Optimal growth and skeletal development during childhood and young adulthood is crucial for avoiding low bone mass and osteoporosis later in life. The influence of dietary protein on bone status has been debated for decades, but remains controversial. Different study designs have been used to investigate this, but conflicting results have been reported( Reference Cao and Nielsen 1 – Reference Jesudason and Clifton 4 ). Experimental studies on the effects of dietary protein on Ca excretion and absorption have been carried out in adults. Based on these studies, high protein intake, especially that of animal origin, has been hypothesised to affect bone mineralisation adversely by increasing bone resorption and thereby urinary Ca excretion( Reference Hegsted and Linkswiler 5 – Reference Zemel, Schuette and Hegsted 10 ). However, some studies in adults( Reference Cao, Johnson and Hunt 11 – Reference Kerstetter, O'Brien, Caseria and Wall 14 ), but not all( Reference Hegsted and Linkswiler 5 , Reference Hegsted, Schuette and Zemel 6 , Reference Allen, Oddoye and Margen 15 , Reference Johnson, Alcantara and Linkswiler 16 ), have shown compensatory increased Ca absorption with increasing intake of dietary protein. When looking at measures of bone status, observational studies in adults( Reference Darling, Millward and Torgerson 2 , Reference Skov, Haulrik and Toubro 17 ) and children( Reference Alexy, Remer and Manz 18 – Reference Vatanparast, Bailey and Baxter-Jones 22 ) and protein supplementation trials in adults( Reference Darling, Millward and Torgerson 2 ) have shown a small positive effect of dietary protein on bone status. In a 7 d intervention study in 8-year-old boys, Budek et al. ( Reference Budek, Hoppe and Michaelsen 23 ) found that a high intake of protein from milk, but not from meat, decreased bone turnover as measured by serum osteocalcin and serum C-terminal telopeptides of type I collagen. So far, no long-term trial in children has been conducted to assess the effect of dietary protein on bone turnover or bone status.
Bone turnover can be assessed by biomarkers in the blood and urine. Osteocalcin is a non-collagenous extracellular matrix protein produced by osteoblasts. It contains three glutamic acid residues, which are post-translationally carboxylated to increase their affinity for mineral ions. In contrast, partial or no carboxylation makes osteocalcin more susceptible to be released from osteoblasts into the circulation( Reference Bugel 24 ). Traditionally, osteocalcin measured in serum or plasma has been considered as a marker of bone formation. However, genetic knockout studies have indicated no direct relationship between osteocalcin and mineral deposition events, but have rather shown that osteocalcin participates in the regulation of the mineralisation process( Reference Gundberg 25 ). Urinary N-terminal telopeptide of collagen type I (U-NTx) is a breakdown product released during the resorption of bone, and is used as a marker of bone resorption. Biomarkers of bone formation and resorption are normally closely related, and the balance between them may reflect whether a higher turnover results in increased or reduced bone mass.
The primary aim of DiOGenes (Diet, Obesity and Genes), a large-scale, European randomised intervention trial, was to examine the effects of diets varying in protein content and glycaemic index (GI) on weight maintenance in adults after a weight-loss period. However, the children of these adults were also included in the study. To assess whether a high-protein diet could be detrimental to bone health in children, the bone markers osteocalcin and U-NTx were analysed in the children's blood and urine samples, respectively. The possible positive effects of protein on body-weight regulation and the risk markers of CVD in adults( Reference Hu 26 ) should be weighed up against concerns about the safety of high-protein diets. The question then arises: what about the GI part of the DiOGenes study – does that mean anything to bone health? Since the initiation of the study, several studies have examined the connection between energy metabolism (including insulin signalling) and bone metabolism( Reference Ng 27 ). Looking at some of the findings in these studies, we postulate that a diet with a low GI might benefit not only body-weight regulation, but also bone growth.
The aim of the present paper was to examine the effects of dietary protein and GI on bone turnover based on blood (osteocalcin) and urine (U-NTx) analyses in children from two of the participating centres in the DiOGenes study. To elucidate the relationship between osteocalcin/U-NTx and bone growth, we also examined the relationship between the baseline levels of osteocalcin and U-NTx and the following changes in height across dietary groups, and examined the relationship between dietary group and changes in height during the intervention. All analyses presented in the study are post hoc analyses.
Experimental methods
Experimental design
Children and their parents were enrolled at eight centres across Europe. In the present study, only data from the centres in Copenhagen and Maastricht were included. These two centres (the so-called ‘shop centres’) did run a more strictly controlled version of the intervention, providing all families with foods for free from specially designed shops, and dietary data indicated that the intervention was only successful among children at these centres.
The study was conducted according to the guidelines in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethical committees in the respective countries. Written informed consent was obtained from all custody holders of the child and from the child, when considered mature enough to understand the procedure. During the screening visit, children and their parents were asked to choose between participation in all planned examinations (‘full’ protocol) or only take part in some of the examinations, excluding blood and urine samples. Only children accepting the full protocol were included in the present study. The trial was registered in the Clinical Trials database (ClinicalTrials.gov no. NCT00390637).
In brief, families with at least one child aged 5–18 years and one or two overweight or obese parents reaching an initial weight loss of ≥ 8 % of their body weight after an 8-week low-energy diet (3347 kJ (800 kcal)) were randomised to one of five intervention diets for 6–12 months: low protein (LP)/low GI (LGI); LP/high GI (HGI); high protein (HP)/LGI; HP/HGI; control. The randomisation was stratified according to centre, the number of eligible parents in each family and the number of parents with a BMI>34 kg/m2 in each family. The five intervention diets were all ad libitum (no restriction on total energy intake), low-fat (25–30 E%) diets. The target dietary differences were 15 GI units between the LGI and HGI diets and 13 E% points from protein between the LP (10–15 E%) and HP (23–28 E%) diets. Families randomised to the control diet were instructed to eat according to some general dietary guidelines: eat fruit and vegetables several times per d; eat fish several times per week; eat potatoes, rice or pasta and whole-grain bread every day; limit the sugar intake especially from liquids, candy and cakes; eat less fat especially from dairy products and meat; eat varied food and keep the weight stable. The dietetic counselling was focused on fat quality and amount, and less on carbohydrate intake and sources, to prevent the control group from becoming just another LP/LGI group.
The participating families were provided with free foods from a specially designed shop during 6 months of intervention. For more details about the study design and the dietary intervention strategies used, see Larsen et al. ( Reference Larsen, Dalskov and van Baak 28 ) and Moore et al. ( Reference Moore, Lindroos and Kreutzer 29 ).
At baseline, two examination days were planned for the children: one before and one after their parents' low-energy diet. For logistic reasons, the majority of children had these two visits combined in one visit around the scheduled second examination day. In the present study, the term ‘baseline’ refers to latest of the two visits, whenever two separate visits were made. In addition to the baseline visit, examinations were scheduled for 1 month and 6 months after the start of the intervention.
Study subjects
Children were excluded from the study if they used prescription medication, suffered from diseases or conditions that might influence the outcome of the study, followed a special diet (e.g. vegetarian or lactose free) or practised elite sports. Children with data from baseline and from at least one of two subsequent visits (month 1 or month 6) were included in the present analyses.
Examinations
Examinations were carried out in the morning after the child had fasted (except for 350–500 ml water) for at least 4 h. Height (to the nearest 0·5 cm) and body weight (to the nearest 0·1 kg) were recorded at each examination day. Children were weighed wearing light clothing. Sex- and age-specific z-scores for height and BMI were calculated using WHO AnthroPlus software( Reference de, Onyango and Borghi 30 , 31 ).
On the examination days, the children delivered a spot urine sample, avoiding the first morning urine. A blood sample was drawn from an antecubital vein. It was not possible to perform blood sampling and urine collection at exactly the same hour in the morning each time a child came in for examination. For the analysis of osteocalcin, Li-heparinised blood was centrifuged within 1 h after collection at 2500 g for 15 min at 4°C, and plasma was stored at − 80°C until analysis. Osteocalcin was measured on an Immulite 2500 using a solid-phase, two-site chemiluminescent immunometric assay (Siemens Medical Solutions Diagnostics, DPC Scandinavia). U-NTx was analysed using an ELISA (Osteomark NTx Urine kit; Wampole Laboratories, Orion Diagnostica). Urinary creatinine was measured by a colorimetric assay on a Vitros 950 analyser (Ortho-Clinical Diagnostics, Johnson & Johnson Medical). To adjust for the concentration of the urine, U-NTx, expressed in nm-bone collagen equivalents, was divided by urinary creatinine in mm. Intra- and inter-assay CV were 3·5 and 5·4 % (osteocalcin) and 4·0 and 7·6 % (U-NTx), as reported by the manufacturer. No information was available for creatinine.
Children and their parents were instructed to register the dietary intake of the children for three consecutive days (two weekdays and one weekend day) at baseline, month 1 and month 6. They were equipped with weighing scales (Soehnle 1208 Actuell Backnang; Leifheit AG), and were instructed to weigh all foods and beverages consumed during the registration periods and to provide cooking methods and recipes for composite meals. When weighing was not possible, the children and their parents were instructed to record the dietary intake in household measures. If the children were not able to perform the dietary registrations themselves, their parents were asked to assist them. The principles of analysis of dietary records in DiOGenes have been described elsewhere( Reference Larsen, Dalskov and van Baak 28 , Reference Aston, Jackson and Monsheimer 32 ). Intakes of protein, carbohydrates and fat were expressed as E%. Since energy intake is dependent on sex, age and body size, it was evaluated relative to an estimated BMR calculated using the formulas suggested by Henry( Reference Henry 33 ).
Statistical methods
Children with data from baseline and from at least one later visit (month 1, month 6 or both) were included in the present analyses.
Baseline characteristics are presented for children included in the analyses of osteocalcin, U-NTx and either or both of these. For children whose 6-month data were available, changes in height and BMI z-score over this 6-month period are also given.
Dietary intake was compared at baseline, month 1 and month 6 between the five dietary groups. Raw data are presented as medians and interquartile ranges. Data were analysed using ANCOVA, with centre as a random effect detecting variations between centres. Outcomes were transformed if necessary to meet model requirements. P values based on likelihood ratio tests are reported for the overall group effect; in addition, P values, estimates and 95 % CI are given for selected pairwise comparisons.
ANCOVA was used for evaluating differences between diets over time. Initially, the effects of dietary protein and GI were assumed to modify how the levels of osteocalcin and U-NTx have changed linearly over time since randomisation (effect modification). To evaluate whether diet effects were modified by sex, an additional sex × diet interaction term was included in the model. To adjust for anticipated child-specific differences, baseline values of the bone markers, BMI z-score at each of the time points and sex-specific linear and quadratic trends in age were included in the model. The adjustment for the BMI z-score in the present analyses was made so that the results were not primarily caused by differences in weight change based on the different diets. Cluster effects were addressed by means of random effects that were included for children, families and centres. Thus, multi-level linear-mixed ANCOVA models were used. Model checking was based on residual plots and normal probability plots. If needed, data were logarithmically transformed to meet model assumptions and, subsequently, estimates were transformed to the original scale. Likelihood ratio tests were used to assess the combined effects and interaction terms, whereas approximate t tests were used for pairwise comparisons between the dietary groups. Adjustment for multiple P values was based on the single-step method( Reference Hothorn, Bretz and Westfall 34 ). Likewise, a linear-mixed ANCOVA model was used to examine whether diet influenced height. However, no adjustment for the BMI z-score over time was made since height is an integral part of BMI. Finally, a linear model was used to investigate whether baseline levels of osteocalcin and U-NTx could predict height at month 6 across all the dietary groups, when adjusting for height at baseline. Estimates and 95 % CI for significant effects of the linear relationships are reported.
The significance level was set at P< 0·05 (two-sided). The statistical environment R version 2.15.1( 35 ) and, in particular, the extension packages lme4 and multcomp, as well as STATA 12.0( 36 ) were used for the analyses.
Results
Data from 191 children were included in the present paper: n 67, osteocalcin analyses; n 180, U-NTx analyses; n 56, both the analyses. The progress of the study participants from screening to month 6 and the selection criteria for the analyses of osteocalcin are illustrated in Fig. 1.

Fig. 1 Flow diagram illustrating the progress of the study participants from screening to month 6, and the selection criteria for the analyses of osteocalcin. LED, low-energy diet; LP, low protein; LGI, low glycaemic index; HGI, high glycaemic index; HP, high protein.
Characteristics
Baseline characteristics and 6-month changes in height-for-age z-scores and BMI-for-age z-scores are presented in Table 1 for the groups of children included in the analyses of either osteocalcin or U-NTx, osteocalcin, U-NTx or both. The median BMI-for-age z-score for the children included in either of the two analyses was 1·13, which was above the cut-off (1·0) for overweight according to the WHO growth reference( Reference de, Onyango and Borghi 30 ). Having a median height-for-age z-score of 0·77, the children were not only thicker, but also taller than the WHO growth reference. None of the children was underweight, which is defined as a BMI-for-age z-score less than − 2, and none of the children was stunted that is defined as a height-for-age z-score less than − 2. Median changes in the height-for-age z-score during the 6-month intervention period were close to 0, and thus it could be considered within normal limits. Baseline median values of osteocalcin and U-NTx for boys and girls at different ages are given in Figs. 2 and 3, respectively. The levels of the biomarkers of bone turnover were lowest among the oldest children.
Table 1 Characteristics of the study participants included in the analyses of either osteocalcin or urinary N-terminal telopeptide of collagen type I (U-NTx), osteocalcin, U-NTx or both (Number of participants, median values and interquartile ranges (IQR))
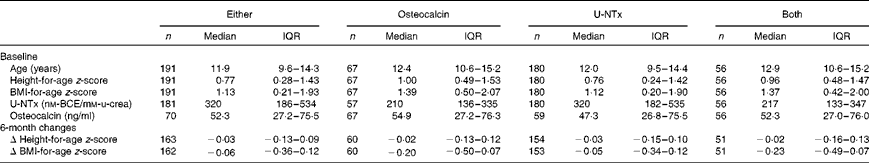
BCE, bone collagen equivalents; u-crea, urinary creatinine.
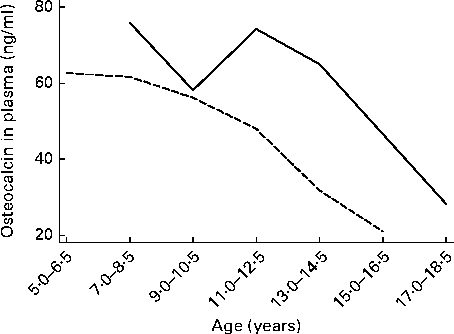
Fig. 2 Median values of osteocalcin for boys (![]() ) and girls (
) and girls (![]() ) at different ages. Subjects were categorised into seven age categories due to the low number of subjects at some ages.
) at different ages. Subjects were categorised into seven age categories due to the low number of subjects at some ages.
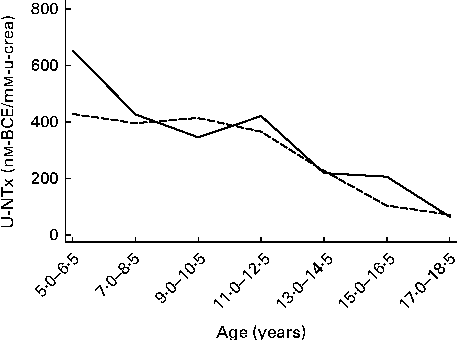
Fig. 3 Median values of urinary N-terminal telopeptide of collagen type I (U-NTx) for boys (![]() ) and girls (
) and girls (![]() ) at different ages. Subjects were categorised into seven age categories due to the low number of subjects at some ages. BCE, bone collagen equivalents; u-crea, urinary creatinine.
) at different ages. Subjects were categorised into seven age categories due to the low number of subjects at some ages. BCE, bone collagen equivalents; u-crea, urinary creatinine.
Dietary intakes
Among the included children, 85, 83 and 45 % registered their dietary intake at baseline, month 1 and month 6, respectively. For the four dietary groups whose baseline characteristics are given in Table 1, these numbers varied from 85 to 91 % at baseline, 82 to 85 % at month 1 and 44 to 54 % at month 6.
Dietary intakes in the different dietary groups were not different at baseline (Table 2). Dietary GI was higher at both month 1 (8·3 (95 % CI 6·1, 10·5) GI units, P< 0·001) and month 6 (7·2 (95 % CI 4·5, 9·9) GI units, P< 0·001) in the HP/HGI group compared with the HP/LGI group, while the GI was higher only at month 1 (5·8 (95 % CI 3·7, 7·8) GI units, P< 0·001) in the LP/HGI group compared with the LP/LGI group. The E% from protein was higher at both month 1 (5·2 (95 % CI 3·6, 6·7) % points, P< 0·001) and month 6 (6·3 (95 % CI 3·6, 9·1) % points, P< 0·001) in the HP/LGI group compared with the LP/LGI group, and the same was the case when comparing the HP/HGI and LP/HGI groups at month 1 (4·0 (95 % CI 2·4, 5·5) % points, P< 0·001) and month 6 (6·5 (95 % CI 3·9, 9·1) % points, P< 0·001).
Table 2 Dietary intakes at baseline, month 1 and month 6 across the dietary groups (Number of participants, median values and interquartile ranges (IQR))

EI, energy intake; LP, low protein; LGI, low glycaemic index; HGI, high glycaemic index; HP, high protein; Ctr, control; E%, percentage of energy.
a,b,cAdjusted median values within a column with unlike superscript letters were significantly different at month 1 and month 6 (P< 0·05; ANCOVA).
* BMR could not be estimated for five children at month 1 and four children at month 6 because of missing data for height and weight.
Osteocalcin
A total of sixty-seven children were included in the osteocalcin analyses (Fig. 1). Of these, fifty-four children provided follow-up data from both month 1 and month 6, nine children from month 1 only and four children from month 6 only. After 6 months of intervention, a close-to-significant change in the level of osteocalcin of − 16·5 (95 % CI − 33·7, 0·74) ng/ml (P= 0·06) was found in the HP/HGI group, whereas the corresponding change in the level of osteocalcin of 12·6 ng/ml in the LP/HGI group was not different from 0 (95 % CI − 8·2, 33·4) ng/ml (P= 0·23) (Fig. 4). Consequently, after 6 months of intervention, the overall difference in the level of osteocalcin between the HP/HGI and LP/HGI groups was 29·1 (95 % CI 2·2, 56·1) ng/ml (P= 0·034). There were no differences between the LP/HGI and LP/LGI (P= 0·45), HP/LGI and LP/LGI (P= 0·40) and HP/HGI and HP/LGI (P= 0·46) groups after 6 months of intervention. There was no effect modification of diet × sex on osteocalcin (P= 0·71).

Fig. 4 Osteocalcin over time in the different dietary groups. There was a significant difference between the low-protein (LP)/high-glycaemic index (HGI) group and the high-protein (HP)/HGI group (P= 0·034). LGI, low glycaemic index; Ctr, control; HP, high protein.
Urinary N-terminal telopeptide of collagen type I
A total of 180 children were included in the U-NTx analyses. Of these, 123 children provided follow-up data from both month 1 and month 6, thirty-seven children from month 1 only and twenty children from month 6 only. There was no effect modification of diet on U-NTx (P= 0·96).
Height
There was no effect of diet on height (P= 0·80). Baseline levels of both osteocalcin and U-NTx were strongly correlated with height at month 6, adjusted for baseline height across the dietary groups (P< 0·001 and P= 0·001, respectively). For every 10 ng/ml increase in the level of osteocalcin at baseline, children grew on average 0·3 cm more during the following 6 months, and for every 100 nm-bone collagen equivalents/mm-creatinine increase in the level of U-NTx at baseline, children grew on average 0·2 cm more during the following 6 months.
Discussion
The present sub-study of the DiOGenes study is the first randomised controlled trial to assess the effects of dietary protein and GI on bone turnover in children. The observed difference in the effects of the HP/HGI and LP/HGI diets on the bone marker osteocalcin (but not between the corresponding LGI diets) could point to a modulating effect of the GI on the effects of dietary protein on bone turnover. However, the diet had no effect on bone resorption and height.
To the best of our knowledge, only one randomised trial has investigated the relationship between dietary protein intake and bone turnover in children. It has shown that an increased intake of protein from milk during 7 d decreased bone turnover in 8-year-old boys as measured by serum osteocalcin and serum C-terminal telopeptides of type I collagen (a measure of bone resorption) when compared with a similar increase in protein intake from meat. Thus, the decrease in bone turnover was not due to protein as such, but to milk proteins or some other component in milk, e.g. Ca( Reference Budek, Hoppe and Michaelsen 23 ). However, in the present study, all children were instructed to eat or drink dairy products corresponding to 0·5 litres of milk daily, and thus the achieved difference in protein between the HP and LP groups is expected to be derived primarily from non-dairy products (meat, nuts and cereals). As in the study in 8-year-old boys by Budek et al. ( Reference Budek, Hoppe and Michaelsen 23 ), studies in postmenopausal women have not found any effect of meat protein on markers of bone turnover( Reference Cao, Johnson and Hunt 11 , Reference Roughead, Johnson and Lykken 37 ).
In an observational study of 17-year-old children, Budek et al. ( Reference Budek, Hoppe and Ingstrup 38 ) found that milk protein was positively associated with size-adjusted bone mineral content, while no association was observed for meat protein. In another observational study, Remer et al. ( Reference Remer, Manz and Alexy 39 ) found that urinary N excretion (a biomarker for protein intake) in 6 to 18-year-old children was a positive predictor of forearm bone mineral content, cortical area, strength strain index and periosteal circumference, but not of bone mineral density based on peripheral quantitative computed tomography.
Considering the apparently different effects of milk protein and meat protein on bone turnover, it cannot be excluded that a decrease in bone turnover due to the intake of dairy products is responsible for the beneficial effects of dairy protein or total protein on bone status in the aforementioned observational studies. If that is the case, then a decline in level of osteocalcin in the HP/HGI group may not be detrimental to bone health (maybe even the opposite). However, we wonder whether the observed decline in the level of osteocalcin without a corresponding decrease in the level of U-NTx indicates a decreased bone turnover, or rather an unbalanced bone turnover with a decrease in the formation part of the modelling and remodelling processes. The latter could have detrimental effects on bone health in these children.
Biomarkers of bone turnover have the advantage that they are more sensitive to short time exposure than measures of bone status. In the present study, the first post-baseline measurement was after 1 month of intervention. According to the literature, one should expect to detect changes in the measures of bone resorption before changes in the measures of bone formation. The full response is typically seen within 1–3 months for the markers of bone resorption v. within 6–9 months for those of bone formation( Reference Christenson 40 ). Thus, the lack of the effect of the dietary intervention in the present study on the bone resorption marker U-NTx cannot be due to a too short follow-up. The different mediums used to measure the levels of osteocalcin and U-NTx (blood v. urine) could be an explanation for the different results obtained for U-NTx and osteocalcin. U-NTx can be measured in both urine and blood. In the DiOGenes study, more children were willing to participate in the urine sampling than in the blood sampling, and thus the U-NTx results reflected a larger fraction of the children. However, the larger variability of measures in the urine than in the blood may offset this larger representativeness of the U-NTx data.
When comparing the levels of U-NTx and osteocalcin for age in this population with those found in other studies, the overall pattern is similar. Equivalent to the study by Mora et al. ( Reference Mora, Prinster and Proverbio 41 ), we found that the U-NTx: creatinine ratio is approximately stable between 5 and 12 years, and then after about 12 years of age, it falls abruptly. Also, the absolute values are very similar in the two populations. With regard to osteocalcin, the present dataset is too small for comparisons of the effects of sex and age with those found in other populations such as that of van der Sluis et al. ( Reference van der Sluis, de Ridder and Boot 42 ).
Results on the biomarkers of bone turnover are difficult to interpret, particularly in growing children. Concentrations cannot be directly translated into amounts of bone gained or lost, and it is not known whether the different biomarkers mainly reflect growth in size, growth in mass or both( Reference Szulc, Seeman and Delmas 43 ). A high bone turnover in late adulthood is considered unfavourable as it results in net bone loss, while in children, a high bone turnover may simply be the result of a high growth velocity. Finally, changes in measures of bone status may not even presuppose changes in biomarkers of bone turnover as indicated by a study by Cadogan et al. ( Reference Cadogan, Eastell and Jones 44 ), where a milk intervention increased bone mass accretion in 12-year-old girls without affecting bone turnover markers.
We found that baseline levels of both U-NTx and osteocalcin were strongly correlated with changes in height during the following 6 months, and thus they indeed seem to be measures of bone growth in children. However, as previously reported among DiOGenes children( Reference Budek, Hoppe and Ingstrup 38 ), diets did not affect these changes in height. Previous studies on osteocalcin( Reference van der Sluis, Hop and van Leeuwen 45 ) and U-NTx( Reference Mora, Prinster and Proverbio 41 ) have shown that these markers do not only depend on age and sex, but also on pubertal development stage. Unfortunately, pubertal status was not assessed in the present study.
In a 1-year lifestyle intervention based on exercise, behaviour and nutrition therapy in sixty obese children, Reinehr & Roth( Reference Reinehr and Roth 46 ) found a significant negative correlation between changes in total osteocalcin and changes in the homeostasis model of assessment for insulin resistance index. Since the initiation of the DiOGenes study, several studies in children have linked bone metabolism with energy metabolism( Reference Reinehr and Roth 46 – Reference Sayers, Lawlor and Sattar 52 ). Osteocalcin is among the bone turnover markers that has attracted most attention. Mechanistic studies in rodents have pointed to an endocrine bone–pancreas loop, through which insulin signalling in the osteoblasts stimulates osteocalcin production, which in turn increases pancreatic insulin secretion and insulin sensitivity to control glucose homeostasis. Thus, on the one hand, osteocalcin-deficient mice have shown decreased insulin secretion and decreased insulin sensitivity – effects that can be reversed by infusions with osteocalcin( Reference Ng 27 ), while, on the other hand, mice lacking the insulin receptor in the osteoblasts have shown reduced postnatal bone acquisition( Reference Fulzele, Riddle and DiGirolamo 53 ). Based on these studies, it would appear that not only the protein component of the DiOGenes dietary intervention may have an influence on bone metabolism, but also the GI component – through the interplay between osteocalcin and insulin. This was also what we observed in the present analyses. The effect of protein on osteocalcin was only evident within the HGI groups. It is possible that the effect of protein on osteocalcin depends on a concurrent high level of insulin. In the present study, only total osteocalcin was measured – not undercarboxylated and carboxylated osteocalcin. On the one hand, it is possible that a decrease in total osteocalcin, primarily caused by a decrease in carboxylated osteocalcin, may pose a threat to bone health. On the other hand, a decrease in total osteocalcin, primarily caused by a decrease in undercarboxylated osteocalcin, may possibly not be harmful to bone health( Reference Bugel 24 ), but could have unfavourable effects on insulin sensitivity( Reference Ducy 54 ). Future research should take into account the possible interaction with insulin, when examining the relationship between protein intake, GI and bone turnover in children.
The median intakes of about 18–20 E% protein in the HP groups were lower than that aimed for these groups (23–28 E%), while the average intakes of 14–16 E% protein in the LP groups were slightly higher or within the intended range for these groups (10–15 E%). Similarly, only approximately one-half (approximately 6–8 GI units) of the aimed difference of 15 GI units between the LGI and HGI groups was achieved, and the difference was not even significant between the LP/HGI and LP/LGI groups at month 6. Thus, the effects of more extreme intakes of protein and GI on bone turnover in children are still unknown. As usually observed in relation to dietary recording, under-reporting was very common (median energy intake:BMR 0·90–1·45). We chose measures for dietary intake that we expected to be less dependent on age and sex of the child and not to be so sensitive to general under-reporting, e.g. E% of protein, fat and carbohydrate instead of using grams. However, it is possible that the study participants were more likely to under-report certain food items than others.
In a mixed diet as used in the present study, other components than protein and the GI such as Ca, vitamin D, vitamin K, P and Na, as well as the sources of protein (dairy products/animal sources other than dairy products/vegetables) may determine whether protein and the GI influence bone turnover or not. Also, it is possible that besides the differences in dietary groups, differences in these other dietary components were actually the reason for an effect on bone markers – not GI or total protein per se. However, we did not find that 3 d dietary records were sufficient to determine protein sources and intakes of specific micronutrients, and for this reason, these dietary components were not included in the analyses.
In conclusion, the present study does not show any effect of increased protein intake on height or bone resorption in children. However, the difference in changes in the level of osteocalcin between the HP/HGI group and the LP/HGI group warrants further investigation and should be confirmed in other studies.
Acknowledgements
The DiOGenes project was supported by a contract (FP6-2005-513946) from the European Commission Food Quality and Safety Priority of the Sixth Framework Program. Local sponsors made financial contributions to the shop centres, which also received a number of foods free of charge from food manufacturers. A full list of these sponsors is available online (www.diogenes-eu.org/sponsors/). The European Commission and the local sponsors had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: W. H. M. S. and A. A. designed the study; W. H. M. S., A. A. and A. P. conducted the study; M. M., C. R. and S.-M. D. conducted the statistical analyses; S.-M. D., C. T. D., K. F. M. and C. M. wrote the paper; S.-M. D. had primary responsibility for the final content. All authors read and approved the final manuscript.
The Department of Nutrition, Exercise and Sports at the University of Copenhagen has received research support from more than 100 food companies for this and other studies. W. H. M. S. is part-time employed by DSM, Inc., The Netherlands. A. A. is currently a member of the following scientific advisory boards: Global Dairy Platform, USA; Jenny Craig, USA; Pathway Genomics, USA; McDonald's, USA. K. F. M. received grants from Arla Foods Ingredients, Denmark and the US Dairy Export Council for studies focusing on undernutrition in low-income countries. S.-M. D., M. M., C. R., C. T. D., A. P. and C. M. declare no conflicts of interest.










