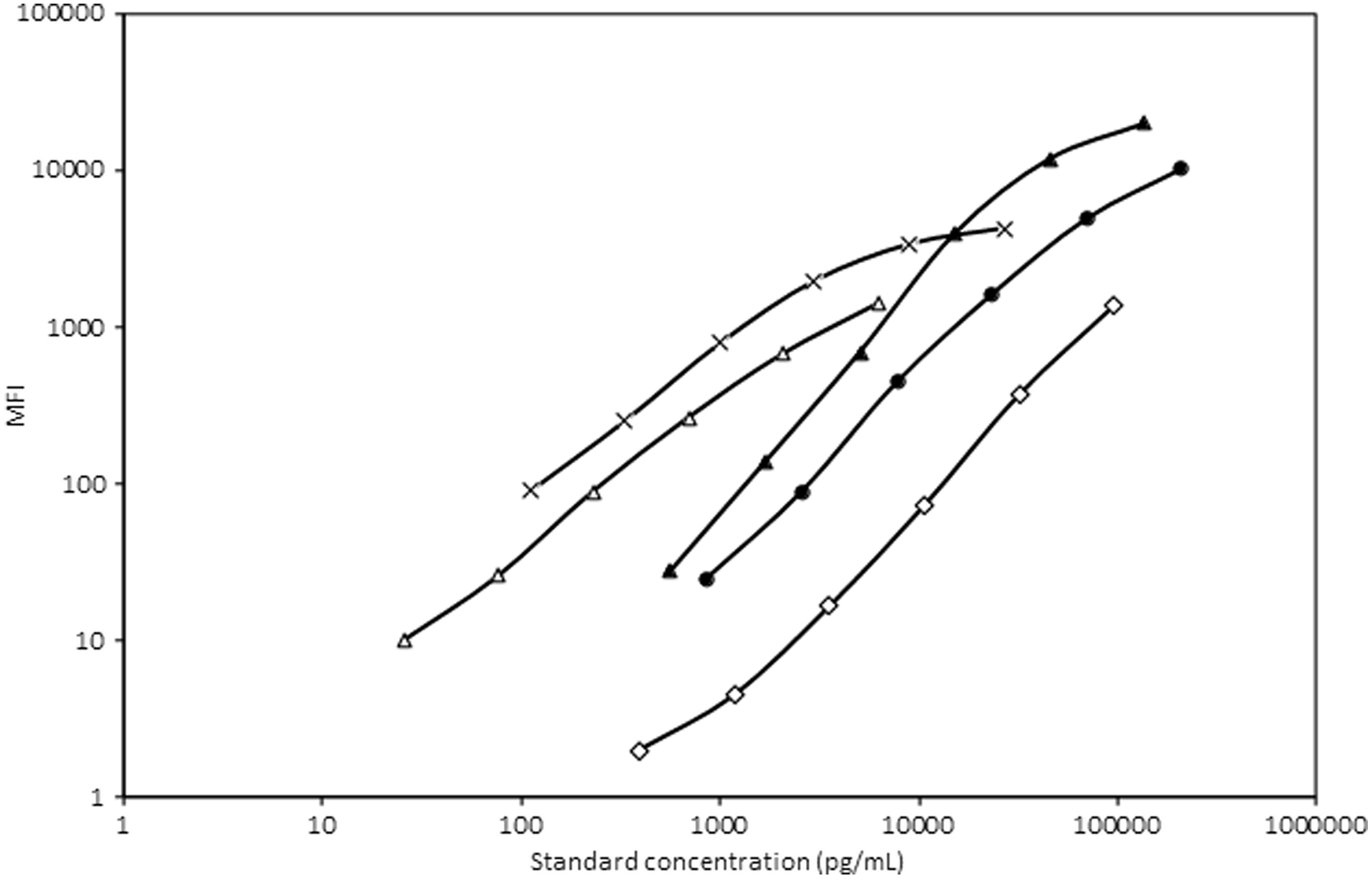
Fig. 4. Standard curve of AGP, ferritin, sTfR, RBP4 and CRP after mixing the AGP detection system with the rest of the system. MFI, median fluorescent intensity; AGP, α-1-acid glycoprotein; sTfR, soluble transferrin receptor; RBP4, retinol binding protein 4; CRP, C-reactive protein. ![]() , AGP-1a;
, AGP-1a; ![]() , CRP;
, CRP; ![]() , RBP4;
, RBP4; ![]() , ferritin;
, ferritin; ![]() , TfR.
, TfR.
Performance characteristics of the Luminex assay for ferritin, soluble transferrin receptor, retinol binding protein 4, C-reactive protein and α-1 acid glycoprotein
The assay showed good linearity, parallelism and acceptable levels of CV in intra- and inter-assay comparisons (Tables 2 and 3).
Table 2. Linearity of biomarkers at different dilutions by in-house Luminex assay

AGP, α-1-acid glycoprotein; CRP, C-reactive protein; RBP4, retinol binding protein 4; sTfR, soluble transferrin receptor.
Table 3. Performance parameters of various biomarkers by in-house Luminex assay
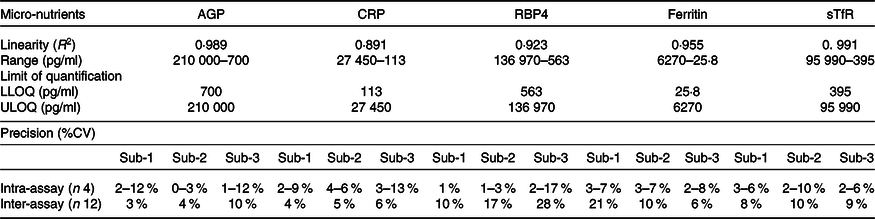
ULOQ, upper limit of quantification; LLOQ, lower limit of quantification; AGP, α-1-acid glycoprotein; CRP, C-reactive protein; RBP4, retinol binding protein 4; sTfR, soluble transferrin receptor.
Comparison with WHO standards
In order to compare the WHO recommended standards for ferritin and sTfR with the purified standards obtained from R&D, dilutions of these were run individually and in a multiplexed format. The readouts of the median fluorescent intensities of the WHO standards were higher than that for R&D standards for similar concentrations of the analytes. WHO standards for ferritin and sTfR also showed cross-reactivity with other analyte standards. The assay system results using WHO standards are shown in Fig. 5.
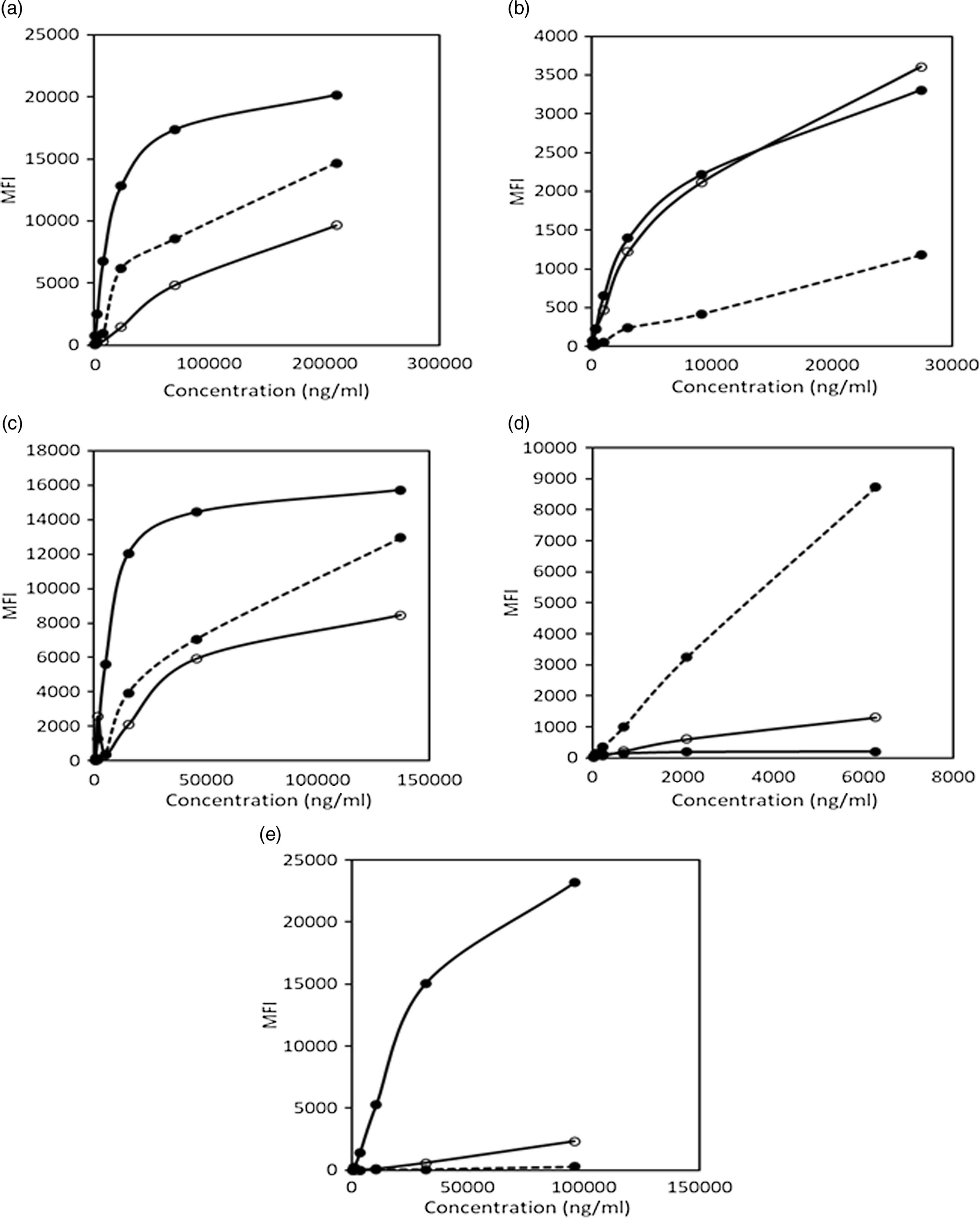
Fig. 5. Comparison of assays using WHO recommended standards and R&D standards. (a) Cross-reactivity of WHO standards for ferritin and sTfR with AGP-1a assay system. (b) Cross-reactivity of WHO standards for ferritin and sTfR with CRP assay system. (c) Cross-reactivity of WHO standards for ferritin and sTfR with RBP4 assay system. (d) Comparison of R&D and WHO standard for ferritin and assessment of cross-reactivity of WHO standard for sTfR with ferritin assay system. (e) Comparison of R&D and WHO standards for sTfR and assessment of cross-reactivity of WHO standard for ferritin with sTfR assay system. MFI, median fluorescent intensity; sTfR, soluble transferrin receptor; AGP, α-1-acid glycoprotein; CRP, C-reactive protein; RBP4, retinol binding protein 4. (a) ![]() , R&D AGP-1a std;
, R&D AGP-1a std; ![]() , WHO ferritin std;
, WHO ferritin std; ![]() , WHO sTfR std. (b)
, WHO sTfR std. (b) ![]() , R&D CRP std;
, R&D CRP std; ![]() , WHO ferritin std;
, WHO ferritin std; ![]() , WHO sTfR std. (c)
, WHO sTfR std. (c) ![]() , R&D RBP4 std;
, R&D RBP4 std; ![]() , WHO ferritin std;
, WHO ferritin std; ![]() , WHO sTfR std. (d)
, WHO sTfR std. (d) ![]() , R&D ferritin std;
, R&D ferritin std; ![]() , WHO ferritin std;
, WHO ferritin std; ![]() , WHO sTfR std. (e)
, WHO sTfR std. (e) ![]() , R&D sTfR std;
, R&D sTfR std; ![]() , WHO ferritin std;
, WHO ferritin std; ![]() , WHO sTfR std.
, WHO sTfR std.
Comparison of Luminex-drop array assay with standard assays for ferritin, soluble transferrin receptor and retinol binding protein 4
As the WHO standards showed significantly higher values compared with standards from R&D systems, we compared the values from the in-house Luminex array with routine assays that are harmonised with WHO standards. Venous samples from volunteers were analysed with both the in-house Luminex-drop array method and standard immunoassays (validated against WHO standards) for ferritin (n 40), and RBP and a nephelometric assay for sTfR (n 35). The values of the samples tested by the in-house Luminex method for ferritin, sTfR and RBP4 showed varying degrees of overestimation by the in-house method for these three analytes when compared with standard reference kits. In order to calculate the extent of overestimation, correction factors were calculated by dividing sample results obtained by the in-house method by results from the immunoassays. The arithmetic means/median of the factors obtained were taken to adjust the values obtained by the in-house Luminex method for the samples. These correction factors were 4·7, 7·4 and 3·6 for ferritin, sTfR and RBP4, respectively. The adjusted dataset was used for all further analysis.
Biomarkers of nutritional status and inflammation in the tribal women
A total of 413 women of reproductive age group in the age range: 15–45 years, from the Sauria Paharia (n 178) and Santhal (n 235) tribes of Godda district, Jharkhand, were sampled for biomarker analysis. A summary of the biomarker levels of the sampled women is given in Table 4.
Table 4. Summary of biomarker estimates among Sauria Paharia and Santhal tribal women
(Mean values and standard deviations; numbers and percentages)
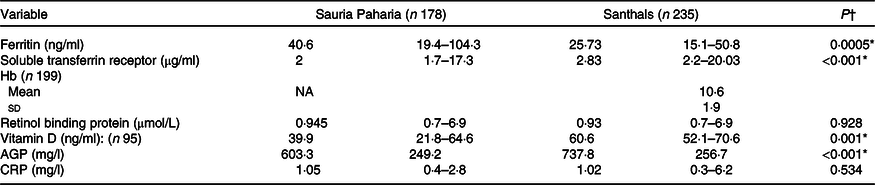
AGP, α-1-acid glycoprotein; CRP, C-reactive protein.
* P < 0·05.
† Variables that followed normal distribution between the tribal groups were compared using unpaired t test and remaining variables which did not follow normal distribution were compared using Wilcoxon rank sum test.
Inflammatory biomarkers
The analysis showed varying levels of inflammatory markers in the women. Approximately 61 % of women had raised levels of CRP and AGP. Among the two groups, a higher percentage of Sauria Paharia women had increased AGP (37 v. 22 %; P < 0·001) levels, while a higher number of Santhal women had raised CRP (14 v. 5·6 %; P < 0·001). The status of inflammatory biomarkers in the two tribes is summarised in Table 5. The levels of ferritin and sTfR were significantly higher in the groups with inflammation compared with those in which the CRP and AGP levels were within normal range (P-values: < 0·001). The values of ferritin and sTfR in different groups with and without inflammation are given in Table 6. Ferritin and CRP levels were found to be significantly positively correlated while those of ferritin and sTfR showed an inverse correlation.
Table 5. Status of inflammatory markers in Sauria Paharia and Santhal women
(Numbers and percentages)
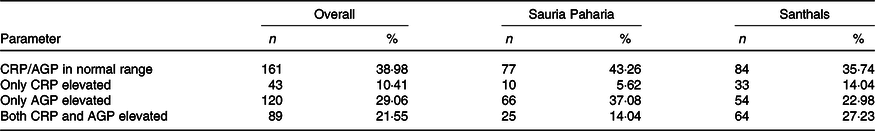
CRP, C-reactive protein; AGP, α-1-acid glycoprotein.
Table 6. Ferritin and sTfR levels in groups with different inflammatory biomarker levels

sTfR, soluble transferrin receptor; CRP, C-reactive protein; AGP, α-1-acid glycoprotein.
Fe status
The prevalence of iron (Fe) deficiency and Fe deficient erythropoiesis among the women based on ferritin and sTfR values is summarised in Table 7.
Table 7. Fe status descriptors in Sauria Paharia and Santhal women
(Numbers and percentages; prevalence and 95 % confidence intervals)
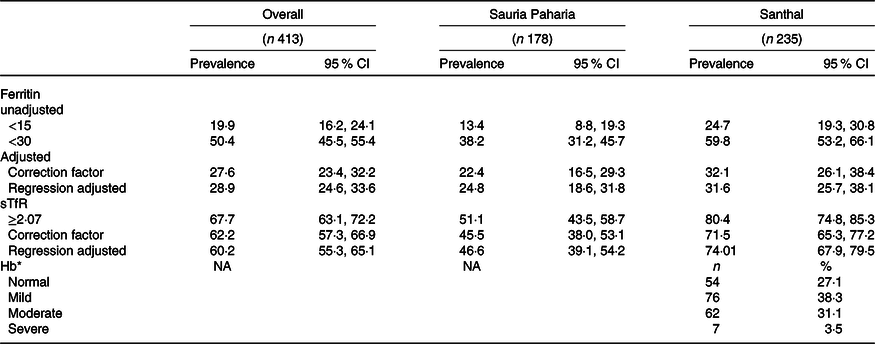
sTfR, soluble transferrin receptor.
* Only available for the Santhal group women; n 199.
The prevalence of Fe deficiency varied based on whether unadjusted or adjusted values (after adjusting for inflammatory status) were used. After adjusting ferritin for inflammation, the proportion of women with Fe deficiency increased in both tribal groups. The change (increase) was greater in Sauria Paharia women (11·4 %) compared with the Santhal women (6·9 %).
The prevalence estimates of Fe deficiency differed significantly based on ferritin and sTfR cut-off values with ferritin-based estimates showing lower prevalence of Fe deficiency compared with when sTfR was used for estimates.
The Sauria Paharia women (25;46 %) had significantly better Fe status compared with Santhal women (31;74%) when either ferritin or sTfR values were considered. The Hb status as estimated by the cyanmethaemoglobin method showed an anaemia prevalence of 72% among the Santhal women. The ferritin and Hb values showed a positive correlation using the spearman correlation coefficient (r 0·21; P = 0·0003).
Vitamin A status
We assessed RBP4 as a measure of vitamin A status which showed an overall prevalence of vitamin A deficiency as 25 % and insufficiency as 34 % among women from both tribes; the prevalence of vitamin A deficiency was similar among Sauria Paharia (25 %) and Santhal (26 %) women.
Vitamin D status
We analysed vitamin D in only a subset (n 35 Sauria Paharia and n 60 Santhal) of samples for which sufficient serum samples were left over after the amount required for the multiplex assay. The majority of women were vitamin D sufficient in both communities.
The vitamin D levels were found to be sufficient in all the Santhal women, while 25 and 20 % of Sauria Paharia women had insufficient and deficient vitamin D levels, respectively.
The vitamin A and vitamin D status for the women are summarised in Table 8.
Table 8. Vitamin status in Sauria Paharia and Santhal women
(Numbers and percentages)
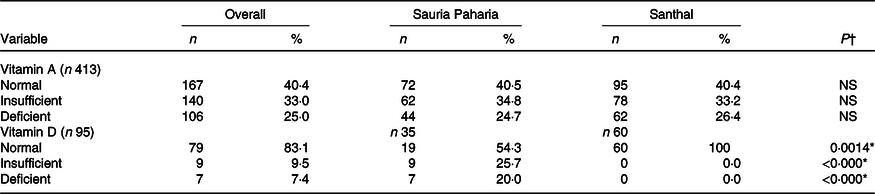
* P < 0·05.
† Variables that followed normal distribution between the tribal groups were compared using unpaired t test and remaining variables which did not follow normal distribution were compared using Wilcoxon rank sum test.
Table 9. Multivariate analysis for household level predictors of anaemia
(Numbers and percentages; odds ratios and 95 % confidence intervals)
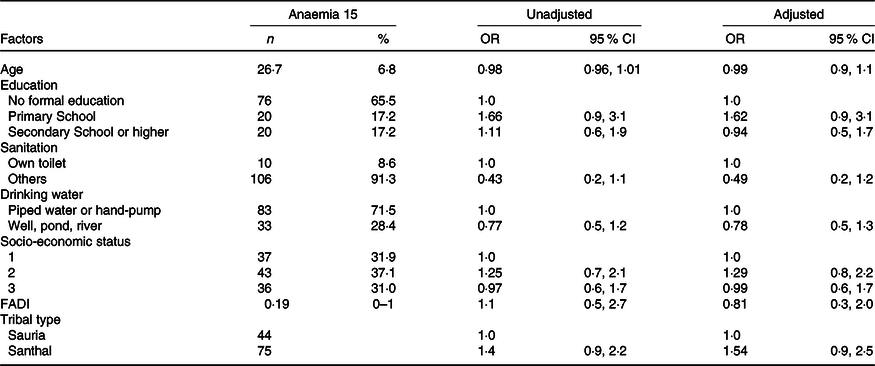
FADI, Food Accessed Diversity Index.
In the multivariate logistic regression analysis, none of the socio-demographic factors or diet diversity (FADI) scores was significantly associated with anaemia or with any other biomarkers analysed, as shown in Table 9.
Discussion
We developed an in-house multiplex assay on the Luminex platform for capillary blood samples which aimed to address the logistic, infrastructure and sample volume requirement challenges in biomarker assessment for nutritionally vulnerable communities residing in difficult terrains. Utilising the developed assay, we estimated Fe status biomarkers, RBP4, vitamin D and biomarkers of inflammation in two tribal groups residing in Godda, Jharkhand, India. The combination of biomarkers was selected based on the appropriateness of these biomarkers as a reflection of status of key micronutrients as well as of inflammation that impacts nutritional status. These have been used frequently in numerous regional and national nutrition surveys across the world. Most recently, a large initiative, the BRINDA project has used the combination of these biomarkers to address knowledge gaps related to the assessment of nutritional status across diverse settings in many countries(Reference Suchdev, Namaste and Aaron38).
Overall, the estimates of Fe deficiency in our study ranged from 30 to 60 % depending upon the cut-offs used and the biomarker taken for reference. A quarter of the women tested had vitamin A deficiency. The number of participants with increased inflammatory markers was over 50 %. Vitamin D levels were largely sufficient with no deficiency observed in the Santhal women and over 75 % of Sauria women having levels above 20 ng/ml. Fe status differed significantly between the women of the two tribal groups, while vitamin A and vitamin D status were not significantly different.
Our multiplex assay was developed to measure ferritin, sTfR, RBP, AGP and CRP, to minimise sample requirements, work repetition and costs. A very limited number of validated multiplex assays measuring multiple micronutrients and inflammatory biomarkers in sample volumes < 25 µl are available worldwide. One of them is the sandwich ELISA assay at the VitMin laboratory(Reference Erhardt, Estes and Pfeiffer39) that has been used in global studies(Reference Namaste, Aaron and Varadhan30,Reference Rohner, Northrop-Clewes and Tschannen40) . Another is the Quansys Biosciences CLIA-based assay requiring its own proprietary platform for analysis and has been undergoing validation(Reference Brindle, Lillis and Barney41,Reference Brindle, Lillis and Barney41,Reference Esmaeili, Zhang and Sternberg42) . These limitations necessitated an attempt to develop an indigenously feasible alternative, with a capacity for high throughput. The assay developed by us in collaboration with our partner lab in Bangalore had robust internal parameters and offers an alternative for measuring these micronutrients in small sample volumes and resource limited settings. We also observed that currently available WHO recommended standards for sTfR and ferritin may not be suitable for multiplex assays as it showed cross-reactivity with other analytes in the assay system. Furthermore, the levels of WHO standards for sTfR and ferritin did not correlate with the standards used for the Luminex assay supplied by the manufacturer which are purified proteins. It could be due to couple of reasons, since the WHO standards showed cross-reactivity, it is possible the higher levels observed in the WHO standards could be coming from non-specific binding or the standards could have higher levels of sTfR and RBP4. Alternatively, the Luminex assay on a drop array platform could be offering enhanced sensitivity as compared with conventional methods(Reference Ghosh-Jerath, Downs and Singh25). In view of this, the assay overestimated the levels when compared with standard reference immunoassays available for ferritin, sTfR and RBP4 and similar challenges were based on other multiplex assays developed, requiring a correction factor for data reporting. Nevertheless, as internal parameters were robust and there are minimal nutritional biomarker data from the population under study, we went ahead with reporting the data after adjustments using correction factors. We derived correction factors by comparing values obtained using standard harmonised kits and the Luminex-drop array platform on samples from a group of volunteers. We consider our derived estimates valid for multiple reasons. The anaemia prevalence based on Hb levels agreed with sTfR estimates, there was a positive correlation between ferritin and Hb and expected differences were observed in Fe biomarker levels in various inflammation-related categories. While using correction factors for the final read-out is not the best approach, our effort is important as it has allowed us to standardise and develop a prototype assay on an instrument that is available across laboratories in major cities. The cost of the estimation using our in-house assay is at par with the other multiplex assay, the Q-plex that is accessible through various vendors. While this cost is higher than the VitMin Lab assay, the latter is not always available due to ethics and data sharing issues. Thus, the validation of this developed prototype against reference assays after addressing the overestimation concerns, cross-reactivity issues and reoptimisation against WHO recommended standards would allow this capacity to be built for government funded core laboratories for national surveys as the instrument used is available in many metropolitan cities.
The analysis of the samples’ Fe status showed a high prevalence of Fe deficiency in both the Santhal and Sauria Paharia women sampled, similar to anaemia data reported from various regions of India(Reference Menon, Skeaff and Thomson13,Reference Rohisha, Jose and Chakrabarty43,Reference Meshram, Kumar and Venkaiah44) NFHS-4 data for Jharkhand show a higher prevalence of anaemia in rural women from Godda (71·6 %)(45) compared with the state average for rural women (67·5 %). Our estimates of anaemia based on Hb estimation for the Santhal women are similar. Some of the difference in estimates could be due to the different methods for Hb used in the NFHS survey (HemoCue) and our method (DBS)(46), as studies show variable degrees of agreement between HemoCue and DBS in Women of Reproductive age (WRA)(Reference Roshania, Mehta and Shete47,Reference Mohanram, Rao and Sastry48) . There was a significant difference in the prevalence estimates for Fe deficiency depending on whether ferritin or sTfR was taken as indicators. These differences can be ascribed to the biomarkers reflecting different biological pathways (i.e. Hb, ferritin, sTfR) and have often been reported in studies(Reference Engle-Stone, Nankap and Ndjebayi49). The discrepancy between Fe status estimates based on ferritin and sTfR differed based on cut-offs used for ferritin. The difference in estimates was much lower when the cut-off was set as 30 compared with when ferritin cut-offs were set at 15 ng/ml. This supports data that cut-offs for SF like < 15 μg/l are specific but poorly sensitive for ID, whereas cut-offs like < 30 μg/l are more sensitive(Reference Daru, Colman and Stanworth50,Reference Hallberg, Bengtsson and Lapidus51) . In addition, modest effects on ID and IDA prevalence estimates have been shown in populations where malaria is endemic when ferritin and sTfR have been used for estimating prevalence(Reference Barffour, Schulze and Coles52).
The status of vitamin A was better than Fe with a quarter of women from either the tribal groups being deficient based on a cut-off of 0·7 micromol/l(Reference Menon, Skeaff and Thomson13) and these estimates are comparable with nationally reported prevalence although little data are available on tribal populations(Reference Longvah, Khutsoh and Meshram12,Reference Daru, Colman and Stanworth50) . Vitamin D status was significantly better in Santhal women who had higher mean vitamin D levels compared with their counterparts among the Sauria tribes. Although the surveys in the two populations were done in January and August, respectively, and there could be some differences due to dietary intakes, both communities reside in a similar geographical location with abundant sunlight, so it is difficult to comment on the reason for the differences observed. This largely sufficient vitamin D status in the majority of women sampled has implications for bone health despite low levels of dietary Ca consumption as reported by our group(Reference Ghosh-Jerath, Singh and Magsumbol17). These data also reveal a much better vitamin D status than that reported from urban areas of India(Reference Gupta53–Reference Chakraborty, Choudhury and Saha55). The status of inflammatory markers reveals that at least 40 % of women from either tribe had one inflammatory marker raised and the levels of ferritin and sTfR were significantly higher in the groups with inflammation compared with those in which the CRP and AGP levels were within normal range. Many areas of Jharkhand that we have surveyed are malaria endemic and hookworm infestation is common(Reference Das, Prajapati and Tiendrebeogo56,Reference Singh, Dhiman and Das57) . Recurrent exposure to inflammatory agents, such as malarial parasites and hookworm, can result in fluctuations in the concentrations of acute-phase proteins and some micronutrient biomarkers(Reference Das, Thurnham and Das58,Reference Muriuki, Mentzer and Webb59) . While we did not find any associations between anaemia and socio-demographic variables like SES, drinking water sources and sanitation practices including open defecation, other, larger studies have found consistent associations(Reference Wirth, Woodruff and Engle-Stone60,Reference Janmohamed, Karakochuk and McLean61) . One of the reasons could be the very homogenous nature of our population with respect to these variables. Finally, our biomarker estimates align with the inadequate intakes that we have been documenting in the dietary surveys done by us previously in various tribes(Reference Ghosh-Jerath, Singh and Lyngdoh18,Reference Ghosh-Jerath, Singh and Bhattacharya62) and as part of the currently ongoing larger study on IF environments(Reference Ghosh-Jerath, Kapoor and Singh19,Reference Ghosh-Jerath, Downs and Singh63) .
The Sauria Paharia community is a historically marginalised tribal group, while the Santhals are relatively more assimilated in mainstream society(9,Reference Gupta64) . Nevertheless, both these communities continue to have sub-optimal health indicators(45). Very few studies have documented nutritional status among the Sauria Paharias and Santhals. Our previous work in these communities reported a high prevalence of undernutrition and sub-optimal dietary intakes among the tribal women. A higher number of Sauria Paharia women were found to have inadequate intake of macro- as well as micronutrients, in comparison with the Santhal women, thus indicating a higher nutritional vulnerability in the Sauria Paharia tribe(Reference Ghosh-Jerath, Singh and Magsumbol17). However, representative and comprehensive information on biomarkers of nutrient status and inflammation is scarcely available from these communities. Some decadal nutrient surveys like NFHS-4(45) document Hb status in sub-samples, but Hb status is an insensitive marker of Fe status(65). Our findings demonstrate a continuing high prevalence deficiency of critical micronutrients that are indispensable to maternal health. The communities are small hold farmers, food insecure and their diets lack diversity despite in-depth knowledge about micronutrient rich IF sources. We have also demonstrated poor consumption of these nutrient-rich IF(Reference Ghosh-Jerath, Singh and Kamboj16–Reference Ghosh-Jerath, Singh and Lyngdoh18). Other factors that may aggravate nutrient deficiencies are endemic tropical diseases. Targeted government programmes like Anemia Mukt Bharat (for Fe and folic acid supplementation)(66), National Vector Borne Disease Control Programme (for Malaria control)(67) and National Prophylaxis Programme Against Nutritional Blindness(68) (for vitamin A supplementation) could be instrumental in addressing this issue among the vulnerable populations.
The chief limitation of our study is that we have not performed an external validation of our method yet. The validation would require performance comparisons in external laboratories and with other assays out there such as Roche, Ramco among others. This limits the generalisability of the method for use in surveys although the prototype is ready and validated for all internal parameters. Further, although the participants were from a malaria endemic region, we did not have information on their recent infection status although we did not enrol women having fever. Finally, since the main focus of our paper was to report the methodology and estimates of biomarker assessments in vulnerable tribal population, we did not include the dietary component as a part of this paper.
Conclusion
We provide the very first data on micronutrient levels from these two communities. In addition, we present a working prototype for a multiplexed assay for measuring these biomarkers in a single assay via multiplexing on instrument that is widely available. The costing also appears to be competitive compared with the very few alternative options available. Local data are imperative for informing programmes for addressing micronutrient deficiencies. These data also support the premise that approaches for infection and inflammation control must be implemented in parallel with measures for alleviating nutritional deficiency disorders. Our study would be useful for major stakeholders in the community health domain, for example, policymakers, programme planners, programme evaluators and researchers to help them interpret biomarkers more accurately, and in the appropriate context(Reference Stoltzfus and Klemm69). Studies have shown possible role of habitual consumption of micronutrient-rich IF towards addressing micronutrient deficiencies in the tribal groups studied(Reference Ghosh-Jerath, Singh and Magsumbol17–Reference Ghosh-Jerath, Kapoor and Singh19). Therefore, precise assessment of biomarkers in these population can provide a strong baseline as well as monitoring indicator for objective assessment of the nutritional profile of these communities and the impact of food based and other nutrition interventions to address widespread malnutrition.
Acknowledgements
The authors would like to thank the team at ‘Ekjut’, a grassroots NGO in Jharkhand, especially Mr. Mohd. Sarfraz Ali, for their support throughout the conduct of the surveys. We would also like to acknowledge the help from Ms Kiran Kumari Pasi, Deputy Commissioner Godda District, Jharkhand who facilitated necessary permissions to conduct the study. We are grateful to Dr. RD Paswan, Civil Surgeon Godda for granting permission to conduct the study. The contribution of Awadesh Kumar, Nakul Sain, Johann Paharia and Haradhan in helping with sample collection, processing and communication with the participants is greatly appreciated. We would like to thank Ayushi Dhasmana and Manoj for their help in formatting and data collation. We would like to thank our field staff, and Anganwadi workers in the study villages for extending their warmth and cooperation. We thank Sneha Sridhar at Indoor Biotechnologies India Private Limited for her efforts in analysing the serum samples. Finally, we are indebted to the Sauria Paharia and Santhal families for agreeing to provide samples and for sharing their invaluable traditional knowledge and wisdom.
This work was supported by the DBT/Wellcome India Alliance Fellowship (https://www.indiaalliance.org/) (IA/CPHI/16/1/502639) awarded to SGJ. The funding source had no role in the design, analysis or writing of this article.
S. G. J and A. S., contributed to the conceptualisation, funding acquisition, methodology, data collection and supervision. A. S. and S. B. were involved in validation of methodology and results. S. G. J., A. S., M. K., R. K. and S. B. contributed to the project administration, data curation and formal data analysis. S. G. J., A. S. and S. B. contributed to the preparation of the original draft. All authors reviewed and edited the final manuscript.
None of the authors had any financial or personal conflicts of interest associated with this manuscript and research.



















