The bioavailability of flavonoids (considering intestinal absorption, metabolic conversion and urinary excretion) is an important factor when evaluating their in vivo activity and possible beneficial effects after intake(Reference Dorne, Walton and Renwick1–Reference Terao3). Flavonoids are generally consumed in food together with other macronutrients, micronutrients and other components. Various studies have shown that some dietary components enhance polyphenol absorption(Reference Lesser, Cermak and Wolffram4–Reference Yamashita, Sakane, Harada, Sugiura, Koda, Kiso and Sezaki7) although others report reduction and null effect of some foods on their bioavailability(Reference Serafini, Ghiselli and Ferro-Luzzi8–Reference Goldberg, Yan and Soleas10). However, few studies have evaluated the influence of food on flavonoid phase II metabolism and flavonoid metabolite excretion profile in urine for humans(Reference Silberberg, Morand, Manach, Scalbert and Remesy2).
During the course of absorption, polyphenols are conjugated in the small intestine and later in the liver, the primary site of drug metabolism in the body(Reference Manach, Scalbert, Morand, Rémésy and Jiménez11). Typically, metabolic conversion of a drug results in inactivation, detoxification and an enhanced likelihood of excretion in urine or faeces. Sulfation, glucuronidation and glutathione conjugation represent the three most prevalent classes of phase II metabolism. In addition, these three conjugation reactions increase the molecular weight and water solubility of the compound. As a result the phase II conjugates tend to have very poor membrane permeability and need carrier-mediated transport for biliary or hepatic basolateral excretion into sinusoidal blood for eventual excretion via urine. The conjugation mechanisms are highly efficient, and generally aglycones are either absent in the blood or only present in low concentrations after the consumption of nutritional doses(Reference Dorne, Walton and Renwick1, Reference Manach and Donovan12–Reference Chan, Cui, Xu and Cai14).
Sulfotransferases catalyse the transfer of a sulfate moiety from 3′-phosphoadenosine-5′-phosphosulfate to a hydroxyl group on polyphenols, although neither the isoforms that are specifically involved in the conjugation nor the positions of sulfation for polyphenols have been clearly identified yet, but sulfation clearly occurs mainly in the liver(Reference Dorne, Walton and Renwick1, Reference Manach, Scalbert, Morand, Rémésy and Jiménez11). Glucuronidation is a biosynthetic reaction in which a suitable functional group on the acceptor molecule (substrate or aglycone) is conjugated with glucuronic acid (GA). This reaction (which requires uridine 5′-diphospho (UDP)-GA as a co-substrate) is catalysed by the enzyme UDP-glucoronosyltranferase. Conjugation with GA is responsible for the deactivation and elimination of a wide range of xenobiotics and endogenous compounds(Reference Dorne, Walton and Renwick1, Reference Manach, Scalbert, Morand, Rémésy and Jiménez11, Reference Miners and Mackenzie15). UDP-glucoronosyltranferases are membrane-bound enzymes that are located in the endoplasmic reticulum in many tissues and that catalyse the transformation of a GA from UDP-GA to polyphenols, and thousands of dietary constituents and xenobiotics. The presence of glucuronidated metabolites in the mesenteric or portal blood after perfusion of polyphenols in the small intestine of rats shows that glucuronidation of polyphenols first occurs in the enterocytes before further conjugation in the liver(Reference Spencer, Chowrimootoo, Choudhury, Debnam, Srai and Rice-Evans16).
Polyphenols and their derivatives are eliminated chiefly in urine and bile. Polyphenols are secreted via the biliary route into the duodenum, where they are subjected to the action of bacterial enzymes, especially β-glucuronidase, in the distal segments of the intestine, after which they may be reabsorbed. This enterohepatic recycling may lead to a prolonged presence of polyphenols in the body(Reference Manach, Scalbert, Morand, Rémésy and Jiménez11).
The balance between sulfation and glucuronidation of polyphenols seems to be affected by species, sex, and food deprivation. Competitive inhibition of conjugation could also occur in the presence of several polyphenols and xenobiotics in the intestine(Reference Manach, Scalbert, Morand, Rémésy and Jiménez11). Dietary components are also known to influence drug metabolism in humans. Diets containing cruciferous vegetables may induce glucuronidation while paracetamol and oxacepam glucuronidation have been shown to be enhanced in subjects fed with a diet containing cabbage and Brussels sprouts(Reference Miners and Mackenzie15). Other factors that may affect drug glucuronidation in humans are age, cigarette smoking, disease, genetic factors and ethnicity. Critchley et al. (2005) showed that Chinese subjects excreted 6 % more sulfate and 5 % less glucuronide than Caucasian subjects after oral ingestion of paracetamol(Reference Critchley, Critchley, Anderson and Tomlinson17). It is not yet known, however, whether these differences are purely genetic or due to lifelong dietary habits modifying the enzymic pathways that affect polyphenol metabolism and excretion.
In many countries, one of the main ways of consuming cocoa is in preparations containing cocoa (called cocoa powder). Spain is the country with the highest consumption of cocoa powder in the world (1·7 kg/person per year), this product being present in more than 80 % of Spanish households with children and it represents the main source of flavonoids in the young population (children and teenagers < 15 years). In fact, cocoa powder consumption (especially consumed during breakfast) with milk represents about 50 % of the daily total flavonoid intake. Other countries with similar consumption of such products are Norway, Sweden, France, Brazil, Austria and Australia(Reference Roura, Andres-Lacueva, Estruch, Mata-Bilbao, Waterhause and Lamuela-Raventos18). Thus, the present study aims to observe the effect of milk on urine excretion of flavonoid metabolites after the intake of a standard portion of cocoa powder, a polyphenol-rich food.
Material and methods
Materials
Reagents were obtained from the following sources: methanol and acetonitrile (HPLC grade) from Scharlau (Barcelona, Spain); o-phosphoric acid from Panreac (Barcelona, Spain); formic acid from Sigma (Steinheim, Germany). Standards were obtained as follows: ( − )-epicatechin (( − )-Ec) from Sigma (St Louis, MO, USA); taxifolin from Extrasynthese (Genay, France); creatinine from Fluka (Seelze, Germany).
All the chemicals used were of analytical or chromatographic grade. The water was purified in a Milli-Q water purification system (Millipore, Molsheim, France). Working standard solutions were filtered with Waters 4 mm polytetrafluoroethylene (PTFE) 0·45 μm (Waters, Milford, MA, USA) filters before being injected into the column and the extraction cartridges were Waters OasisTM hydrophilic–lipophilic-balanced (HLB) 3 cc (60 mg).
Subjects and study design
Twenty-one non-smoking healthy volunteers (nine women and twelve men) with an average age of 25·7 (sd 6·9; range 18–50) years and with an average BMI of 21·59 (sd 2·1; range 19·1–27·7) kg/m2 were selected. None of them reported any history of heart disease, homeostatic disorders or other medical conditions. None of the subjects was receiving any medication or taking any vitamin supplements. The Institutional Review Board of the Hospital Clinic in Barcelona approved the study protocol and all the volunteers gave informed, written consent before they were included in the trial.
The study was an open, prospective, randomised cross-over clinical trial. Participants were instructed to abstain from alcoholic beverages and any polyphenol-rich foods for at least 48 h before the study as well as during the day of the study. The day before the study, all the participants were given two menus and a list showing permitted and forbidden foods in order to help them follow the polyphenol-free diet correctly. The subjects fasted for at least 8 h before consuming the test meals.
On three different days (with a 1-week interval between them), using a cross-over experimental design, the twenty-one subjects received the three different meals in a random order: (1) cocoa beverage containing 40 g cocoa powder (Nutrexpa, Barcelona, Spain) and 250 ml whole milk (CC–M); (2) cocoa beverage containing 40 g of the same cocoa powder (Nutrexpa) and 250 ml water (CC–W); (3) 250 ml whole milk as a control. In order to avoid differences in the rate of stomach emptying (which in turn would influence absorption kinetics) sugar was added to balance energy content, thus making the three test meals isoenergetic. Test meals were prepared on each day of the study following a standardised procedure. The CC–M and CC–W macronutrient composition (in 250 ml) was: carbohydrates, 30·75 and 58·4 g; fat, 10·91 and 2·16 g; protein, 13·54 and 5·64 g; energy, 1152 kJ (275·35 kcal) and 1158 kJ (276·6 kcal) respectively. Flavonoid composition was as follows: ( − )-Ec, 28·2 mg; procyanidin B2, 25·5 mg; (+)-catechin, 8·4 mg; flavonols, 2 mg. The flavonols included isoquercitrin, quercetin, quercetin-3-glucoside and quercetin-3-arabinoside. Phenolic compounds were not detected in the milk used.
Urine samples were obtained before consumption and during the 0–6, 6–12 and 12–24 h periods after test meal consumption. The volunteers remained in the clinical ward for over 6 h to avoid the possibility of transgressing the proscribed diet in the first study period. For the remaining 18 h, all the volunteers followed a standardised polyphenol-free diet (as they had done the day before the study).
Urine metabolite content determination by liquid chromatography–mass-spectrometry/mass spectrometry analysis
After collection and storage of urine samples, they were treated and analysed as described in a previous study(Reference Roura, Andres-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos19) with some modifications. Briefly, 1 ml homogenised urine was subjected to a solid-phase extraction procedure (SPE) with a Waters OasisTM hydrophilic–lipophilic-balanced (HLB) ninety-six-well SPE plate (30 mg), preconditioned with 1 ml methanol and equilibrated with 1 ml of 1·5 m-formic acid. The plate was washed with 1 ml of 1·5 m-formic acid and 1 ml of 5 % methanol in water. The analytes were collected in a ninety-six-well collection plate by elution with 1·5 ml methanol containing formic acid (1 ml/l). The eluate was evaporated to dryness using a Techne sample concentrator (Duxford, Cambs, UK) at 30°C under an N2 stream. The residue was reconstituted with a sample of 100 μl taxifolin dissolved in mobile phase as additional internal standard to assess the performance of the mass spectrometer. It was then vortexed briefly and left in a refrigerated autosampler for HPLC–MS/MS analysis.
A triple quadrupole mass spectrometer was used for the analysis and quantification of ( − )-Ec metabolites. Liquid chromatography analyses were performed using a Perkin Elmer series 200 (Norwalk, CT, USA) with a quaternary pump. An API 3000 triple quadrupole mass spectrometer (Perkin-Elmer Sciex, Concord, ON, Canada) equipped with a Turboionspray source in a negative ion mode was used to obtain the MS and MS/MS data. The column that was selected for the analyses was a Luna C18 column (50 × 2 mm internal diameter, 5 μm) (Phenomenex, Torrance, CA, USA).
The MS/MS analyses were rigorous and described in detail in a previous study(Reference Roura, Andres-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos19). Briefing, a urine sample from 6 h after the cocoa beverage intake was investigated using liquid chromatography–MS/MS in multiple-reaction monitoring mode to check the traces of all cocoa ( − )-Ec metabolites described in the literature; ( − )-Ec (m/z 289/245), O-methyl-epicatechin (m/z 303/289), ( − )-Ec-glucuronide (m/z 465/289), O-methyl-( − )-Ec-glucuronide (m/z 479/289), ( − )-Ec-sulfate (m/z 369/289), sulfated-O-methyl-epicatechin (m/z 383/289), sulfate-epicatechin-glucuronide (m/z 545/289) and sulfate-O-methyl-epicatechin-glucuronide (m/z 559/289), to identify the metabolites and to establish their retention time. Once identified, the urine metabolites were confirmed in a second experiment, a product-ion scan, which only confirmed the sulfate (m/z 369/289) and glucuronide (m/z 465/289) metabolites.
Statistical analysis
All statistical analysis was performed using SPSS for Windows software, version 11·5 (SPSS Japan Inc., Tokyo, Japan). A two-tailed paired t test was used to compare ( − )-Ec metabolite concentrations excreted during the 0–6, 6–12 and 12–24 h periods after the intake of the test meals. Differences between the three meals were also studied by analysis of covariance using general linear models; baseline values and sex were used as covariates. Significance was recognised at P < 0·05 and the variables are presented as arithmetic means and standard deviations.
Results
( − )-Ec metabolites excreted are expressed per g creatinine excreted in urine instead of per litre (volume) of urine, i.e. μg ( − )-Ec/g creatinine. We used the classical Jaffé alkaline picrate method to determine the amount of creatinine in the urine(Reference Roura, Andres-Lacueva, Estruch and Lamuela-Raventos20).
Quantitative analysis of (−)-epicatechin metabolite excretion in urine
Four ( − )-Ec metabolites were detected in the urine samples collected during the 0–6, 6–12 and 12–24 h periods after the ingestion of the two cocoa beverages (CC–W and CC–M). One ( − )-Ec-glucuronide (( − )-Ec-G) and three ( − )-Ec-sulfates (( − )-Ec-S) were identified and confirmed in the urine samples (Table 1). The following names were assigned to the three ( − )-Ec-S)detected: ( − )-Ec-S1, ( − )-Ec-S2 and ( − )-Ec-S3, where the numbers 1, 2 and 3 correspond to the order of appearance in the liquid chromatography analysis, number 1 being the most polar sulfate and number 3 the least. Both before consumption of the test meals and after consumption of the control meal (milk, control), no ( − )-Ec urine metabolites were detected in any of the subjects.
Table 1 (−)-Epicatechin ((−)-Ec) metabolite concentrations in the three urine excretion periods after intake of the two cocoa beverages*
(Mean values and standard deviations)
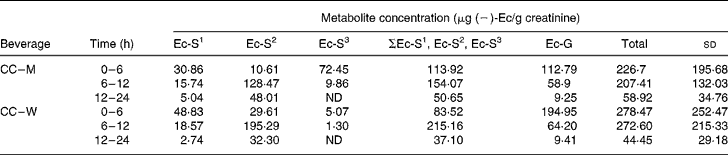
Ec-S1, epicatechin-sulfate 1; Ec-S2, epicatechin-sulfate 2, Ec-S3, epicatechin-sulfate 3; ΣEc-S1, Ec-S2, Ec-S3, sum of the three detected sulfates; Ec-G, epicatechin-glucuronide; Total, sum of the four excreted metabolites; CC–M, 40 g cocoa powder dissolved in 250 ml whole milk; CC–W, 40 g cocoa powder dissolved in 250 ml water; ND, not detected.
* For details of subjects and procedures, see Materials and methods.
After statistical analysis of the data applying a significance test (paired t test) for each treatment (CC–M and CC–W) and excretion time, we observed that there were no significant differences in total metabolite excretion (considering the sum of all metabolites) between the two treatments for any of the time periods (P < 0·05; all). However, when we considered the metabolite profile (the qualitative data) as shown in Fig. 1, we observed that even though milk does not modify the total excretion of ( − )-Ec metabolites, it modifies the ( − )-Ec metabolite excretion profile. Although the maximum concentration of total metabolites excreted was observed during the 0–6 h period after intake of both CC–W and CC–M, statistically there were no differences between total excretion during the 0–6 h and during the 6–12 h periods. Thus, after consumption of the cocoa beverage, with milk or with water, the maximum concentration of metabolites excreted in urine occurs between 0 and 12 h after intake. During the following 12–24 h period, the metabolite concentrations returned to their base levels.
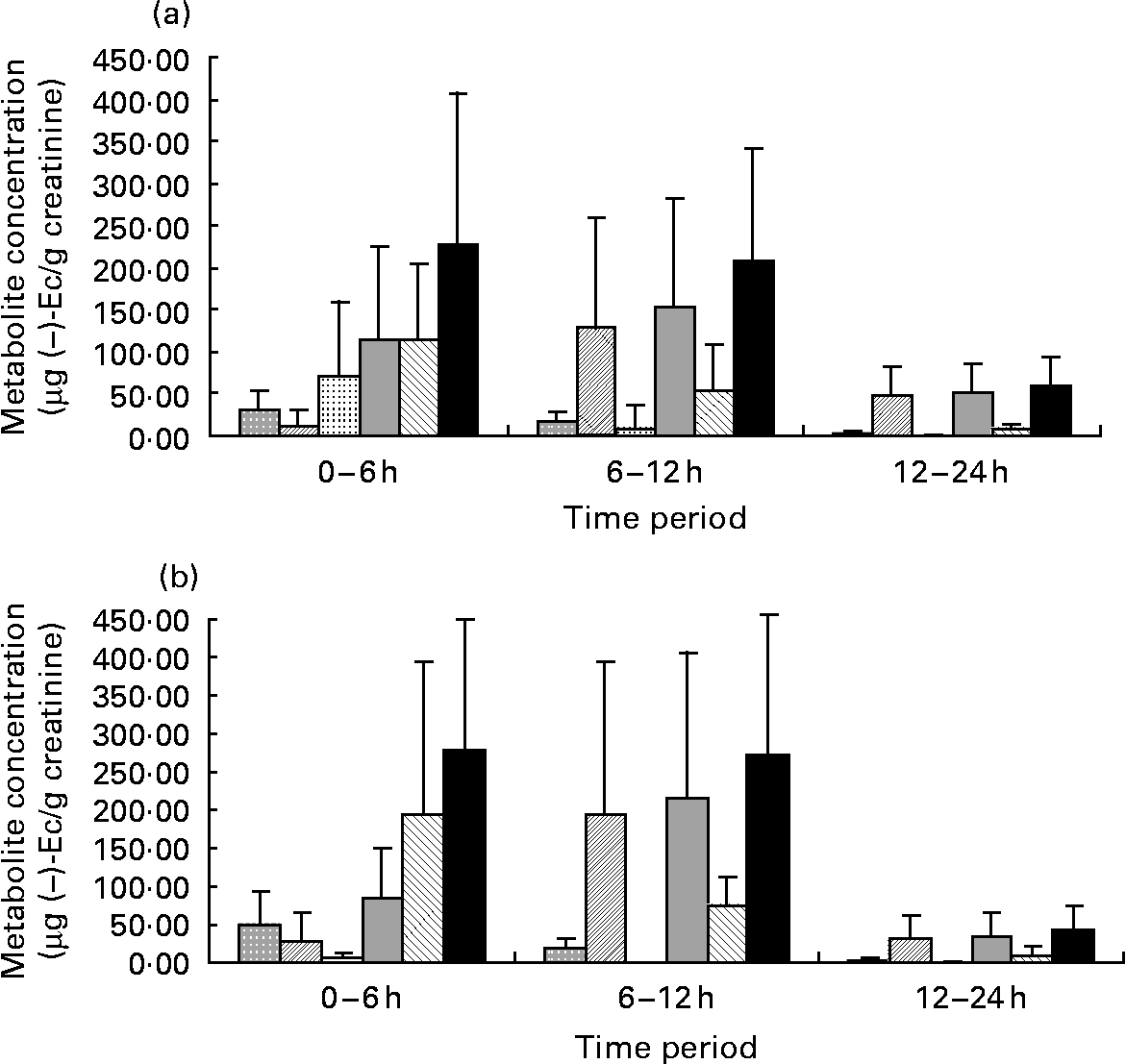
Fig. 1 Graphic representations of ( − )-epicatechin (( − )-Ec) metabolite concentrations (μg ( − )-Ec/g creatinine) during the three urine excretion periods after intake of the two cocoa beverages: 40 g cocoa powder dissolved in 250 ml whole milk (a) and 40 g cocoa powder dissolved in 250 ml water (b). Values are means, with standard deviations represented by vertical bars. (![]() ), Epicatechin-sulfate 1; (
), Epicatechin-sulfate 1; (![]() ), epicatechin-sulfate 2; (
), epicatechin-sulfate 2; (![]() ), epicatechin-sulfate 3; (
), epicatechin-sulfate 3; (![]() ), sum of the three detected sulfates; (
), sum of the three detected sulfates; (![]() ), epicatechin-glucuronide; (■), sum of the four excreted metabolites.
), epicatechin-glucuronide; (■), sum of the four excreted metabolites.
The most interesting finding, however, did not concern the total metabolite excretion, but rather the excreted metabolite profile. Up until 6 h after CC–W intake, ( − )-Ec-G is the main metabolite observed (Fig. 1 (a)), followed by ( − )-Ec-S1 and ( − )-Ec-S2. In this case, the sum of the sulfates does not reach half of the excreted ( − )-Ec-G concentration. In contrast, although up until 6 h after the intake of the cocoa with milk beverage (CC–M), ( − )-Ec-G is still the main excreted metabolite (see Fig. 1 (b)), the sum of excreted sulfates has the same concentration: 6 h after the intake of the cocoa with milk beverage, the same quantity of glucuronide and sulfate metabolites had been excreted.
Another difference in metabolite excretion after the meals is that at 0–6 h after CC–M intake, ( − )-Ec-S3 was the main sulfate, whereas it was barely present in the urine samples corresponding to CC–W after the same time period.
During the period 6–12 h after the ingestion of both cocoa meals, the main excreted metabolite was ( − )-Ec-S2, followed by ( − )-Ec-G, although the latter at much lower concentrations. Therefore, there is a change in the excretion profile: while from 0–6 h after consumption the main excreted metabolite was ( − )-Ec-G, 6–12 h after intake the main metabolite was ( − )-Ec-S2. During both the 6–12 h period and the 0–6 h period, urine samples corresponding to the CC–M treatment contained the ( − )-Ec-S3 metabolite, which was not detected in the urine samples corresponding to the CC–W treatment during the same periods.
For both treatments, after 24 h, ( − )-Ec-S2 is still the main metabolite (despite low concentrations, < 50 μg/l) followed by small traces of ( − )-Ec-G ( < 15 μg/l).
Discussion
The pharmacokinetics of ( − )-Ec and its metabolites in human plasma after consumption of the same cocoa beverages have been studied in a previous study where we did not find significant differences between the amounts of ( − )-Ec metabolites found in plasma 2 h after CC–M and CC–W consumption(Reference Roura, Andres-Lacueva, Estruch, Mata-Bilbao, Waterhause and Lamuela-Raventos18). However, the present study reports the urinary excretion of ( − )-Ec and its metabolites after CC–M and CC–W consumption during periods 0–6, 6–12 and 12–24 h after consumption in the human subjects. While the total amount of ( − )-Ec and its metabolites excreted by urine did not change after CC–M and CC–W consumption, the metabolite profile differed. We observed that milk affects flavonoid metabolism pathways, increasing sulfation compared with glucuronidation in the first 6 h excretion period.
Other studies have observed that matrices may affect excretion of polyphenols. Donovan et al. (2002) reported that 20 % more catechin metabolites were excreted in urine over a period of 8 h after the intake of red wine compared with dealcoholised red wine, indicating that ethanol enhances the rate of catechin elimination. However, alcohol did not significantly affect the percentage of the individual conjugate forms(Reference Donovan, Kasim-Karakas, German and Waterhouse21). Urine volume was increased by 17 % after consuming wine with ethanol, suggesting that this increase is due to a diuretic effect(Reference Yamashita, Sakane, Harada, Sugiura, Koda, Kiso and Sezaki7). On the other hand, Goldberg et al. (2003) investigated the total urinary excretion of resveratrol, catechin and quercetin after consumption in three different matrices (grape juice, white wine and vegetable homogenate) and showed that 24 h resveratrol urinary excretion after oral consumption did not show any matrix effect, while catechin excretion varied with the matrix (being higher when consumed with wine). No changes were observed in the excreted metabolite profiles due to the matrix effect(Reference Goldberg, Yan and Soleas10), because they also determined polyphenol excretion after urine enzymic treatment with β-glucuronidase and sulfatase, and the individual metabolite forms were thus lost.
Polyphenols can interact with proteins that might decrease the bioavailability of these antioxidants. The interaction, resulting in protein–polyphenol complexes, can be either reversible or irreversible depending on pH, temperature, type of protein and flavonoid concentrations. The fate of these complexes in the gastrointestinal tract is not known(Reference Donovan, Kasim-Karakas, German and Waterhouse21). Serafini et al. (1996) found that the addition of milk to black tea negates the increased antioxidant potential observed when tea is consumed without milk, explaining that the mitigation of this effect by milk is thought to be due to the interaction of tea polyphenols with milk proteins(Reference Serafini, Ghiselli and Ferro-Luzzi8). However, Arts et al. (2002) showed no relevant effect of proteins on the bioavailability or on the antioxidant capacity of tea polyphenols(Reference Arts, Haenen, Wilms, Beetstra, Heijnen, Voss and Bast22) and Shroeter et al. (2003) described that the presence of milk in cocoa products does not counteract the absorption and biological activity of monomeric flavanols from cocoa products, nor does it affect plasma antioxidant capacity(Reference Schroeter, Holt, Orozco, Schmitz and Keen23).
Drug metabolism is categorised in two phases; phase I for chemical modification such as oxidation, reduction and hydroxylation, and phase II for conjugations such as glucuronidation, methylation and sulfation at specific functional groups. The ultimate goal of hepatic metabolism is detoxification and the conversion of xenobiotics into more soluble or hydrophilic forms to facilitate excretion(Reference Chan, Cui, Xu and Cai14). The present study provides no information on the mechanisms involved or their efficiency when these compounds enter the enterocyte for hydrolysation and later enter the hepatocyte to be conjugated and reconjugated.
Antioxidant activity of polyphenols is directly related to their chemical structure, thus the antioxidant capacity of their metabolites would be very different. Several studies of the structure–activity relationship on radical-scavenging activity of flavonol-type flavonoids revealed that the o-dihydroxyl structure in the B ring is mostly responsible for their radical-scavenging activity. Yamamoto et al. (1999) described an increasing order of inhibition of 2,2′-azobis (2-amidinopropane) dihydrochloride-induced lipid peroxidation in LDL by different quercetin metabolites: Q4′G < isorhamnetin < Q3G < rhamnetin = Q7G = quercetin, confirming that the o-dihydroxyl structure in the B ring is mostly responsible for scavenging free radicals involved in lipid peroxidation of LDL. This implies that the capacity of free-radical scavenging of quercetin is significantly lowered by the introduction of a substituents group into the o-dihydroxyl structure in the B ring(Reference Yamamoto, Moon, Tsushida, Nagao and Terao24). Natsume et al. (2004) described that the ( − )-Ec-G metabolites have different antioxidant activities; they described that concentrations of 42·9 μm-Ec–7-glucuronide and Ec-3-glucuronide caused 61 and 20 % inhibition of thiobarbituric acid-reactive substance production; thus the position of the conjugated position can modify the antioxidant capacity of the metabolites(Reference Natsume, Osakabe, Yasuda, Baba, Tokunaga, Kondo, Osawa and Terao25).
It is evident, however, that ( − )-Ec is subjected to glucuronidation and sulfation. The enzymes involved in the synthesis of these metabolites are glucuronosyltransferase and sulfotransferase, which are also found in the human intestine. It is, therefore, feasible that all the ( − )-Ec metabolites that appear in plasma are the result of conversions that occur in the lumen of the small intestine. Once these metabolites have been produced in the small intestine, they pass into the portal vein and are further converted and reconjugated because human hepatocytes contain glucuronyl-, sulfo- and methyltransferases as well as β-glucuronidase activity(Reference Mullen, Edwards and Crozier26). Ex vivo incubation of quercetin-3-glucuronide with human hepG2 hepatonoma cells results in cleavage of the glucuronide moiety and the formation of quercetin-3′-sulfate(Reference O'Leary, Day, Needs, Mellon, O'Brien and Williamson27). Milk does not have any effect on the absorption of ( − )-Ec in cocoa powder, because 2 h after cocoa consumption, the only metabolite found was ( − )-Ec-G. However, differences were observed in the metabolite profile of urine; milk may therefore alter some enzymic pathways. Further investigation is necessary to understand possible milk interactions in phase II drug metabolism clearly.
The present study shows for the first time that the matrix does affect the metabolic phenolic profile. The present study provides detailed quantitative information regarding concentrations of glucuronyl- and sulfo-conjugates, ( − )-Ec metabolites, in the urine of human subjects after ingestion of a standard portion of cocoa powder dissolved in water or milk. These data should enable better and more relevant studies of the bioactivity and role of dietary flavanols in their possible interaction with other food or food components. The excreted metabolite profile is affected when the cocoa powder is consumed with milk, which seems to induce the excretion of sulfates before glucuronides. The position of sulfation is also affected. However, further studies are necessary to elucidate the possible biological consequences of these differences.
Acknowledgements
The authors wish to thank all the volunteers involved in the study. The authors' responsibilities were as follows: E. R., C. A.-L. and M. L. M.-B. conceived, designed and devised the study and contributed to the analysis and writing of the manuscript. R. M. L.-R. and R. E. analysed the data and supervised the study. R. E. collected the samples and supervised the analysis. M. I.-P. obtained funding; all authors revised and approved the manuscript. None of the authors had a conflict of interest.
This present study was supported by grants from the Fundació Bosch i Gimpera FBG-302218, Nutrexpa S.A., CDTI (P-02-0277), PROFIT (FIT-060000-2002-99) and CICYT (AGL2004-08 378-C02-01/02). E. R. is supported by a UB Recerca i Docència fellowship.






