In 2006, breast cancer was the fourth leading cause of death in Taiwan and more than 5200 new cases of breast cancer had been diagnosed 2 years earlier. With such a high rate of incidence and prevalence, breast cancer remains an important public health issue in Taiwan. For high-risk cancer patients, they typically undergo surgery, followed by systemic chemotherapy and/or local radiotherapy as a standard adjuvant prophylaxis against disseminated tumour cells(Reference Karrison, Ferguson and Meier1). As a result, the combination of the cancer itself, as well as treatments including surgery, chemotherapy and/or radiation, impairs a patient's immunity(Reference Mafune and Tanaka2). For example, chemotherapy has cytostatic or cytotoxic effects on rapidly dividing cells, leading to adverse side effects. These side effects can include leucopenia, neutropenia and thrombocytopenia, which are attributable to bone marrow suppression, as well as suppression of the proliferation or function of lymphocyte subsets. Moreover, overall T cell levels have been found to be significantly lower in patients who received adjuvant systemic therapy v. patients with primary breast cancer or normal healthy donors(Reference Solomayer, Feuerer and Bai3). The immune system may also play an important role in mediating tumour growth and metastasis(Reference Mccoy, Rucker and Petros4, Reference Von Mensdorrl-Pouilly, Verstraeten and Kenemans5). Accordingly, cell-mediated immunity to tumour-associated antigens has been shown to be a better predictor of patient survival than tumour stage, grade and lymph node status(Reference Mccoy, Rucker and Petros4).
Many patients undergoing cancer therapy consider complementary and alternative medicine options, which can include vitamins, Chinese medical herbs or other biological products(Reference Tagliaferri, Cohen and Tripathy6). Usually, these products are taken to reduce side effects and organ toxicity, to protect and stimulate immunity or to prevent further development of additional cancers or recurrence(Reference Newman, Rock and Faerber7, Reference Von Gruenigen, White and Kirven8). However, scientific evidence regarding the efficacy and safety of herbs and nutritional supplements remains limited.
Ganoderma tsugae (also known as Lingzhi), Codonopsis pilosula (also known as Dang shen) and Angelica sinensis (also known as Dong quai) are often used in Chinese medicine, and are a source of food in Taiwan. Moreover, Lingzhi, which was recorded in traditional Chinese medicine as early as 2000 years ago, has been used to cure many diseases and to promote human vitality. Correspondingly, many therapeutic effects have been associated with mushrooms of diverse Lingzhi species, and these include immunomodulatory, anti-tumour, hepatoprotective, antioxidant, cholesterol-lowering, hypoglycaemia-inducing and appetite-suppressing activities(Reference Wasser and Weis9). Moreover, these observed therapeutic effects have been attributed to triterpenoid, polysaccharide and/or glycoprotein content of Lingzhi, although the exact mechanisms responsible for the observed biological activities have not been determined. Nevertheless, Lingzhi has traditionally been consumed as a luxurious functional food supplement in Chinese society.
C. pilosula belongs to the Campanulaceae family, and its root is commonly used in Chinese medicine. It has been used for the treatment of dyspepsia, poor appetite, fatigue and psychoneurosis. More recently, it has also been shown to mediate immunomodulatory activities of mouse splenocytes(Reference Wang, Ng and Yeung10). A. sinensis Diels is another well-known Chinese herbal medicine that has been used for the treatment of various diseases and as a tonic medicine for thousands of years, especially for gynaecological diseases(Reference Hardy11). It has also been used to treat anaemia due to enhanced haematopoiesis by stimulating the secretion of haematopoietic growth factors(Reference Bradley, Cunniff and Pereira12).
Rose geranium (Pelargonium graveolens) is a multi-harvest, high-value, aromatic plant cultivated for its essential oil that is widely used in the fragrance industry, for aromatherapy and for extraction of commercial rhodinol (a mixture of linalool, citronellol and geraniol). Essential oils from rose geranium have also been associated with strong antioxidant effects(Reference Sun, Xu and Wang13). Therefore, in the present study, the Chinese medicinal herb complex, RG-CMH, which includes extracts of rose geranium, G. tsugae, C. pilosula and A. sinensis, was evaluated in a randomised, double-blind, placebo-controlled study of fifty-eight patients with breast cancer and those that were prescribed chemotherapy or radiotherapy. Immune cell counts before, and 6 weeks after, treatment were assayed.
Experimental methods
Subjects
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the Institutional Review Board of the Chung Shan Medical University Hospital. Written informed consent was obtained from all the patients.
A total of sixty-six patients ≥ 18 years of age with stage I–IV breast cancer (according to the International Union Against Cancer TNM classification of malignant tumours) were treated with surgery followed by chemotherapy or radiotherapy at the Chung Shan Medical University Hospital (Taichung, Taiwan) and were then enrolled in the present study. Exclusion criteria for participation included in situ breast carcinoma patients, terminally ill patients, renal failure, congestive heart failure or hepatic failure, as well as intolerance or poor compliance with herbal medicine.
Study design
Between December 2004 and November 2005, a randomised, placebo-controlled study was conducted. A physical examination was performed at the beginning of the study, which recorded body height, body weight, vital signs, blood pressure and anamnesis. Patients confirmed that other systemic diseases had not been diagnosed, and that adverse effects had not previously been experienced following the use of any herbal medicines. Enrolled patients were randomly assigned to receive either nine capsules of RG-CMH (experimental group) or placebo (control group) each day, and patients received medicinal herb treatment and chemotherapy concomitantly. Patients who received chemotherapy by injection were required to have an overnight stay in the hospital, followed by a 3-week recovery period. The treatment period for each patient was 6 weeks (which included two chemotherapy cycles). Blood samples were collected before the first/next chemotherapy cycle for the two groups for biochemical analysis. The patients were also requested to visit their physician every 7 d to have leucocytes counts monitored, and to return patient diaries. Tumour location and staging details were obtained from patient medical records.
Compliance control
Compliance control was performed on the basis of empty packaging and unused investigational products documented for each patient. The compliance of each patient was also evaluated based on the daily feedback recorded and their presence at assigned examination appointments.
Efficacy evaluation
Primary efficacy criterion was defined as a reduction in levels of leucocytes, neutrophils, lymphocytes and lymphocyte subsets, and a decrease in the number of adverse events (AE) related to chemotherapy. Venous blood samples were obtained to assay the complete blood count and each patient's immunity status was analysed before, and following, 6 weeks of treatment. Erythrocytes, Hb, haematocrit, platelets, leucocytes, neutrophils and lymphocytes were evaluated using an automated blood analyser. Lymphocyte subsets (e.g. T cells, helper T cells, cytotoxic T cells), B cells and natural killer (NK) cells were evaluated using flow cytometry. Peripheral blood specimens were labelled with Trucount reagent (BD Biosciences, San Jose, CA, USA) and incubated with antibodies to lymphocyte subsets (e.g. CD3+, CD3+/CD4+ and CD3+/CD8+), B cells (CD19+) and NK cells (CD3−/CD16+/CD56+) for 15 min at room temperature, were then subjected to erythrocyte lysis. Four-colour flow cytometric immunophenotyping was performed using a FACSCalibur flow cytometer and Multiset software (BD Biosciences). At least 16 500 ungated events were collected and were expressed as a percentage of the total lymphocyte population detected. Both the percentage and absolute count were obtained for each subtype.
Common Terminology Criteria for Adverse Events (CTCAE v3.0) were used (http://ctep.cancer.gov/reporting/ctc.html) to assess AE related to chemotherapy. An AE was defined as any unfavourable and unintended sign (including an abnormal laboratory finding), symptom or disease temporally associated with the use of a medical treatment or procedure that may or may not be considered a medical treatment or procedure. The CTCAE v3.0 displays grades 1–5 with unique clinical descriptions of the severity associated with each AE grade. For example, grade 1 represents a mild AE, grade 2 a moderate AE, grade 3 is a severe AE, grade 4 is a life-threatening or disabling AE and grade 5 is a death-related AE. Information regarding AE related to cancer treatment was obtained from patient medical records. AE included anorexia, vomiting, diarrhoea, distension/abdominal bloating, changes in hearing, and changes in saliva and/or the salivary gland.
Test material
A placebo capsule containing maize starch (115·6 mg), magnesium stearate (2·5 mg), β-cyclodextrin (363·7 mg) and caramel (18·2 mg) was administered to the control group. In contrast, the experimental group received an RG-CMH capsule that contained A sinensis extract (64·5 mg), C. pilosula extract (27·1 mg), G. tsugae extract (3·0 mg), rose geranium power (273·6 mg), maize starch (129·3 mg) and magnesium stearate (2·5 mg). Liquid extracts of G. tsugae, C. pilosula, and A sinensis were obtained following extraction with boiling water (v/w 10) for 1 h. After the extracts were freeze-dried, levels of ferulic acid were detected. Compared with the placebo capsules, RG-CMH capsules were standardised according to the level of ferulic acid detected (e.g. 0·12 mg), and all capsules were made by Sun Ten Pharmaceutical Company Limited (Taoyuan, Taiwan), and provided by Medigreen Biotechnology Corporation. Each patient was required to consume nine capsules per d.
Statistical analysis
All analyses were carried out in accordance with current clinical practice guidelines and were applied to all variables. Biochemical data are presented as the means and standard deviation. ANCOVA were also applied to erythrocytes, Hb, haematocrit, and platelet counts were determined before, and following, 6 weeks of treatment for both the experimental and the placebo groups. The dependent Student's t test was used to compare differences within the same group, and an independent t test was used to compare differences between the biochemical analyses performed for the control and experimental groups. Statistical analyses of toxicity related to chemotherapy/radiotherapy for the control and experimental groups were also determined using the χ2 test. A P value < 0·05 was considered statistically significant. The Statistics Package for the Social Sciences (SPSS) statistical software package version 10.0 (Chicago, IL, USA) was used for all statistical analyses performed.
Results
Study group characteristics
A total of sixty-six subjects were enrolled in the present study, and were randomised into two groups. Patients in the experimental group (n 33) were assigned to take nine capsules containing RG-CMH a day, while patients in the control group (n 33) were assigned nine placebo capsules each day.Out of sixty-six patients, fifty-eight completed this 6-week study; two from the experimental group and four from the control group withdrew from the study due to changes in their medical therapy programmes; one control patient withdrew due to the development of a mild rash, and another control patient due to intolerance of the capsule flavour. Patient characteristics evaluated for both the experimental group (n 31) and the control group (n 27) at the start of the study are presented in Table 1. Treatment regimens received by patients of the control group included: one cycle of chemical therapy (n 25), two cycles of chemotherapy (n 1) and radiotherapy (n 1). For the experimental group, twenty-nine patients underwent one cycle of chemical therapy, one patient underwent two cycles of chemotherapy and one patient underwent radiotherapy. There were no significant differences in any of the variables compared between the two patient groups at the start of the study (Table 1).
Table 1 Baseline characteristics for this cohort
(Mean values and standard deviations or numerical value for each group)
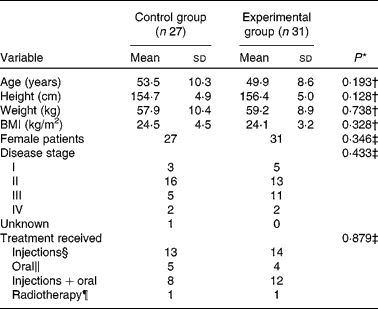
* There was no significant difference in sex, age, height, weight, BMI, type of carcinoma, tumour stage or type of therapy between the two groups.
† According to t test between control and experimental groups.
‡ According to χ2 test between control and experimental groups.
§ Pharmorubicin, endoxan and fluorouracil were injected with dosages determined by body surface area.
∥ Novofen and UFUR were administered according to body weight.
¶ Radiotherapy: 57·6 GY/32 FX was administered to control group patients and 55·8 GY/31 FX was administered to experimental group patients.
Compliance control
All fifty-eight patients remained in the study for the required duration, returned the empty packaging and unused investigational product, and filled out their diaries carefully. For both groups, the ratio of empty packaging to total packaging was 99·9 %.
Administration of RG-CMH affected haematological indicators
The mean platelet concentration for the control group was 247·11 (sd 59·21) × 103/mm3 at the start of the study, yet decreased significantly to 227·67 (sd 52·97) × 103/mm3 by week 6 (P =0·047; Table 2). In contrast, there was no difference in the platelet concentrations detected for the experimental group at the start of the study v. week 6. Nevertheless, according to ANCOVA tests (which included adjustments for Hb and haematocrit baseline values), concentrations of platelets, as well as of erythrocytes, Hb and haematocrit, showed no significant differences between the two groups between the start of the study and at week 6.
Table 2 Haematology analysis performed†
(Mean values and standard deviations)
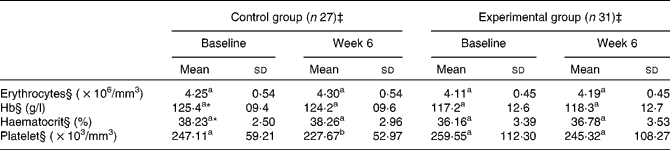
a,b Mean values within a column with unlike superscript letters were significantly different within each group (baseline v. week 6).
* Mean values were significantly different between the control group and the experimental group at baseline (P < 0·05).
† The dependent Student's t test was used to compare differences within the same group.
‡ The independent t test was used to compare the control and experimental groups.
§ ANCOVA (adjusted for Hb and haematocrit baseline values) were performed for erythrocytes, Hb, haematocrit, and platelet levels at week 6 for the experimental and control group, and no significant differences were found between the two groups.
Administration of RG-CMH affected immunological parameters
Using flow cytometry, we detected a significant decrease in the percentage of leucocytes and neutrophils for the control and experimental groups after 6 weeks of treatment, and this observation was independent of supplement (Table 3). For example, leucocyte levels decreased 31·5 (sd 14·1) % in the control group, while a 13·4 (sd 20·1) % decrease was detected in the experimental group after 6 weeks. For neutrophils, the control group exhibited a 35·6 (sd 18·3) % decrease, and the experimental group exhibited an 11·0 (sd 39·7) % decrease. In both the cases of change in leucocytes and of neutrophils during the study, the difference between experimental and control groups was statistically significant. In combination, these results demonstrate that a significant reduction in leucocytes and neutrophils accompanied the administration of RG-CMH capsules in this cohort.
Table 3 Immunological parameters for the two treatment groups
(Mean values and standard deviations)

NK, natural killer.
* The independent t test was used to calculate the percent change for the two groups.
† CD3+.
‡ CD3+/CD4+.
§ CD3+/CD8+.
∥ CD19+.
¶ CD3−/CD16+/CD56+.
For lymphocyte levels, a decrease of 21·9 (sd 20·5) % and 12·1 (sd 28·8) % was detected for the control and experimental groups respectively. Similarly, levels of T lymphocytes were found to decrease 18·3 (sd 20·9) % and 7·5 (sd 26·1) %, and helper T lymphocyte levels decreased 18·7 (sd 20·4) % and 7·1 (sd 30·3) % in each case. For cytotoxic T lymphocytes, the control group was associated with a 16·2 (sd 24·0) % decrease, while the experimental group exhibited a decrease of 4·7 (sd 31·6) %. Moreover, for both the control and the experimental groups, B lymphocyte levels decreased 33·7 (sd 41·3) % and 33·2 (sd 39·7) %. Furthermore, levels of NK cells decreased 18·9 (sd 39·1) and 6·4 (sd 58·4) %. Overall, despite change patterns that were similar to those observed for leucocytes and neutrophils, no significant differences in lymphocytes and lymphocyte subsets were detected for the control group or the experimental group after 6 weeks of treatment.
RG-CMH treatment affected chemotherapy/radiotherapy-related toxicities
Various toxicity-related conditions due to the chemotherapy/radiotherapy received by patients in this study were reported (Table 4). For example, anorexia was experienced by all patients in the placebo group (e.g. 81·5 % of patients had grade 1, 18·5 % of patients had grade 2) by week 6 of treatment. Similarly, anorexia was experienced by all of the experimental patients (e.g. 90·3 % patients had grade 1, 9·7 % patients had grade 2) throughout the treatment period. In addition, patients in both groups also experienced vomiting (14 ·8 and 13·0 % respectively), as well as diarrhoea (7·1 and 7·5 % respectively) by week 6. Both groups also reported distension, with 85·2 % of the control group experiencing grade 1 distension, 11·1 % experiencing grade 2 and 3·7 % experiencing grade 3 distension by week 6. Similarly, 74·2 % of experimental patients suffered from grade 1 distension, and 25·8 % experienced grade 2 distension. No significant differences in the rates of anorexia, vomiting, diarrhoea, distension, hearing or changes in saliva or the salivary gland were found between the two groups. Moreover, there were no significant differences in the toxicity related to chemotherapy/radiotherapy experienced by the two groups.
Table 4 Toxicity-related conditions due to chemotherapy/radiotherapy received by patients*
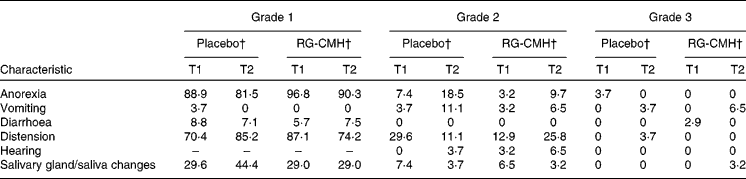
T1, baseline; T2, week 6.
Values were significantly different: P < 0·05.
* Data listed represent percentages and no adverse event grade 4 conditions reported.
† The χ2 test was used to compare placebo and RG-CMH groups.
Discussion
Cancer patients exposed to chemotherapy/radiotherapy are at higher risk of experiencing therapy-induced toxicity. Adverse side effects can include mucositis, xerostomia, changes in taste, dysphagia, nausea, vomiting, diarrhoea and anorexia, which compromise nutritional status and bodily functions. Many patients will take complementary and/or alternative medicines to reduce side effects and organ toxicity, to protect and stimulate immunity or to prevent the further development of cancer or recurrences. However, most studies that have investigated side effects of chemotherapy/radiotherapy have used animal models, and only a few studies have involved human clinical trials.
G. tsugae, C. pilosula, A. sinensis and rose geranium are often used in Chinese medicine, and are also a food source in Taiwan. However, research regarding these Chinese herbs and immunity has been limited to in vitro studies. Therefore, the present study is the first to evaluate these Chinese herbs in breast cancer patients in a randomised, controlled study. As a result, administration of RG-CMH was found to be associated with a significant reduction in levels of leucocytes and neutrophils (with decreases from 31·5 to 13·4 %, and 35·6 to 11·1 % respectively in each case). In comparison, another study of patients that received paclitaxel reported a 60 % decrease in leucocyte levels after 2 weeks of treatment, and a decrease of 24 and 57 % was detected 3 and 4 weeks after the start of treatment(Reference Tong, Seamour and Lawson14).
Ganoderma, a traditional Chinese medicine, has recently received considerable attention from both the health care and cancer research communities in Taiwan. For centuries, Ganoderma has been used for medicinal purposes in Asian countries to treat various human diseases, including cancer. Ganoderma lucidum and G. tsugae are the most widely cultivated species of the Ganoderma genus in Taiwan, and both have a long history of being used in folk medicine throughout Asia. Triterpenoids from G. tsugae have been shown to induce apoptosis and arrest the cell cycle in human hepatoma cells(Reference Gan, Fann and Hsu15), while FIBP-gts, a fungal protein from G. tsugae, has been associated with immunomodulatory activity(Reference Jinn, Wu and Tu16). In the latter case, pre-clinical studies have established that the polysaccharide fractions of G. tsugae specifically mediate immunomodulatory effects(Reference Kino, Yamashita and Yamaoka17–Reference Lin, Hung and Hsu20). In combination, these results demonstrate that RG-CMH intervention has the potential to reduce leucopenia.
For approximately 80 % of cancer patients undergoing treatment, neutropenia is a dangerous complication that is often reported(21). However, the exact incidence of neutropenia and the number of neutropenia-related cancer deaths is not clear, with rates of mortality ranging from 5 to 30 % among patients older than 70 years(Reference Balducci and Yates22). In a study by Tong et al. (Reference Tong, Seamour and Lawson14), cancer patients received docetaxel, and after 1 week, untreated levels were detected in 21 % of cases. In comparison, a decrease in neutrophil levels of 35·6 % and 11·0 % for the placebo and RG-CMH groups, respectively, was detected at week 6 in the present study. On the basis of these results, it appears that RG-CMH treatments may delay, or ease, reductions in neutrophil levels during cancer treatment. Furthermore, it has been hypothesised that the maintenance of leucocyte and neutrophil levels associated with RG-CMH is mediated by the polysaccharide of G. tsugae.
NK cells have previously been associated with a spontaneous cytolytic activity that influences unsensitised lymphoid cells. Correspondingly, this process plays an important role in the immune surveillance of malignant tumours(Reference Herberman and Ortaldo23), and in the immune defence against certain infections, particularly viral infections(Reference Minato, Bloom and Jones24). Women with breast cancer are often at risk for elevated levels of depression and anxiety, as well as reduced levels of NK cells. NK cells are associated with anti-cancer activities that include the lysis of tumour and virus-infected cells, as well as a capacity for monitoring and targeting neoplastic (both new and abnormal) growth(Reference Locke, Kraus and Leserman25, Reference Brittenden, Heys and Ross26). Correspondingly, higher levels of NK cell activity in patients with breast cancer were associated with a lower rate of cancer recurrence after 5 years(Reference Levy, Herberman and Lippman27). Although other studies have indicated that the G. tsugae polysaccharide can enhance splenic NK cell activity and serum interferon production in mice(Reference Won, Lin and Wu28), the present study detected no significant difference between the levels of NK cells for the control and experimental groups following treatment.
Chemotherapeutic medicines for treating breast cancer also target B and T lymphocytes, which are critical for the adaptive immune response. However, T helper lymphocytes and CD4+ cells are especially slow to recover following chemotherapeutic treatments(Reference Hakim, Cepeda and Kaimei29). For patients with solid tumours that have achieved high levels of CD3+ cells during an extended follow-up period, these patients have also been found to experience a longer time to progression(Reference Pierelli, Perillo and Ferrandina30). In another study by Hakim et al. (Reference Hakim, Cepeda and Kaimei29), levels of CD4+ cells were found to decrease more than 60 % following cancer treatment, and these levels did not return to normal 18 months after treatment was completed. Ferrandina et al. (Reference Ferrandina, Pierelli and Perillo31) also showed that the recovery of CD3+, CD4+ and CD8+ lymphocytes represented independent markers of a longer time to progression, and was also survival markers in ovarian cancer patients that had received high doses of chemotherapy with peripheral blood stem cell and growth factor supplements. In combination, these results suggest that enhancing the recovery of CD3+, CD4+ and CD8+ lymphocytes not only increases a patient's immunity against infection, but also increases patient survival.
C. pilosula is another commonly used Chinese herbal medicine. The root of C. pilosula, of the family Campanulaceae, is beneficial to the immune, digestive and haematopoietic systems. It also induces saliva production, and has been used to treat fatigue, thirst and loss of appetite(Reference Wang, Du and Xu32). In a study by Wang et al. (Reference Wang, Ng and Yeung10), C57BL/6 mice that received drinking water containing a polysaccharide-enriched fraction prepared from C. pilosula root exhibited lower mitogenic responses by splenocytes to concanavalin A and lipopolysaccharide. A. sinensis is another herb that has been used in traditional Chinese medicine for the treatment of a wide variety of diseases over thousands of years. Correspondingly, a polysaccharide of A. sinensis has been shown to increase the proliferation of total spleen cells, macrophages and T lymphocytes(Reference Hardy11). Furthermore, treatment with this polysaccharide was associated with an increase in the production and expression of IL-2 and interferon-γ, and a concomitant decrease in IL-4 expression and production. Flow cytometry assays have also detected an increase in the percentage of CD4+ lymphocytes in total spleen populations following treatment with the A. sinensis polysaccharide(Reference Yang, Jia and Meng33). In combination, these results demonstrate that Chinese herbal medicine can provide valuable insights into the capacity for modulation of inflammatory cytokine secretion, lymphocyte proliferation and antibody production to improve an individual's immunity. In the present study, it is hypothesised that the maintenance of T lymphocyte levels associated with RG-CMH may be due to the action of polysaccharides from both C. pilosula and A. sinensis.
In summary, the administration of RG-CMH was associated with an improvement in the conditions of leucopenia and neutropenia experienced by breast cancer patients during treatment. As such, it appears that administration of RG-CMH to patients receiving chemotherapy/radiotherapy would represent a strategy for improving patient immunity. It is hypothesised that the limited sample size of this study did not affect the differences observed between the control and the experimental groups regarding the immune cell counts monitored. However, a larger sample size will be needed to confirm the effect of RG-CMH on lymphocytes and lymphocyte subsets in future studies.
Acknowledgements
The authors would like to thank all cancer patients who participated in this study. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. S. R. Z. and S. L. C. designed the study and revised the manuscript, H. F. C. and J. H. T. conducted the research. M. Y. L. and H. S. L. analysed the data. Y. C. S., Y. Y. Y. and G. T. S. did the laboratory work and prepared the manuscript under the supervision of C.-K. W. All the authors read and approved the final manuscript. None of the authors has any conflicts of interest in the writing and publication of the present study.








