Interest in vitamin D has intensified lately, with a growing body of evidence suggesting that adequate vitamin D status is required for optimal health(Reference Holick1, Reference Michos and Melamed2). The vitamin D axis affects vascular smooth muscle cell proliferation, inflammation, vascular calcification, the renin–angiotensin system and blood pressure, all of which affect risk of CVD(Reference Zittermann, Schleithoff and Koerfer3). The Health Professionals Follow-up Study(Reference Giovannucci, Liu and Hollis4), Min-Finland Health Study(Reference Kilkkinen, Knekt and Aro5), and Ludwigshafen Risk and Cardiovascular Health Study(Reference Dobnig, Pilz and Scharnagl6) indicate that low vitamin D levels are associated with an increased risk of CVD. However, the Third National Health and Nutrition Examination Survey(Reference Melamed, Michos and Post7, Reference Martins, Wolf and Pan8) reported no association between vitamin D status and the risk of CVD, and the Women's Health Initiative trial(Reference Hsia, Heiss and Ren9, Reference Newhouser, Wassertheil-Smoller and Thomson10) observed that vitamin D supplementation did not decrease the incidence and mortality of CVD. Thus, studies regarding the relationship between vitamin D and CVD are inconclusive at this point.
A major source of vitamin D is endogenous production via the action of the sun's UV-B rays on the 7-dehydrocholesterol precursor in the skin, which is then converted to vitamin D. Vitamin D undergoes 25-hydroxylation in the liver to form 25-hydroxyvitamin D, the metabolite that reflects stores of vitamin D. Few foods naturally contain vitamin D and oily fish, such as salmon, sardines, mackerel and tuna, are good sources of vitamin D(Reference Holick1). Oily fish contain not only vitamin D, but also long-chain n-3 PUFA, EPA, 20 : 5n3 and DHA, 22 : 6n3. Higher fish intake and erythrocyte EPA and DHA levels(Reference Harris and Von Schacky11) have been shown to be a significant and independent discriminator of CVD(Reference Park, Lim and Lee12, Reference Park, Park and Yi13). The Diet and Reinfarction Trial(Reference Burr, Fehily and Gilbert14), Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Prevenzione (Italian Group for the Study of the Survival of Myocardial Infarction)(Reference Marchioli, Barzi and Bomba15), and the Japan EPA Lipid Intervention Study(Reference Yokoyama, Origasa and Matsuzaki16), have shown that oily fish, EPA and DHA and EPA alone significantly reduce cardiac death.
The Korean population consumes greater amounts of fish (average consumption 67·7 g/d)(17) than most Western populations, and therefore Koreans tend to have higher tissue n-3 PUFA levels(Reference Park, Lim and Lee12, Reference Park, Park and Yi13–Reference Nogi, Yang and Limei18), which may be positively correlated with serum 25-hydroxyvitmain D levels. In the present study, we investigated the hypothesis that tissue levels of n-3 PUFA are positively associated with 25-hydroxyvitamin D levels, and 25-hydroxyvitamin D levels are independently associated with the risk for CVD in Koreans with and without a first event of CVD, after adjusting for other risk factors.
Experimental methods
Subjects
Subjects were recruited consecutively from among patients admitted to Hanyang University Seoul Hospital between May 2007 and May 2009. Cases consisted of patients diagnosed with first-event myocardial infarction or stroke (n 60), and controls (n 60) were matched by age, sex, season during which blood was drawn and BMI. Patients were excluded if they had a history of CVD, cancer, hyperlipidaemia or diabetes. The present study was conducted according to the guidelines laid out in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of Hanyang University Seoul Hospital. Written informed consent was obtained from all participants. Anthropometric data, medical history and socioeconomic status were obtained by medical chart reviews and interviews. ‘Drinker’ was defined by drinking more than a glass a month during the last year, ‘current smoker’ meant currently smoking and smoking more than five packs in the lifetime, and ‘exercise’ meant more than three times a week of exercising for at least 20 min.
Laboratory measurements
Blood samples were collected in EDTA and SST (serum separated tube) blood collection tubes on the day of admission. The samples were centrifuged and divided into aliquots for storage at − 80°C. Serum lipid profiles (TBA-30FR; Toshiba, Tokyo, Japan), blood chemicals (Coulter LH 750; Beckman Coulter, Inc., Fullerton, CA, USA), liver function (Variant II; Bio-Rad, Hercules, CA, USA) and C-reactive protein concentrations (IMMAGE Immunochemistry System; Beckman Coulter, Inc.) were measured with auto analysers. Levels of IL-6 and TNF-α were measured at 490 nm with an ELISA reader (E max precision Molecular Device Company, Sunnyvale, CA, USA) using commercially available high-sensitivity ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). Mean coefficients of variation for IL-6 were 7·8 and 6·5 % at levels of 2·45 and 5·65 pg/ml, respectively. For TNF-α, the CV were 8·5 and 10·6 % at levels of 1·96 and 1·83 pg/ml, respectively.
Serum 25-hydroxyvitamin D levels were measured by RIA using a commercially available test kit (Biosource, Inc., Nivelles, Belgium) and radioactivity was determined with a gamma counter (COBRA QUANTUM 5010; Packard Instrument Company, Meriden, CT, USA). Total (intra- and inter-assay) CV for control values of 22·8 and 57·9 ng/ml were 3·2 and 5·3 %, respectively.
Erythrocytes were directly methylated by adding boron trifluoride methanol-benzene (B1252; Sigma-Aldrich, St Louis, MO, USA) and heating for 10 min at 100°C. Fatty acid methyl esters were analysed by GC (Shimadzu 2010AF; Shimadzu Scientific Instrument, Tokyo, Japan) with a 100 m SP2560 capillary column (Supelco, Inc., Bellefonte, PA, USA). Fatty acids were identified by comparison to known standards (GLC-727; Nu-Check Prep, Elysian, MN, USA). In the standard, the 18 : 1-trans (t) peak was a mixture of 18 : 1n-12t, 18 : 1n-9t and 18 : 1n-7t, while the 18 : 2n-6t peak contained 18 : 2n-6tt. The omega-3 index was calculated as the sum of erythrocyte concentrations of EPA and DHA and expressed as a percentage of total fatty acids in the erythrocytes(Reference Harris and Von Schacky11). The quality control sample comprised pooled erythrocytes with a CV of 5·0 %.
Statistical analysis
The statistical analysis was performed using SPSS, version 12.0 (SPSS, Inc., Chicago, IL, USA). P values < 0·05 were considered statistically significant. Continuous variables were expressed using the mean and standard deviation, and proportions of nominal variables were compared using the χ2 test. ANOVA with the Bonferroni post hoc test was used to determine the significance of differences for continuous variables. The trend in rates for categorical variables was tested with the Mantel–Haenszel extension test. OR and 95 % confidence intervals for the risk of CVD according to serum levels of 25-hydroxyvitamin D were obtained from multiple logistic regression models, after adjusting for age, sex, BMI and smoking. The lowest quartile of serum 25-hydroxyvitamin D levels was considered as a reference, and a likelihood ratio test was used to detect trends.
Results
Table 1 shows the baseline characteristics and metabolic parameters of the subjects according to serum 25-hydroxyvitamin D level quartiles. As serum 25-hydroxyvitamin D levels increased, we observed that there were significantly more men and smokers in the quartile which may be explained by the fact that most of the smokers were male. However, there were no significant differences in age, BMI, exercise, drinking and education levels between the quartiles. Although there were no significant trends with the 25-hydroxyvitamin D level quartiles, erythrocyte counts, haematocrit levels, Hb concentrations and aspartate transaminase:alanine transaminase ratios were significantly lower in the fourth quartile than the first quartile. K concentrations were significantly higher in the fourth quartile than the first quartile of serum 25-hydroxyvitamin D levels. Leucocytes count, HbA1c, glucose, albumin, Na, Ca, aspartate transaminase, alanine transaminase, total cholesterol, HDL-cholesterol, TAG, C-reactive protein, IL-6 and TNF-α levels did not differ significantly between the serum 25-hydroxyvitamin D level quartiles.
Table 1 Characteristics and metabolic parameters of subjects by serum 25-hydroxyvitamin D level quartiles (Q)
(Mean values and standard deviations or percentage distribution)

AST, aspartate transaminase; ALT, alanine transaminase; CRP, C-reactive protein.
a,b Mean values with unlike letters within a row are significantly different (P < 0·05).
* From a linear model.
Table 2 shows erythrocyte fatty acid composition according to serum 25-hydroxyvitamin D level quartiles. As serum 25-hydroxyvitamin D levels increased, erythrocyte concentrations of docosapentaenoic acid, DHA, omega-3 index and total n-3 PUFA increased significantly, while erythrocyte concentrations of stearic acid and total SFA decreased significantly, after adjusting for age, sex, BMI and smoking. Although there were no significant trends between trans-fatty acid and serum 25-hydroxyvitamin D levels, 16 : 1n7t was significantly higher in the first quartile of serum 25-hydroxyvitamin D levels than the fourth quartile. Consistently, partial correlation analysis showed that erythrocyte n-3 PUFA levels were positively correlated, while total SFA content was negatively correlated, with serum 25-hydroxyvitamin D levels (Fig. 1). In addition, stearic acid (r − 0·335, P < 0·001), total n-6 PUFA (r 0·185, P = 0·048) and docosapentaenoic acid (r 0·237, P = 0·011) were correlated with serum 25-hydroxyvitamin D levels (data not shown). Multiple logistic regression analysis showed that serum 25-hydroxyvitamin D levels were not significantly associated with the risk of CVD in this population, after adjusting or not adjusting for age, sex, BMI and smoking (Table 3).
Table 2 Erythrocyte fatty acid composition of subjects by serum 25-hydroxyvitamin D level quartiles (Q)
(Mean values and standard deviations)

a,b Mean values with unlike letters within a row are significantly different.
* From a linear model after adjusting for age, sex, BMI and smoking.

Fig. 1 Pearson's correlation coefficients between serum 25-hydroxyvitamin D concentrations and levels of total (a) n-3 PUFA (r 0·215; P = 0·021) and (b) SFA (r − 0·263; P = 0·004) in erythrocytes after adjusting for age, sex, BMI and smoking.
Table 3 Association of serum 25-hydroxyvitamin D levels with risk of CVD by multivariable regression analysis
(Odds ratios and 95 % confidence intervals)*
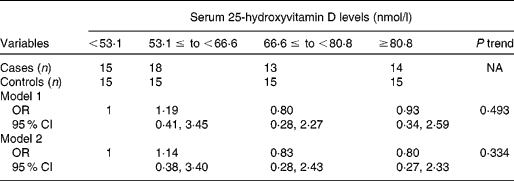
NA, not applicable.
* Model 1, unadjusted; model 2 adjusted for age, sex, BMI and smoking.
Discussion
In the present study, we detected a significant positive association between serum 25-hydroxyvitamin D and erythrocyte n-3 PUFA levels after adjusting for confounding factors; however, no association between serum 25-hydroxyvitamin D levels and risk of CVD was found in this population. Oily fish is the major dietary source of both vitamin D and n-3 PUFA, consumption of which is suggested to be protective against CVD. Previously, Lym & Joh(Reference Lym and Joh19) observed that frequent fish intake was positively associated with serum 25-hydroxyvitamin D concentrations in healthy Korean men. Van der Meer et al. (Reference van der Meer, Boeke and Lips20) also reported that fatty fish intake was the greatest contributor to serum 25-hydroxyvitamin D levels in a multiethnic population in the Netherlands. This raises the possibility that vitamin D and n-3 PUFA are potential confounding factors in CVD risk. Frequency of fish intake probably resulted in a random misclassification of exposure and would attenuate any association, and thus tissue levels of n-3 PUFA could be a better indicator of fish intake. The present study was the first to investigate the relationship between tissue levels (erythrocytes) of n-3 PUFA and serum 25-hydroxyvitamin D levels.
Although the exact mechanisms by which an adequate vitamin D status may protect against CVD are not fully understood, experimental studies indicate that vitamin D is one of the most potent chemicals for suppressing the renin–angiotensin system and thus for regulating blood pressure(Reference Li, Kong and Wei21). In addition, vitamin D may influence vascular function and the development or progression of atherosclerosis. Vitamin D receptors have a broad tissue distribution that includes vascular smooth muscle cells(Reference Carthy, Yamashita and Hsu22), macrophages(Reference Shioi, Katagi and Okuno23) and lymphocytes(Reference Rigby, Denome and Fanger24). Vitamin D induces prostacyclin in vascular smooth muscle cells, which prevents thrombus formation, cell adhesion and smooth muscle cell proliferation(Reference Wakasugi, Noguchi and Inoue25). Vitamin D suppresses pro-inflammatory cytokines, including IL and TNF-α in vitro and in vivo (Reference Zittermann and Koerfer26). However, there was no association between 25-hydroxyvitamin D levels and cytokines in the present study. The lack of association may be partly due to the elevated inflammatory profile as a result of acute trauma, such as heart attack and stroke in the subjects.
There is limited epidemiological evidence of an association between vitamin D and the risk of CVD. In the Health Professionals Follow-up Study, men with high circulating levels of 25-hydroxyvitamin D had half the risk of myocardial infarction as men with vitamin D insufficiency(Reference Giovannucci, Liu and Hollis4). The Min-Finland Health Study(Reference Kilkkinen, Knekt and Aro5) showed that low serum 25-hydroxyvitamin D levels might have a more important role in the prevention of CVD, particularly cerebrovascular disease. Similarly, a study of German adults who were undergoing elective cardiac catheterisation showed a twofold risk of CVD death among persons in the lowest quartile of baseline vitamin D levels compared with those in the highest quartile(Reference Dobnig, Pilz and Scharnagl6). However, vitamin D deficiency was associated with an increased risk of CVD in hypertensive subjects, but not in those without hypertension in the Framingham Offspring Study cohort(Reference Wang, Pencina and Booth27). The Third National Health and Nutrition Examination Survey(Reference Melamed, Michos and Post7) also did not find a statistically significant association between vitamin D status and the risk of CVD.
There are a few plausible explanations for these inconsistent findings. First, the Diet and Reinfarction Trial(Reference Burr, Fehily and Gilbert14) has shown that oily fish intake significantly reduced the risk of cardiac death and CVD events, and our previous studies involving a similar population suggested that high erythrocyte n-3 PUFA levels were associated with the risk of myocardial infarction(Reference Park, Lim and Lee12) and stroke(Reference Park, Park and Yi13). However, previous studies have not investigated the association of vitamin D and n-3 PUFA with CVD, the potential confounding factors. Second, optimal levels of serum 25-hydroxyvitamin D levels for CVD protection are not known, but relatively high concentrations of serum 25-hydroxyvitamin D (>75 nmol/l) are required to maintain normal parathyroid hormone levels and even higher levels (83–121 nmol/l) are suggested to be desirable for preventing cancer(Reference Bischoff-Ferrari, Giovannucci and Willett28). The mean levels of serum 25-hydroxyvitamin D in the present study were approximately of the same order of magnitude as those previously found in the general Korean population(Reference Kim, Kang and Oh29), but somewhat higher than those generally observed in American(Reference Giovannucci, Liu and Hollis4, Reference Melamed, Michos and Post7), German(Reference Dobnig, Pilz and Scharnagl6) and Finnish(Reference Kilkkinen, Knekt and Aro5) subjects. It is noteworthy that a vitamin D and CVD association was observed in a population with lower vitamin D levels. Third, serum 25-hydroxyvitamin D levels measured at a single point in time reflect only recent exposure rather than long-term exposure. A single serum measurement could be a useful tool in epidemiological studies, but such a measurement fails to take into account the intra-individual seasonal variation in serum 25-hydroxyvitamin D levels. To account for seasonality as a potential confounder, we included twenty cases and twenty controls with blood samples drawn during winter; the rest were drawn between April and November.
Interestingly, we found that erythrocyte SFA levels were negatively associated with serum 25-hydroxyvitamin D concentrations. SFA provide about 50 % of the fatty acids found in meat(30), and Welch et al. (Reference Welch, Shakya-Shrestha and Lentjes31) reported that non-fish-eating meat-eaters had lower intakes of n-3 PUFA compared with fish-eaters. Thus, meat-eaters may have higher SFA and lower n-3 PUFA intake, suggesting that differences in diet could partly explain the negative association between SFA and vitamin D in the present study.
A limitation of the present study was the small sample size. We also acknowledge that the cross-sectional design of the present study limits the ability to understand causal inference, and therefore does not allow for the establishment of a cause–effect relationship between vitamin D and the risk of CVD. Differences in the selection criteria and the demographics of the study population may be responsible for our inconsistent findings, and the present findings may be limited to Koreans and not applicable to other groups or other geographic areas. While the homogeneity of our sample may limit the external validity of the present findings, it minimises the potential for residual confounding by unmeasured characteristics.
In conclusion, the present study demonstrates for the first time, to our knowledge, that serum 25-hydroxyvitamin D levels are positively associated with tissue levels of n-3 PUFA. However, we failed to show a significant association between serum 25-hydroxyvitamin D levels and risk for CVD in this population. Further investigations involving a greater number of subjects from different populations with repeated measurements of vitamin D status are warranted. To demonstrate a possible link between vitamin D status and risk of CVD, large clinical trials that adjust for dietary intake, sun exposure and the effects of n-3 PUFA are needed.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2010-0008656). The authors state that there are no conflicts of interest. Y. P. was the principal investigator for this project and wrote the manuscript, and M. K. conducted laboratory and statistical analyses. The authors thank Dr Hyengjoong Yi, Dr Hyun Young Kim and Dr Jaeung Lee at the Hanyang University Hospital for their assistance in recruiting patients and collecting medical information.








