According to recent studies, several major complications could occur during and after long-term hormone replacement therapy, including the increased incidence of venous thromboembolism and breast cancer(Reference Stevenson1). Phyto-oestrogens, such as lignans, may constitute an alternative to hormone replacement therapy.
Sesame (Sesamum indicum, Pedaliaceae) is one of the most important oilseed crops with worldwide production reaching about 3·3 Mt annually and mainly cultivated in developing countries(2). The interest in sesame oil arises from its high content of unsaturated fatty acids and antioxidant lignans, exemplified by sesamin, sesamolin and sesaminol, that are also classified as phyto-oestrogens(Reference Dachtler, van de Put and van Stijn3). Usually, for the preparation of the various sesame products, the pericarp (hull) is removed. However, sesame oil from seeds with pericarp was more stable than that extracted from dehulled seeds(Reference Abou-Gharbia, Shahidi and Shahata4). This observation indicates that antioxidative components exist in the sesame perisperm, also confirmed by Chang et al. (Reference Chang, Yen and Chyn Huang5). Chemical analysis of the obtained extracts led to the identification of more than sixteen lignans(Reference Lazarou, Grougnet and Papadopoulos6), while a polyphenol-rich extract, appearing to be a by-product of great importance due to its antioxidant and anti-mutational properties, has been obtained from the sesame perisperm(Reference Grougnet, Magiatis and Mitaku7).
Oestrogens have diverse effects on many tissues in both males and females and the majority of these effects are mediated by oestrogen receptors that are ligand-dependent transcriptional activators belonging to the superfamily of nuclear receptors(Reference McDonnell8, Reference Pettersson and Gustafsson9). The two subtypes of oestrogen receptors, oestrogen receptor α (ERα) and oestrogen receptor β (ERβ), contain certain structurally conserved functional domains such as the regions responsible for DNA binding, dimerisation, ligand binding and ligand-dependent transactivation of gene expression. The tissue distribution of the two subtypes is not completely coincident as some tissues (for example, uterus, vagina) express predominantly ERα, whereas others (for example, lung, prostate, ovarian granulosa cells) express predominantly ERβ. Other tissues, such as bone and the pituitary, express both ERα and ERβ(Reference Pettersson and Gustafsson9). The relative levels of ERα and ERβ are important determinants of cellular sensitivity to oestrogens. Although, at normal concentrations of oestradiol, ERα is the strongest transcriptional activator of the two oestrogen receptor isoforms, co-expression of ERβ results in suppression of both the efficacy and the potency of hormone-stimulated responses(Reference Hall and McDonnell10).
Phyto-oestrogens are plant-derived compounds that promote oestrogenic actions mediated through the oestrogen receptors ERα and ERβ in mammals(Reference Hall, Couse and Korach11). They are structurally similar to mammalian oestrogen 17β-oestradiol and are, therefore, considered to play an important role in the prevention of hormone-dependent diseases such as cancer, heart disease, menopausal symptoms and osteoporosis(Reference Adlercreutz12). Due to interaction with oestrogen receptors, these compounds also have the potential to disrupt oestrogenic signalling, acting either oestrogenically as oestrogen agonists or anti-oestrogenically as antagonists and can also, therefore, be classified as selective oestrogen receptor modulators(Reference Brzezinski and Debi13).
According to our previous report(Reference Papadakis, Lazarou and Grougnet14), the levels of mammalian lignans (enterodiol and enterolactone) increased by several-fold in the plasma of experimental animals provided for a time period of 8 weeks with a diet rich in sesame pericarp (30 %), among other experimental diets. In the present study we attempted to further investigate the effect of this diet upon the expression of ERα and ERβ in certain tissues of the experimental animals.
Materials and methods
Animals
All animal procedures were performed in accordance with the guidelines of the Greek Local Authority and Municipality of the Central Region of Macedonia.
Animals and diets
Special care was taken in order to eliminate any unwanted effects due to the provided diet. Commercial rat chow was used (type 510K; Hellenic Feed Company, Plati, Greece) containing 21 % total protein from beetroots, 6·2 % lipids, 4·5 % fibres, 7·5 % ash, 1·1 % Ca, 0·9 % P, 0·35 % Na and 1·1 % methionine. The pellets were grounded and one part (experimental diet) was mixed with the sesame pericarp (Haitoglou SA, Thessaloniki, Greece) at a final concentration of 30 % (w/w), while the rest (control diet) was kept as it was. Subsequently, in both parts, the ground material was mixed with a minimum amount of tap water and by the use of a meat mincing machine, new pellets were created that were dried overnight at 45°C in a well-ventilated incubator.
Male Wistar rats were obtained from the Medical School of Aristotle University of Thessaloniki. The animal experimentation was conducted according to the Regional Veterinary Directorate (licence no. 13/9749/12-07-2006).
Animals, aged 6 weeks and of about 170 g body weight, were housed under controlled conditions of temperature (20–25°C) and lighting (12 h light–dark cycle). Animals were fed on a commercial non-purified diet for 1 week after which they were fed on either the control diet or the experimental diet. At the end of the experiment, animals were fasted for 12 h and killed after being anaesthetised using diethyl ether.
RNA extraction and cDNA synthesis
Total RNA was extracted from frozen tissues according to the method described by Chomczynski & Sacchi(Reference Chomczynski and Sacchi15). RNA was quantified spectrophotometrically at 260 nm and its integrity was verified also spectrophotometrically by measuring the ratio of 260/280 nm and by electrophoresis in 1·5 % (w/v) agarose gel. A quantity of 1 μg total RNA was used to prepare cDNA. Synthesis of cDNA was performed with the iScript Select cDNA Synthesis Kit (170-8896; Bio-Rad Laboratories, Hercules, CA, USA) in a final volume of 20 μl. For reverse transcription, mixtures of RNA samples (1 μg total RNA), random primer and nuclease-free water (total volume 15 μl) were incubated at 65°C for 5 min and were then cooled on ice immediately. 5 × iScript select reaction mix (4 μl) and iScript reverse transcriptase (1 μl) were added to each reaction according to the manufacturer's instructions. The mixture was further incubated for 5 min at 25°C, following a 30 min incubation at 42°C and a final 5 min incubation at 85°C in order to heat-inactivate the reverse transcriptase. The finally derived cDNA products were stored at − 20°C until further analysed by means of real-time PCR.
Quantitative real-time polymerase chain reaction
To verify changes in gene expression in the uterus and prostate tissues of the experimental animals due to the administration of sesame pericarp, quantitative real-time PCR (qRT-PCR) was performed using the iCycler iQ Real-Time PCR detection system (Bio-Rad Laboratories).
Oligonucleotide primer pairs for ERα were selected in accordance with previous references(Reference Tena-Sempere, Navarro and Mayer16). Primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed from the published sequence of the rat GAPDH gene (GenBank reference AF106860). Primer pairs for ERβ were designed on the basis of the common sequence of seven isoforms according to the published sequence of the rat ERβ gene (ensembl.org: ENSRNOG00000005343). The primer pairs used were as follows:
ERα sense, 5-AAT TCT GAC AAT CGA CGC CAG-3; ERα antisense, 5-GTG CTT CAA CAT TCT CCC CCT C-3 with PCR product size (bp) 345.
ERβ sense, 5-GCT CCT CTA TGC AGA ACC TCA AA-3; ERβ antisense, 5-CAG AAG TGA GCA TCC CTC TTT G-3 with PCR product size (bp) 137.
GAPDH sense, 5-GCC ACTC AGA AGA CTG TGG A-3; GAPDH antisense, 5-AGG TGG AAG AAT GGG AGT TG-3 with PCR product size (bp) 337.
The position of the primers on cDNA is given below:
ERα sense, 681; ERα antisense, 1003.
ERβ 1, 2, 3, 6, 7 sense, 288; ERβ 1, 2, 3, 6, 7 antisense, 425; ERβ 4 sense, 568; ERβ 4 antisense, 705; ERβ 5 sense, 388; ERβ 5 antisense, 525.
These sets of primers were designed to span over intronic DNA sequences.
The synthesised cDNA were further amplified (1:10) in triplicate by PCR using SYBR green I as a fluorescent dye (SYBR Green Supermix, 170-8880; Bio-Rad Laboratories) in a final volume of 20 μl.
The PCR cycling conditions were as follows: initial denaturation and enzyme activation at 95°C for 3 min, followed by thirty-five cycles of denaturation at 95°C for 30 s, annealing for 30 s, and extension at 72°C for 40 s. The specific annealing temperature of 60°C was the same for each target. Product purity was confirmed by dissociation curve (melting curve) followed by agarose gel electrophoresis. No template controls, as well as negative PCR controls without cDNA, were included in all assays, yielding no consistent amplification.
Calculation of relative expression levels of each target was conducted based on the cycle threshold (CT) method according to the equation 2− ΔΔCT(Reference Livak and Schmittgen17). Normalisation of the cDNA input was performed by using GAPDH gene expression as the internal control.
Gel electrophoresis and Western blot analysis
Western blot analysis was performed on uterus and prostate tissue samples. Tissues were removed from the animals and immediately immersed in liquid N2. The frozen tissues were pulverised in a mortar and homogenised further on ice in lysis buffer consisting of 50 mm-2-amino-2-hydroxymethyl-propane-1,3-diol-HCl (pH 7·4), 1 % (v/v) Nonidet P-40, 0·25 % (w/v) deoxycholic acid, 150 mm-NaCl, 1 mm-Na3VO4, 1 mm-EDTA, 1 mm-NaF and protease inhibitor cocktail from Sigma (P2714). The samples were kept on ice for 30 min and centrifuged further at 10 000 g for 10 min at 4°C to obtain the supernatant fraction. The protein concentration of samples was estimated by means of the Quick Start Bradford dye reagent (500-0205; Bio-Rad Laboratories). The samples were electrophoresed on 10 % SDS-PAGE. Prestained precision protein standards (161-0305; Bio-Rad Laboratories) were used as molecular weight markers.
After electrophoresis, the proteins were transferred onto a nitrocellulose membrane. Blots were probed with rabbit polyclonal antibody against ERα and against ERβ. The anti-ERα antibody (ab34575) was purchased from Abcam (Cambridge, MA, USA) and raised by use of an immunising peptide corresponding to amino acid residues 21–32 from human ERα which is completely conserved between human, rat, rabbit, sheep, porcine and bovine species and cross-reacts with human and rat protein. The anti-ERβ antibody (ab786) was purchased from Abcam and it was raised in rabbit against a synthetic immunising peptide corresponding to amino acids 459–477 of human ERβ, and it cross-reacts with human, rat and baboon and possibly other species. As a secondary antibody goat anti-rabbit IgG conjugated with horseradish peroxidase was used (Goat Anti-Rabbit, 170-5046; Bio-Rad Laboratories), and was visualised by means of enhanced chemiluminescence (catalogue no. 2600; Chemicon International, Temecula, CA, USA). Signals were quantified by image analysis (Gel-Pro version 3.0; Media Cybernetics, Silver Spring, MD, USA). Protein loads were normalised by using the actin signal as the internal control.
Statistical analysis
For statistical analysis of the results the Student's t test and ANOVA were applied.
Results
Immunoblotting studies were performed with total prostate and uterus protein from male and female rats provided daily with a sesame pericarp-enriched diet (experimental diet). After targeting the trans-blotted proteins with a specific antibody and visualising it by means of the enhanced chemiluminescence method, immuno-reactive full-length ERα and ERβ proteins of approximately 64–66 kDa and 54–56 kDa respectively were clearly observed in all the tissues studied except in the case of prostate, where ERα could not be detected under the described conditions.
The administration of sesame pericarp for a time period of 8 weeks resulted in a significant increase in the expression of ERβ in prostate. It was evident that the surface area corresponding to prostate ERβ protein from the treated animals was more dense and broad, as shown in Fig. 1. The histogram shows the mean values of the area corresponding to ERβ for all the four experimental animals of each group quantified by image analysis (Gel-Pro version 3.0; Media Cybernetics). The mean value for the control group was set as 100 %. The difference among the mean values corresponding to each group was statistically significant as judged by Student's t test and ANOVA.

Fig. 1 Expression of oestrogen receptor β (ERβ) protein in the prostate of two control animals (PC1 and PC2; fed with the control diet) and two experimental animals (PS1 and PS2; fed with the experimental diet) as investigated by means of Western blotting. Actin was used as an internal control. In both cases, 100 μg of total prostate protein was used. In the histogram is shown the visualised area (quantified by image analysis; Gel-Pro version 3.0; Media Cybernetics, Silver Spring, MD, USA) of all the animals from the control group (PC) and the sesame (Sesamum indicum) pericarp-fed group (PS). The area corresponding to the protein of ERβ in the prostate of the control group animals was taken as 100 %. Values are means, with standard deviations represented by vertical bars.
As far as the uterus is concerned, the effect of the provided experimental diet upon the expression of ERβ in the experimental animals studied was similar (Fig. 2). The difference between the mean values corresponding to the visualised area for each group was statistically significant (P < 0·01). The area corresponding to the ERβ zone in the sesame pericarp-treated animals was increased by two-fold (Fig. 2).

Fig. 2 Expression of oestrogen receptor β (ERβ) protein in the uterus of two control animals (UC1 and UC2; fed with the control diet) and two experimental animals (US1 and US2; fed with the experimental diet) as investigated by means of Western blotting. Actin was used as an internal control. In both cases, 100 μg of total uterus protein was used. In the histogram is shown the visualised area (quantified by image analysis; Gel-Pro version 3.0; Media Cybernetics, Silver Spring, MD, USA) of all the animals from the control group (UC) and the sesame (Sesamum indicum) pericarp-fed group (US). The area corresponding to the protein of ERβ in the uterus of the control group animals was taken as 100 %. Values are means, with standard deviations represented by vertical bars.
Concerning the expression of ERα in prostate tissue homogenate, no specific protein was detected with this method while in the uterus, the observed changes were not statistically significant (P < 0·1; Fig. 3) in spite of the fact that the visualised surface area corresponding to this receptor was broader in some of the treated animals.

Fig. 3 Expression of oestrogen receptor α (ERα) protein in the uterus of two control animals (UC1 and UC2; fed with the control diet) and two experimental animals (US1 and US2; fed with the experimental diet) as investigated by means of Western blotting. Actin was used as an internal control. In both cases 100 μg of total uterus protein was used. In the histogram is shown the visualised area (quantified by image analysis; Gel-Pro version 3.0; Media Cybernetics, Silver Spring, MD, USA) of all the animals from the control group (UC) and the sesame (Sesamum indicum) pericarp-fed group (US). The area corresponding to the protein of ERα in the uterus of the control group animals was taken as 100 %. Although the surface area corresponding to ERα protein in the uterus of the treated animals appears to be lower, the difference was not statistically significant (P < 0·5). Values are means, with standard deviations represented by vertical bars.
Oestrogen receptor α and oestrogen receptor β mRNA in prostate and uterus of treated animals
By means of qRT-PCR, the level of transcription of the two receptors, ERα and ERβ, was monitored.
mRNA in prostate
cDNA was synthesised from samples of eight prostate tissues by means of random primers and subjected to qRT-PCR amplifying ERα, ERβ and GAPDH. As it is mentioned in the Materials and methods section, the primers were designed to detect either of the two receptors. Four of the samples were derived from control animals (aged 14 weeks) provided with the control diet, and four were from experimental animals (aged 14 weeks) provided with the experimental diet. The qRT-PCR reactions were set up for thirty-five cycles.
As far as the ERα mRNA levels are concerned, although an increase by almost two-fold was observed, the difference was not statistically significant in prostate tissue (P < 0·1; Fig. 4). However, a very dramatic increase in prostate tissue by more than four-fold of the levels of ERβ mRNA was revealed after sesame pericarp administration (P < 0·001; Fig. 4).
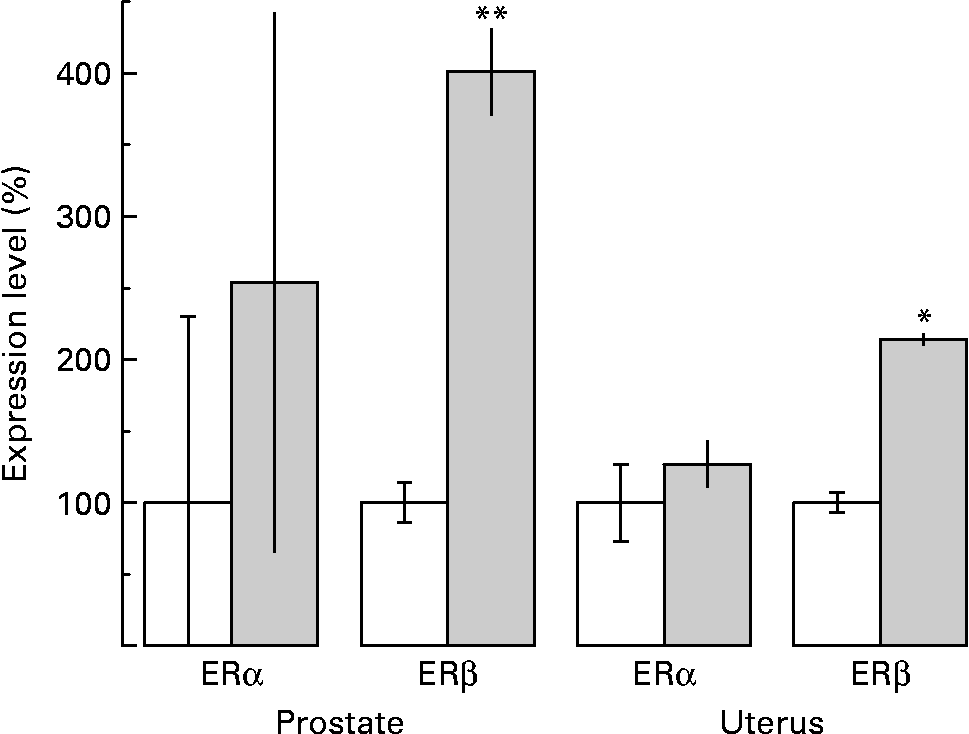
Fig. 4 Effect of sesame (Sesamum indicum) pericarp diet on the expression of oestrogen receptor α (ERα) and oestrogen receptor β (ERβ) mRNA in the prostate and uterus as investigated by means of quantitative real-time PCR. Calculation of relative expression levels of each target was conducted based on the cycle threshold (CT) method according to the equation 2− ΔΔCT. Each one of the histograms represents the variation in expression levels of oestrogen receptors observed in the prostate and uterus of treated (![]() ) as related to untreated (□) animals, that were set to 100 %. The untreated animals were provided with rat chow while the experimental ones were provided with rat chow enriched with 30 % sesame pericarp. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that for the control animals: *P < 0·005, **P < 0·001. The observed difference between control and experimental animals was not statistically significant for ERα in the prostate and uterus tissues (P < 0·1).
) as related to untreated (□) animals, that were set to 100 %. The untreated animals were provided with rat chow while the experimental ones were provided with rat chow enriched with 30 % sesame pericarp. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that for the control animals: *P < 0·005, **P < 0·001. The observed difference between control and experimental animals was not statistically significant for ERα in the prostate and uterus tissues (P < 0·1).
mRNA in uterus
The levels of mRNA were investigated in uterus tissue by following the same procedure and applying the same set of primers. In this course of experiments also, four of the samples were derived from control animals (aged 14 weeks) provided with the control diet, and four were from experimental animals (aged 14 weeks) provided with the experimental diet. The qRT-PCR reactions were set up for thirty-five cycles.
According to the results, both ERα and ERβ mRNA are expressed in the uterus. Administration of sesame pericarp for 8 weeks caused induction of ERβ mRNA by more than two-fold in uterus tissue (P < 0·005) while the levels of ERα mRNA were not statistically altered (P < 0·1) (Fig. 4).
Discussion
Despite the suggestion that the oestrogenic and/or anti-oestrogenic activity of phyto-oestrogens may reduce but also stimulate oestrogen-dependent tumour growth depending on dose and time of exposure(Reference Bhat, Lantvit and Christov18), reports providing information on the effects of a diet rich in plant products containing lignans upon the expression of oestrogen receptors in the organism are very rare. In the present paper we report on the effects of a diet rich in sesame pericarp that contains significant amounts of lignans(Reference Lazarou, Grougnet and Papadopoulos6) with phyto-oestrogenic activity upon the expression of ERα and ERβ(Reference Hall, Couse and Korach11, Reference Cappelletti, Fioravanti and Miodini19) in the prostate and uterus which express predominantly different classes of oestrogen receptors (ERβ for prostate, ERα for uterus)(Reference Weihua, Saji and Makinen20, Reference Weihna, Andersson and Cheng21).
According to our previous report, the levels of enterolignans (i.e. enterolactone and enterodiole, which are the end metabolites of lignans in mammals and express strong phyto-oestrogenic activity) were increased dramatically in the plasma of Wistar rats receiving for 8 weeks the same, as in the present study, experimental diet. More specifically, the levels of enterodiole from zero reached 2663·4 mmol/l while those of enterolactone increased from 68·5 mmol/l to 2133·9 mmol/l(Reference Papadakis, Lazarou and Grougnet14).
In the present study, sesame pericarp consumption led to a significant increase in the expression of ERβ in the prostate. The increase was more than two-fold as judged by means of Western blotting and even higher, by four-fold, when the levels of mRNA were measured by means of qRT-PCR.
In the uterus tissue, expression of ERβ was also elevated, although the observed difference was less prominent than in prostate (increase by two-fold), a finding that may be attributed to the variation in the natural levels of 17β-oestradiol due to the oestrus cycle of the experimental animals that were used.
No statistically significant difference was observed in the expressed levels of ERα in either tissue. The rather high differences observed in the band density of ERα protein from the uterus of animals deriving from the same experimental group are also attributed to the variation of the natural levels of 17β-oestradiol present in each animal due to the oestrus cycle. On the other hand, the very high variation of mRNA for ERα observed in prostate tissue is attributed to the very low levels that are naturally present in this tissue.
These findings are in agreement with previous reports that ERα and ERβ display marked differences in binding affinity and sensitivity to certain ligands and that phyto-oestrogens preferentially bind and activate ERβ(Reference Kuiper, Lemmen and Carlsson22).
According to accumulated knowledge, the observed alterations in the expression of oestrogen receptors after administering an experimental diet are beneficial to the organism. Increased expression of ERβ is associated with increased likelihood of response to endocrine therapy, while in tumour tissues v. normal ones in many cancers, including breast, ovary, colon and prostate, expression of ERβ mRNA and ERβ protein is decreased(Reference Bardin, Boulle and Lazennec23). Co-expression of ERα and ERβ results also in the formation of receptor heterodimers (instead of homodimers) modifying, therefore, the ERα- and ERβ-mediated responses(Reference Bardin, Boulle and Lazennec23). Emerging data support different functions between ERβ when it is expressed alone and ERβ when it is co-expressed with ERα(Reference Murphy and Watson24). ERβ seems to play a key role in the mitogenic action of oestrogen by providing protection against ERα-induced proliferation(Reference Pettersson, Delaunay and Gustafsson25, Reference Peng, Lu and Leygue26).
The imbalance in ERα:ERβ expression observed in oestrogen-dependent cancer opens up a new field in the hormone therapy of cancer. Targeted ERβ therapies, including the development of Erβ-specific ligands, may constitute a new therapeutic approach particularly for pre-invasive or proliferative lesions(Reference Weigel and Zhang27). According to the present results, it becomes evident that consumption of sesame pericarp that is rich in lignans and phenols(Reference Grougnet, Magiatis and Mitaku7) is combined with increased levels of enterolignans in the plasma(Reference Papadakis, Lazarou and Grougnet14) that induce the expression of ERβ and alter the balance between the two types of oestrogen receptors. The balance of ERα:ERβ is very important to the sensitivity of the cell to hormonal action and by shifting it to the benefit of ERβ may help either to prevent cancer development on even to decrease its rate of development. The clinical value of ERβ in cancer prognosis and its possible usefulness for prediction of the hormone response should be assessed in large-scale and prospective clinical studies. In general, these results offer additional information supporting the concept that consumption of vegetables and legumes rich in lignans and phenols is beneficial for the maintenance of good health.
Acknowledgements
A. A. performed the major part of the experimental work and designed the study. A. I. P. contributed to the performance of some of the experiments, to the completion of the design, to data analysis and to the drafting of the paper. The authors would also like to acknowledge the expert advice of Dr E. Boukouvala and Dr I. Paspaltsis, and the expert assistance of G. Pasientzis.
The present study was supported by a grant (05 DSVEPRO-113) from GSRT Greece and from the food company Haitoglou Bros SA (Thessaloniki, Greece).
The authors state that there are not any conflicts of interest.








