In the process of β-oxidation of long-chain fatty acids to generate energy, l-carnitine (LC) plays an essential role by acting with carnitine palmitoyltransferase 1 (CPT1) to transport long-chain fatty acids from the cytosol into the mitochondrial matrix(Reference Bremer1,Reference Zammit, Ramsay and Bonomini2) . LC also regulates lipid and glucose metabolism by modulating the ratio of acetyl-CoA/CoA. Previous studies proved that a deficiency in LC could induce severe systemic metabolic syndrome, such as hyperlipidemia and diabetes, in mammals(Reference Karpati, Carpenter and Engel3–Reference Mamoulakis, Galanakis and Dionyssopoulou5). Therefore, supplementary LC can widely improve lipid metabolism and treat metabolic diseases mainly through promoting fatty acid (FA) β-oxidation in land animals(Reference Owen, Nelssen and Goodband6–Reference Stephens, Constantin-Teodosiu and Greenhaff10).
Recently, there has been increased understanding regarding how LC regulates endoplasmic reticulum (ER) stress in mammals. In rats fed with high-fructose corn syrup or in human aortic endothelial cells incubated with high glucose, curative effects have been obtained by dietary LC through suppressing X-box binding protein 1 (XBP1) or activating transcription factor 6 (ATF6) signalling(Reference Gao, Li and Zhu11,Reference Unsal, Deveci and Ozmen12) . In H2O2-treated lens epithelial cells or neurons, LC reduced the apoptosis rate or offered neuroprotection via alleviation of glucose-regulated protein 78 (GRP78) and/or C/EBP-homologous protein (CHOP) signalling(Reference Kong, Zheng and Liu13,Reference Ye, Han and Chen14) . LC, when combined with bisoprolol, exerted protective effects on myocardial damage in rats related to an inhibition of GRP78 and CHOP(Reference Zhang, Wu and Shen15). However, whether LC ameliorates adverse effects induced by high-fat diets (HFD) through regulating ER stress is still unknown. The positive effect of LC on mitochondrial β-oxidation and ER stress, simultaneously or separately, has not yet been elucidated.
Due to their high energy, HFD are currently used as substitutions for superior protein sources to save cost in aquaculture(Reference Morais, Bell and Robertson16,Reference Boujard, Gelineau and Coves17) . However, in farmed fish, HFD are prone to trigger adverse effects on growth performance and lipid accumulation in tissues, mainly in liver and abdominal adipose tissue(Reference Regost, Arzel and Cardinal18–Reference Liu, He and Ning20). The excessive lipid deposition induced by HFD occurs due to decreased FA β-oxidation capacities in herbivorous grass carp (Ctenopharyngodon idellus), blunt snout bream (Megalobrama amblycephala) and medaka (Oryzias latipes)(Reference Du, Clouet and Zheng21–Reference Matsumoto, Terai and Oishi25). It was recently found that activating ER stress associated with inositol-requiring kinase enzyme 1 (IRE1)-XBPl and/or the ATF6 signalling pathway caused a fat utilisation disturbance in blunt snout bream, yellow catfish (Pelteobagrus fulvidraco) and zebrafish (Danio rerio) fed with HFD(Reference Cao, Dai and Liu26–Reference Dai, Wang and Zheng28). It was also recently reported that simultaneously impairing FA β-oxidation and increasing ER stress at the mRNA expression level contributed to fatty liver disease in blunt snout bream and tilapia (Oreochromis niloticus) fed with HFD(Reference Cao, Liu and Zheng29,Reference Jia, Cao and Du30) . The metabolic syndromes caused by HFD in farmed fish were associated with dysfunction of mitochondria and the ER. Therefore, it is necessary to seek suitable therapy for alleviating the harmful symptoms of lipid metabolism disorder that can result from fish diets via simultaneous regulation of FA β-oxidation and ER stress.
The goal of fish nutritionists is to invoke effective nutritional regulating pathways with lipid-lowering effects, especially through enhancing lipid catabolism. The potential nutritional function of LC has been studied in many fish species(Reference Li, Limbu and Ma31). Dietary supplementation of LC improves lipid catabolism and/or alleviates HFD-associated excess lipid accumulation in fish, not only through increasing the concentrations of acetyl-CoA and ATP in tissues but also stimulating mitochondrial β-oxidation efficiency and mRNA and/or protein expression relative to key β-oxidation processes(Reference Fujisawa, Takami and Matsuzaki32–Reference Li, Li and Qin34). Conversely, the endogenous carnitine concentration was reduced by more than 80 % when a carnitine synthesis inhibitor was fed to tilapia and zebrafish and could be further induced to inhibit mitochondrial β-oxidation efficiency and increase lipid accumulation in tissues(Reference Li, Li and Qin35–Reference Li, Li and Ning37). The two different sides of these studies confirmed the critical roles of LC in fish nutrition, as it regulates the mitochondrial FA β-oxidation. However, whether LC can regulate the ER stress in fish fed with HFD remains unclear. Therefore, it is worthy to investigate the regulatory mechanism used by dietary LC to relieve the lipid metabolism disturbance at the organelle level in fish fed with a HFD.
The large yellow croaker (Larimichthys crocea) is an important commercially cultured marine fish that is sensitive to dietary lipid levels and sources in diets(Reference Yan, Liao and Wang38,Reference Liao, Yan and Mai39) . Previous studies also proved that: (1) mitochondrial dysfunction was closely associated with the variation in lipid accumulation in the liver(Reference Liao, Yan and Mai39,Reference Liao, Yan and Mai40) ; (2) ER stress was involved in the response to dietary FA(Reference Liao, Yan and Li41,Reference Zhang, Liu and Pang42) and (3) tea polyphenols, bile acid, curcumin and LC reduce the lipid content through increasing FA β-oxidation at the gene or protein expression level(Reference Ji, Li and Li43–Reference Li, Chen and Chen46). Therefore, the large yellow croaker could be considered as an ideal animal model to explore the regulation of lipid metabolism at the organelle level. The present study aimed to explore whether dietary LC can reduce lipid deposition via simultaneous regulation of mitochondrial FA β-oxidation and ER stress in large yellow croaker fed with HFD. This is the first study to simultaneously investigate the function of FA β-oxidation and ER stress in regulating lipid metabolism by using LC along with a nutritional background of dietary HFD in fish.
Materials and methods
2·1 Animal ethics
All experimental procedures were performed on fish, and animal care was conducted in compliance with the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised on 1 March 2017). This study was approved by the Animal Research and Ethics Committees of the Ocean University of China (Permit Number: 20141201).
2·2 Diet preparation
In order to explore whether dietary supplementation with LC improved liver lipid metabolism through simultaneously promoting mitochondrial β-oxidation and inhibiting ER stress in large yellow croaker fed with HFD, the lipid level in diets was chosen to be 18 %, in accordance with previous studies(Reference Yan, Liao and Wang38,Reference Liao, Yan and Mai40,Reference Wang, Li and Hou47,Reference Li, Xu and Lai48) . The diets used were a basic diet containing 18 % lipid (HF), a basic diet containing 18 % lipid and supplemented with 1·2 ‰ LC (HF/L-LC), and a basic diet containing 18 % lipid and supplemented with 2·4 ‰ LC (HF/H-LC). The LC levels used were selected according to the ratio of HFD to normal fat diets and 0·8 g/kg LC used in previous studies with large yellow croakers(Reference Li, Chen and Chen46,Reference Sang, Deng and Shentu49) . The main lipid source was a mixture of fish oil and soya lecithin (weight ratio at 9:1), and the ingredients and nutrient composition of the experimental diets are presented in Supplementary Table 1.
For the first step in preparing the fish diets, all raw ingredients were carefully crushed and then passed through a 200-μm sieve so that a fine powder was formed. They were then precisely weighed according to the experimental diet formulations and well mixed. After that, the lipid sources (fish oil and soya lecithin) and LC dissolved in a small amount of water added into the mixture. Next, the mixture was sifted through a 400-μm sieve and mixed well with water (250 ml/kg) to produce a stiff dough. The dough was then pelleted into two forms (2 mm × 5 mm and 4 mm × 5 mm, which were formulated for fish participating in the earlier feeding trial and the later stage trial, respectively) using an automatic fish granulator (F-26, South China University of Technology). The pellets were dried overnight in a ventilated oven at 55°C, sealed in plastic bags and stored at −20°C for the feeding trials. The procedure for diet preparation was followed according to the protocol of the previous study(Reference Li, Xu and Lai48).
Fish feeding procedure and sampling
Juvenile large yellow croaker fish with an average initial body weight of 8·59 g were chosen as the subjects, and 270 healthy fish were randomly distributed into nine floating sea cages (1 m × 1 m × 2·5 m). There were thirty fish per cage, and each diet was administered to all the fish in triplicate cages. The three groups of fish fed with different diets were named the HF group, HF/L-LC group and HF/H-LC group. Fish were fed to visual apparent satiation twice per day at 05:00 and 17:00 for 10 weeks. During the experimental period, the environmental conditions (temperature: 23·4–30·2°C, salinity: 31·2–35·7 ‰ and oxygen level: 6·6–7·5 mg/l) were natural and optimum for marine fish feeding. This trial was conducted at the Aquatic Seeds Farm of the Marine and Fishery Science and Technology Innovation Base, Ningbo, China.
After the feeding trial, fish were fasted for 24 h, and then the total fish weight per cage was obtained, and fish in each cage were counted. Thereafter, each fish was anesthetised (MS-222:20 mg/l) for sample collection of liver and plasma. These samples were used for histochemical, histological, biochemical and molecular analysis.
Biochemical analysis of whole body and tissues
The moisture, crude protein, and crude lipid in diets, and crude lipid in whole body from six fish in each group were measured following the methods of the Association of Official Analytical Chemists(50). A ventilated drying oven was set at a constant temperature of 105°C to measure the moisture content. The Kjeldahl method was used to determine the crude protein content in diets (FOSS, KjeltecTM 8200). The Soxhlet extraction method was used to test the crude lipid in diets and whole body of the fish (FOSS, Soxtec 2050). The methanol and chloroform (1:2, v/v) method was used to extract and measure the total lipids in the liver from six fish in each group, as previously described(Reference Folch, Lees and Sloane Stanley51,Reference Li, Li and Zhang52) .
Specific commercial kits were used to assess liver TAG, malondialdehyde (MDA), superoxide dismutase (SOD), catalase, total antioxidant capacity, monoamine oxidase, succinate dehydrogenase, ATP, Na+K+ATPase, Mg++-ATPase, Ca++ATPase and 8-hydroxydeoxyguanosine (Jiancheng Bioengineering Institute) from six fish in each group.
An automatic biochemical analyser (Roche Cobas c311) was used to measure the serum total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, glucose, total protein, alanine transaminase and aspartate transaminase. Specific commercial kits were used to assess serum β-hydroxybutyrate (D3-H), total amino acid, MDA and SOD (Jiancheng Bioengineering Institute) from six fish in each group.
Histochemical and histological analysis
Pieces of liver (5 × 5 mm) from three fish in each group were cut and quickly fixed in 4 % paraformaldehyde. Sections of 6 μm were cut using a cryostat microtome and then immersed in cold 10 % buffered formalin. These sections were further stained with Oil Red O for liver histochemical observation. For liver histological observation, liver samples were dehydrated using graded ethanol and were then embedded in paraffin. Sections of 5-μm thickness were cut with an ultrathin semiautomatic microtome and then stained with haematoxylin and eosin. Histochemical and histological observations were photographed with an Olympus BX53 optical microscope. Histochemical and histological observations were performed as previously described(Reference Betancor, Sprague and Sayanova53,Reference Song, Gao and Hogstrand54) .
Quantitative real-time PCR and western blot
The total RNA isolation, measurement of total RNA quality and quantity, and synthesis of cDNA from six fish in each group were performed following a protocol given in a previous study(Reference Li, Xu and Lai48). Quantitative real-time PCR was performed using a mix that contained 1 μl primer, 2 μl cDNA product, 10 μl SYBR-Premix ExTaq II (Takara) and 6 μl RNAse-free water (CFX Connect Real-Time System, Bio-Rad). The reaction programme was set at 95°C for 2 min, followed by forty cycles of 95°C for 10 s, 59°C for 10 s and 72°C for 20 s, and then melting curve analysis. The sequences of the primers used in this study are provided in Supplemental Table 2. The data for mRNA expression were calculated and normalised via the 2−ΔΔCT method, with the HF group being used as the control(Reference Livak and Schmittgen55).
Pieces of liver (approximately 20 mg) from three fish in each group were prepared for testing protein expression. The western blot was conducted following a previously described method(Reference Yang, Zhou and Wu56). Total protein was extracted with the use of a commercial kit (Sangon Biotech), and then the proteins were quantified using a BCA Kit (Beyotime Institute of Technology). The protein sample was separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Millipore), blocked with 5 % skimmed milk and then incubated with shaking at room temperature for 2 h. The polyvinylidene difluoride membranes were incubated with primary antibodies overnight at 4°C against the following proteins: CPT1a (15 184–1-AP) was purchased from Proteintech; acyl-CoA oxidase (ACO, ab184032), estrogen-related receptor α (ERR-α, ab76228) and peroxisome proliferator-activated receptor γ coactivator 1 (PGC1, ab188102) were purchased from Abcam; GRP78 (3183), p-eukaryotic translational initiation factor 2a (p-eIF2α, 3398), PTEN-induced putative kinase 1 (PINK1, 6946) and p-AMP-activated protein kinase (p-AMPKα, Thr172, 50081) were purchased from Cell Signaling Technology; ATF6 (bs-1634R) was purchased from Bioss; P-PKR-like eukaryotic initiation factor 2a kinase (p-PERK, abs137056), peroxisome proliferator-activated receptor α (PPARa, abs117362) and 70-kDa peroxisomal membrane protein (PMP70, abs137792) were purchased from Absin; glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AB-P-R001) was purchased from GoodHere; and β-actin (AF7018) was purchased from Affinity. After that, the membranes were incubated with secondary antibodies at room temperature for 2 h against horseradish peroxidase(HRP)-labelled IgG(H + L) (A0208, Beyotime Institute of Technology). Finally, protein bands were visualised with an ECL kit (Beyotime Institute of Technology) and scanned using the Microtek Bio-5000 Plus system (Microtek). GAPDH and β-actin served as the internal control. All the protein band intensities were calculated using ImageJ software.
Mitochondrial DNA copy number analysis
The liver mitochondrial number from six fish in each group was determined by relative mitochondrial DNA (mtDNA) copy number and measured by quantitative PCR assay using mitochondrial gene/nuclear β-actin. Total DNA was first extracted from the liver tissue using a commercial kit (TaKaRa MiniBEST Universal Genomic DNA Extraction Kit, TaKaRa). Quantitative real-time PCR was performed as described above. Primers for the mitochondrial displacement loop (D-loop), cytochrome b (Cyt b), 16S ribosomal RNA (16S rRNA) and nuclear β-actin gene were synthesised using a previously described method(Reference Hartmann, Reichwald and Wittig57) and are listed in Supplemental Table 2.
Calculations and statistical analysis
Weight gain (WG, %) = (wt − wo) × 100/wo
Specific growth rate (SGR%, day–1) = (Ln (wt)-Ln (wo))/70 days × 100
Feed efficiency ratio (FER) = (wt − wo)/F
Survival rate (SR, %) = (FN/IN) × 100
Hepatosomatic index (HSI, %) = wet liver weight/wet fish weight × 100
where wt is the final weight of total fish, wo is the initial weight of total fish and F is the feed intake (g). FN is the final number and IN is the initial number of total fish in each cage.
All results are presented as the mean and standard error of the mean. The data for the three groups were first tested for the normality and homogeneity of variances with the Levene test and were then analysed using one-way ANOVA, and finally, Tukey’s multiple range test was used to estimate the differences. P < 0·05 indicates that the differences were significant. All statistical analyses were conducted using SPSS Statistics 19.0 software (IBM).
3 Results
Dietary l-carnitine acts as a lipid-lowering factor
During the 10-week feeding trial, the fish among the three groups were in good health, and no different survival rate (P = 0·514) or growth performance (P = 0·547 in WG, P = 0·539 in SGR and P = 0·395 in PER) was observed (online Supplementary Fig. 1). To further test the effect of dietary LC on reducing lipid deposition, the total lipid concentration in whole body and liver, liver TAG content and weight, and liver histochemical results stained by Oil Red O were measured. The total lipid concentration in whole body was not affected by dietary LC (P = 0·249) (Fig. 1(a)). Interestingly, liver total lipid (P = 0·004), TAG (P = 0·000) concentration and liver weight (HSI, P = 0·037) in the HF/H-LC group were lower than those in the HF group (Fig. 1(a)–(c)), but not the HF/L-LC group. Histochemical results of tissue stained by Oil Red O showed that the size and/or numbers of lipid droplets were smaller in the dietary LC-treated groups than in the HF group (Fig. 1(d)). These above data indicated that dietary LC does reduce the lipid deposition in the liver of large yellow croaker fed with HFD.

Fig. 1. Effect of dietary l-carnitine (LC) on the lipid content in whole body and liver of large yellow croaker. (a) Total lipid in whole body and liver; (b) liver TAG content; (c) HSI; and (d) liver histochemical characteristics (Oil Red O staining). Data are expressed as the means ± sem (n 6, but n 3 in HSI and liver Oil Red O). Mean values with unlike letters are significantly different (P < 0·05). HIS, hepatosomatic index.
The serum lipid profile is another important index used to evaluate the lipid-lowering effect of dietary LC in fish nutrition. The serum total cholesterol (P = 0·856), LDL-C (P = 0·564) and D3-H (P = 0·119) were not significantly different among the three groups (Fig. 2(a) and (b)). Compared with the HF group, serum HDL-C increased only in the HF/H-LC group (P = 0·008) (Fig. 2(a)). Serum TAG in the HF/H-LC group (P = 0·045) was significantly lower than that in the HF group, but there was not a significant tendency in the HF/L-LC group (P = 0·085) (Fig. 2(b)). These results showed that dietary LC improved the serum lipid profile of large yellow croaker fed with HFD.

Fig. 2. Effect of dietary l-carnitine (LC) on the serum metabolite profiles of large yellow croaker. (a) Serum lipid profiles; (b) serum D3-H; (c) serum glucose; and (d) serum TP and TAA. Data are expressed as the means ± sem (n 6). Mean values with unlike letters are significantly different (P < 0·05). TC, total cholesterol; D3-H, β-hydroxybutyrate; TP, total protein; TAA, total amino acid.
There was significantly decreased serum glucose in the dietary LC-treated groups as compared with the HF group (P = 0·000) (Fig. 2(c)). Conversely, the dietary LC-treated groups exhibited significantly increased serum total protein compared with the HF group (P = 0·012) (Fig. 2(d)). Compared with the HF group, the serum total amino acid in the HF/H-LC group was increased (P = 0·054) (Fig. 2(d)). These results suggested that dietary LC may be potentially used to increase glucose utilisation, and protein and amino acid deposition.
Dietary l-carnitine increased the antioxidant capability and then relieved liver damage
The two dietary LC-treated groups exhibited lower serum MDA than the HF group (P = 0·004) (Fig. 3(a)). The liver MDA content in the HF/H-LC group was lower than that in the HF group, but the difference was not significant (P = 0·073) (Fig. 3(b)). The serum SOD activity (P = 0·436), liver SOD (P = 0·637) and catalase (P = 0·113) activity were not different among the three groups (Fig. 3(a) and (b)). However, the serum total antioxidant capacity in the HF/L-LC (P = 0·055) and HF/H-LC (P = 0·067) groups was higher than that in the HF group by at least 200 % (Fig. 3(a)). The serum and liver indexes demonstrated that dietary LC improved the liver antioxidant capability.

Fig. 3. Effect of dietary l-carnitine (LC) on the liver antioxidant capability of large yellow croaker. (a) Serum indexes and (b) liver indexes. Data are expressed as the means ± sem (n 6). Mean values with unlike letters are significantly different (P < 0·05). MDA, malondialdehyde; SOF, superoxide dismutase; CAT, catalase; T-AOC, total antioxidant capacity.
Interestingly, the serum aspartate transaminase activity in the HF/L-LC (P = 0·009) and HF/H-LC (P = 0·011) groups was significantly lower than that in the HF group. However, compared with the HF group, the serum alanine transaminase activity in the HF/L-LC (P = 0·050) and HF/H-LC (P = 0·056) groups was reduced (Fig. 4(a)). Furthermore, the sizes of vacuoles decreased, but nuclei numbers increased in the HF/L-LC and HF/H-LC groups compared with the HF group (Fig. 4(b)). These results indicated that dietary LC relieved liver damage in fish fed with HFD.

Fig. 4. Effect of dietary l-carnitine (LC) on liver damage in large yellow croaker. (a) Serum indexes of liver damage and (b) histological characteristics (haematoxylin and eosin (HE)) of liver damage. Data are expressed as the means ± sem (n 6, but n 3 in liver HE). Mean values with unlike letters are significantly different (P < 0·05). ATL, alanine transaminase; AST, aspartate transaminase.
Dietary l-carnitine improved the mitochondrial function by stimulating mitochondrial β-oxidation and biogenesis
Mitochondria play a central role in regulating lipid metabolism and energy production. To investigate the effect of dietary LC on mitochondrial function, indexes relative to mitochondrial biogenesis and β-oxidation were tested. Liver monoamine oxidase activity was comparable among the three groups (P = 0·103) (Fig. 5(a)). The liver succinate dehydrogenase activity in the HF/H-LC group was higher than that in the HF group by at least 50 %, but the difference was not significant (P = 0·108) (Fig. 5(a)).
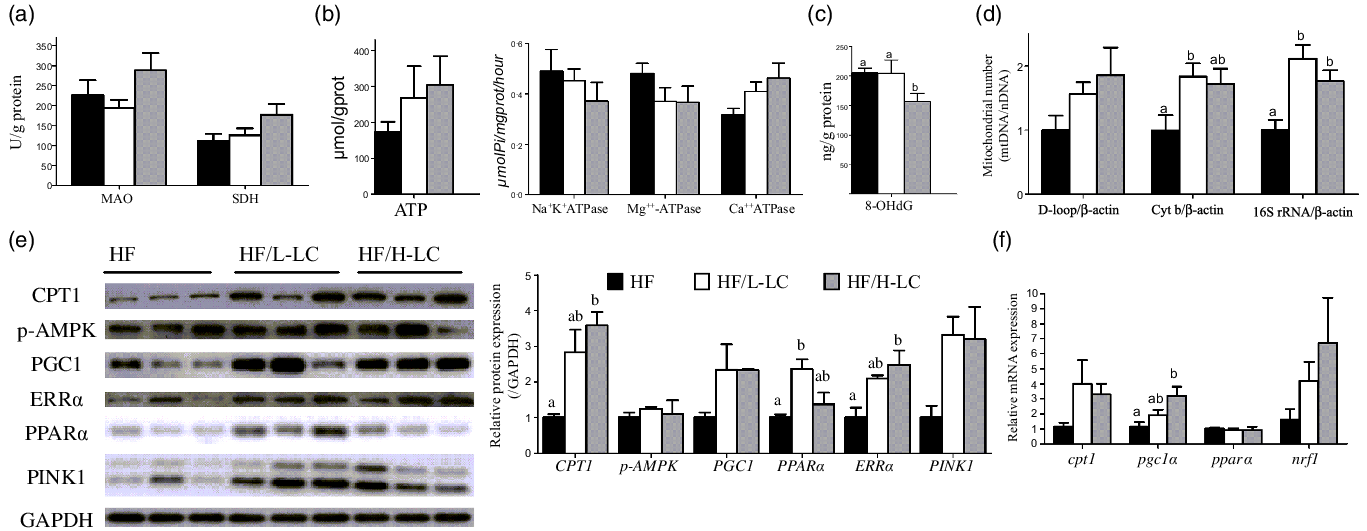
Fig. 5. Effect of dietary l-carnitine (LC) on liver mitochondrial β-oxidation and biogenesis in large yellow croaker. (a) MAO and SDH activity; (b) ATP level and ATPase; (c) level of oxidative mtDNA damage; (d) mitochondrial number; (e) protein level of mitochondrial β-oxidation and biogenesis, and mitophagy; and (f) mRNA level of genes relative to mitochondrial β-oxidation and biogenesis. Data are expressed as the means ± sem (n 6, but n 3 in protein expression). Mean values with unlike letters are significantly different (P < 0·05). MAO, monoamine oxidase; SDH, succinate dehydrogenase; Cyt b, cytochrome b; 16S rRNA, 16S ribosomal RNA; AMPK, AMP-activated protein kinase; ERRα, estrogen-related receptor α; PINK1, PTEN-induced putative kinase 1; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α; PPARα, peroxisome proliferator-activated receptor α; CPT1, carnitine palmitoyltransferase 1; NRF1, nuclear respiratory factor 1.
The ATP production was not significantly different among the three groups, but the two dietary LC-treated groups exhibited higher ATP content compared with the HF group by at least 50 % (P = 0·411) (Fig. 5(b)). Activities of liver Na+K+ATPase (P = 0·496) and Mg++-ATPase (P = 0·266) were comparable among the three groups, but the activity of liver Ca++ATPase was higher in the HF/H-LC group as compared with the HF group (P = 0·064) (Fig. 5(b)).
In examining the level of oxidative mtDNA damage, compared with the HF group, the liver 8-hydroxydeoxyguanosine content was only lower in the HF/H-LC group (P = 0·034) (Fig. 5(c)). As for the relative quantity of mitochondrial number, the ratio of D-loop to β-actin in the liver was comparable among the three groups (P = 0·154). The ratios of Cyt b to β-actin in the livers of the HF/L-LC group (P = 0·047) and 16S rRNA to β-actin in livers of the HF/L-LC (P = 0·002) and HF/H-LC (P = 0·025) groups were significantly higher than those of the HF group (Fig. 5(d)). However, compared with the HF group, the ratio of Cyt b to β-actin in the livers of the HF/H-LC group showed an increasing trend (P = 0·089) (Fig. 5(d)). These results showed that dietary LC accelerated the ATP dynamic process, reduced mtDNA damage and increased mitochondrial numbers.
As for protein related to mitochondrial β-oxidation, biogenesis and mitophagy, the liver protein levels of CPT1 (P = 0·014), ERRα (P = 0·026) and PINK1 (P = 0·067) were dramatically higher in the two dietary LC-treated groups than in the HF group by at least 100 % (P < 0·05) (Fig. 5(e)). The protein level of PPARα in the liver was lower in the HF group than that in the HF/L-LC group (P = 0·019), but comparable with that of the HF/H-LC group (P = 0·557) (Fig. 5(e)). Crucially, the protein level of PGC1 in the livers of the HF/L-LC (P = 0·141) and HF/H-LC (P = 0·146) groups was higher than that of the HF group by more than at least 100 %, but the variation was not significant (Fig. 5(e)).
The mRNA level of genes relative to mitochondrial biogenesis and β-oxidation showed that the mRNA levels of pgc1α in the liver were higher in the HF/H-LC group than in the HF group (P = 0·019), whereas the differences between the HF and HF/L-LC groups were not significant (P = 0·534) (Fig. 5(f)). Compared with the HF group, the mRNA levels of cpt1 in the liver were higher in the HF/L-LC (P = 0·332) and HF/H-LC (P = 0·151) groups by at least 200 %, but the difference was not significant (Fig. 5(f)). The mRNA expression of pparα (P = 0·881) and nuclear respiratory factor 1 (nrf1) (P = 0·208) was comparable among the three groups (Fig. 5(f)). These data demonstrated that dietary LC improved mitochondrial biogenesis and β-oxidation at the mRNA and protein levels.
Dietary l-carnitine-activated peroxisomal biogenesis and β-oxidation
To further study the ability of dietary LC to affect the β-oxidation of FA, the parameters associated with peroxisomal β-oxidation and biogenesis were measured. The mRNA expression of ATP binding cassette subfamily D member 4 (abcd4) was higher in the HF/H-LC group than that in the HF (P = 0·006) and HF/L-LC (P = 0·036) groups (Fig. 6(a)). The mRNA expression of aco was higher in the HF/H-LC group than that in the HF group (P = 0·001), but not in the HF/L-LC group (P = 0·12) (Fig. 6(a)). The mRNA expression of peroxisomal biogenesis factor 5 (pex5) (P = 0·02), mitochondrial fission factor (mff) (P = 0·044) and peroxisomal biogenesis factor 11 beta (pex11β) (P = 0·006) was only higher in the HF/H-LC group as compared with the HF group, but not in the HF/L-LC group (Fig. 6(a)). The mRNA expression of acetyl-CoA acyltransferase (acca) (P = 0·101) and peroxisomal biogenesis factor 11 gamma (pex11γ) (P = 0·071) in the HF/H-LC group was higher than that in the HF group by at least 80 %, but the difference was not significant.

Fig. 6. Effect of dietary l-carnitine (LC) on peroxisomal β-oxidation and biogenesis in large yellow croaker. (a) mRNA level of genes relative to peroxisomal β-oxidation and biogenesis and (b) protein level of ACO and PMP70. Data are expressed as the means ± sem (n 6, but n 3 in protein expression). Mean values with unlike letters are significantly different (P < 0·05). ACO, acyl-CoA oxidase; ABCD4, ATP binding cassette subfamily D member 4; HSD17b4, hydroxysteroid 17-beta dehydrogenase 4; EHHADH, enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase; SCP2, sterol carrier protein 2; ACCA, acetyl-CoA acyltransferase; PEX5, peroxisomal biogenesis factor 5; PEX7, peroxisomal biogenesis factor 7; MFF, mitochondrial fission factor; PEX11a, peroxisomal biogenesis factor 11 alpha; PEX11β, peroxisomal biogenesis factor 11 beta; PEX11γ, peroxisomal biogenesis factor 11 gamma; PMP70, 70-kDa peroxisomal membrane protein.
The protein level of ACO was higher in the HF/L-LC (P = 0·038) and HF/H-LC (P = 0·062) groups as compared with the HF group (Fig. 6(b)). Additionally, the protein level of PMP70 was higher in the HF/L-LC group than in the HF group (P = 0·023), but no significant difference was found in the HF/H-LC and HF groups (P = 0·574) (Fig. 6(b)). These findings indicated that dietary LC can activate peroxisomal biogenesis and β-oxidation in fish fed with HFD.
Dietary l-carnitine suppressed the endoplasmic reticulum stress signalling pathway
ER stress can also regulate lipid metabolism in fish nutrition. We further investigated the effect of dietary LC on inhibiting the ER stress pathway in fish fed with HFD. The protein levels of GRP78 (P = 0·000) and p-eIF2α (P = 0·001) were decreased in the dietary LC-treated groups compared with the HF group (Fig. 7(a)). The protein levels of ATF6 were higher in the HF group as compared with the other two groups, but the difference between the HF and HF/L-LC groups was not significant (P = 0·255) (Fig. 7(a)). Additionally, the protein level of p-PERK was not significant among the three groups (P = 0·367) (Fig. 7(a)). The data demonstrated that dietary LC inhibited the GRP78, p-eIF2α and ATF6 signalling pathways in fish fed with HFD.

Fig. 7. Effect of dietary l-carnitine (LC) on ER stress in large yellow croaker. (a) Protein level of ER stress pathway and (b) mRNA level of genes relative to SREBP1 and its downstream pathway. Data are expressed as the means ± sem (n 6, but n 3 in protein expression). Mean values with unlike letters are significantly different (P < 0·05). GRP78, glucose regulated protein 78; PERK, PKR-like eukaryotic initiation factor 2a kinase; eIF2a, eukaryotic translational initiation factor 2a; ATF6, activating transcription factor 6, SREBP1, sterol-regulatory element binding protein 1; FAS, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; DGAT2, acyl-CoA: diacylglycerol acyltransferase 2.
ER stress regulates lipogenesis by activating SREBP1, and therefore, we tested the mRNA expression of sterol-regulatory element binding protein 1 (srebp1) and its downstream genes to assess the effect of dietary LC on lipogenesis in fish fed with HFD. The mRNA expression of srebp1 was lower in the HF/H-LC group than in the HF group by at least 30 %, but the difference was not significant (P = 0·064) (Fig. 7(b)). The mRNA expression of fatty acid synthase (fas) was higher in the HF group than in the HF/L-LC (P = 0·012) and HF/H-LC (P = 0·116) groups, but the difference was not significant between the HF and HF/H-LC groups (Fig. 7(b)). The mRNA expression of stearoyl-CoA desaturase 1 (scd1) was higher in the HF group than in the HF/L-LC (P = 0·012) and HF/H-LC (P = 0·09) groups, but the difference was only significant for the HF and HF/L-LC groups (Fig. 7(b)). The mRNA expression of acyl-CoA: diacylglycerol acyltransferase 2 (dgat2) was significantly lower only in the HF/H-LC group as compared with the HF group (P = 0·024) (Fig. 7(b)). These results suggest that dietary LC could potentially reduce hepatic lipogenesis in fish fed with HFD.
Discussion
Dietary l-carnitine showed potential lipid-lowering and antioxidant effects
Fatty liver disease is common in mammals and fish and is characterised by abnormal lipid deposition in the liver(Reference Angulo58,Reference Du59) . Previous studies showed that abnormal lipid deposition was prone to occur in the liver of large yellow croaker after feeding with HFD(Reference Yan, Liao and Wang38,Reference Wang, Li and Hou47) . The large size of liver lipid droplets stained with Oil Red O also proved that HFD induced severe lipid accumulation in this study. Thus, it is necessary to study the function of dietary LC in alleviating excessive lipid accumulation.
In this study, after feeding with dietary LC, the sizes of lipid droplets in the liver became smaller. As a consequence, the liver TAG and total lipid content were significantly reduced. Combined with the lower serum TAG levels, an efficient lipid-lowering effect from dietary LC was observed in large yellow croaker. Similar phenomena have also been reported in other farmed fish(Reference Li, Limbu and Ma31), medaka(Reference Fujisawa, Takami and Matsuzaki32), zebrafish(Reference Li, Li and Qin34), humans(Reference Liang, Li and Shan7), mice(Reference Su, Chang and Chou60) and other land animals(Reference Owen, Nelssen and Goodband6,Reference Seccombe, James and Hahn8) . Hence, dietary LC indeed is an indispensable optional nutritional factor that can be used to alleviate the dyslipidemia parameters induced by HFD in vertebrates.
Interestingly, through analysis results of MDA and total antioxidant capacity, the potential antioxidant capability of dietary LC was also determined in this study. Previous studies determined that LC reduced MDA levels, elevated enzymatic antioxidants (SOD, glutathione peroxidase, catalase, etc.) or upregulated the mRNA expression of genes related to the nuclear factor-erythroid 2-related factor 2 and Kelch-like ECH-associated protein 1 (Nrf2-Keap1) pathway in black sea bream (Sparus macrocephalus), common carp (Cyprinus carpio) and Amur minnow (Rhynchocypris lagowski)(Reference Wang, Luo and Ghonimy61–Reference Zhang, Guo and Zhao64). These studies in fish were also in accordance with those conducted using mammals, and commercial poultry and pigs that indicated that the antioxidant action of LC may be related to the scavenging of lipid peroxidation products and boosting of redox signalling via the activation of the Nrf2-Keap1 pathway(Reference Gulcin65–Reference Surai67). The decreased oxidative damage may protect liver integrity and slow the excessive release of alanine transaminase and aspartate transaminase from hepatocytes into the blood. Dietary LC can enhance the hepatic ability to cope with HFD intake in fish by relieving liver dysfunction.
Dietary l-carnitine can promote fatty acid β-oxidation
Enhancing FA oxidation is the primary mechanism used by LC to decrease the fat content in tissues and increase the ability to scavenge lipid peroxidation products(Reference Li, Limbu and Ma31). In the present study, dietary LC boosted the activity of CPT1 and its regulatory factor, PPARα, enhanced succinate dehydrogenase activity and increased ATP levels. The previous study showed that dietary LC was used to evaluate the mRNA expression and enzymatic activity of CPT1 in large yellow croaker fed with a terrestrial oil mixture(Reference Li, Chen and Chen46). LC also enhanced mitochondrial β-oxidation activities in zebrafish and Atlantic salmon (Salmo salar) and significantly upregulated the mRNA expression of CPT1 in zebrafish(Reference Ji, Bradley and Tremblay33,Reference Li, Li and Qin34) . Moreover, increased levels of acetyl-CoA and ATP were present in LC-treated medaka(Reference Fujisawa, Takami and Matsuzaki32). Thus, dietary LC can enhance mitochondrial β-oxidation efficiency to increase acetyl-CoA levels and further boost the tricarboxylic acid cycle to generate more ATP in fish, and these results were in accordance with results from previous experiments with mammalian subjects(Reference Zammit, Ramsay and Bonomini2,Reference Su, Chang and Chou60,Reference Arduini, Bonomini and Savica68) .
Mitochondrial biogenesis is another side of mitochondrial function(Reference Brand and Nicholls69). High fructose, free PA, HFD, hypoxia, ageing or disease can cause mitochondrial damage in vivo or in vitro in mammals(Reference Brand and Nicholls69–Reference Marcovina, Sirtori and Peracino71). Treatment with LC and its derivatives (e.g. acetyl-LC) can promote characteristics of mitochondrial biogenesis, such as increased mitochondrial DNA and AMPK activity, and increased PGC1α, NRF-1 or mitochondrial transcription factor A protein levels, to counteract these mitochondrial dysfunctions(Reference Montesano, Senesi and Vacante72–Reference Hota, Hota and Chaurasia77). However, the regulatory metabolism of dietary LC on mitochondrial biogenesis is still unknown in fish. This study in large yellow croaker indicated that dietary LC increased the number of mitochondria and enhanced the protein expression of mitochondrial biogenesis regulatory factors such as PGC1, PPARα and ERRα. Dietary LC can promote mitochondrial biogenesis via regulating the pathway of PGC1 and its downstream pathway, and then facilitating mitochondrial β-oxidation.
Mitophagy regulates mitochondrial number and energy through removing impaired mitochondria(Reference Palikaras, Lionaki and Tavernarakis78). In the present study, LC increased protein levels of PINK1, which is a marker of mitochondrial autophagy. Notably, LC decreased hepatic 8-hydroxydeoxyguanosine, which is one marker of oxidative mtDNA damage in this study. This suggested that LC may regulate mitophagy in fish. In mammals, LC treatment activated the main marker proteins of mitophagy, such as PINK1, Parkin, BCL2/adenovirus E1B 19 kDa protein interacting protein 3 (BNIP-3) and microtubule-associated protein 1 light chain 3 B-II (LC3B-II)(Reference Sabry, Ahmed and Maksoud73,Reference Choi, Ohn and Jung75) , and decreased hepatic 8-hydroxydeoxyguanosine in rats(Reference Chang, Nishikawa and Nishiguchi79). LC treatment may activate autophagy and eliminate impaired mitochondria. However, the regulatory mechanism used by LC on autophagy in fish requires further study in the future.
Mitochondrial function is the key to regulating FA oxidation. Peroxisomes do not require carnitine for the import of FA into their matrix and act with mitochondria to regulate FA β-oxidation(Reference Van Den Branden and Vamecq80–Reference Fransen, Lismont and Walton82). In this study and other studies involving mammals fed with HFD, LC treatment increased peroxisomal FA β-oxidation while mitochondrial FA β-oxidation increased(Reference Su, Chang and Chou60,Reference Mun, Soh and Cha83) , which are results similar to those obtained with 3T3-L1 adipocytes(Reference Lee, Lee and Lee84). However, in zebrafish and large yellow croaker not fed with HFD, the peroxisomal β-oxidation was not influenced by LC treatment(Reference Li, Li and Qin34,Reference Li, Chen and Chen46) . Thus, LC treatment may enhance peroxisomal FA β-oxidation to subsequently interact with mitochondria and elevate FA β-oxidation, but the positive effect may be prone to occur in the nutritional background of a HFD. Interestingly, LC treatment also regulated peroxisomal biogenesis in the present study. To the best of our knowledge, this is the first report that indicates that LC treatment can regulate peroxisomal β-oxidation and biogenesis in lipid metabolism.
Therefore, it is reasonably presumed that dietary LC improved mitochondrial and peroxisomal interaction via regulating their β-oxidation and biogenesis, as well as mitophagy, to maintain highly efficient FA utilisation in vertebrates.
Dietary l-carnitine inhibited the endoplasmic reticulum stress pathway
ER stress also regulates lipid metabolism(Reference Hotamisligil85,Reference Basseri and Austin86) . However, most studies examining the effect of dietary LC on ER stress were relative to reducing apoptosis, oxidative stress and myocardial damage(Reference Gao, Li and Zhu11,Reference Kong, Zheng and Liu13–Reference Zhang, Wu and Shen15) . The ability of dietary LC to affect lipid metabolism by regulating ER stress was only studied in rats fed with high-fructose corn syrup(Reference Unsal, Deveci and Ozmen12). This study showed that dietary LC decreased abdominal fat and liver weight and was accompanied with lower liver XBP1 activity but did not examine the effect on serum TAG or measure the amount of liver lipid. These studies further suggest that it is necessary to explore whether dietary LC can improve lipid metabolism via inhibiting ER stress, especially in animals fed a HFD.
In the present study, dietary LC inhibited GRP78, p-eIF2α and ATF6. Dietary LC regulated two of three major unfolded protein response transducers in fish fed with HFD. The downregulated mRNA expression of srebp1 and its downstream genes also indicated that there is an inhibitory effect of dietary LC on ER stress. The current study is the first to indicate that ER stress inhibition is involved in the process whereby dietary LC regulates lipid metabolism in vertebrates.
In the present study, dietary LC decreased the mRNA expression of genes relative to lipogenesis, such as fas, scd1 and dgat2. These results indicated that dietary LC can inhibit lipid synthesis and resulted in a lipid-lowering effect, which was consistent with the results of previous studies using zebrafish and large yellow croaker(Reference Li, Li and Qin34,Reference Li, Chen and Chen46) . Nevertheless, whether dietary LC can depress lipid synthesis through regulating ER stress requires careful evaluation in further studies.
To the best of our knowledge, there are no reports describing the regulation of dietary LC on the function of the mitochondria and ER in mammals or fish. This study systemically illustrated that dietary LC can improve lipid metabolism via simultaneously promoting mitochondrial β-oxidation and suppressing ER stress pathways in fish fed with HFD. There is close crosstalk and interconnection between the ER and mitochondria(Reference Rocha, Diaz-Morales and Rovira-Llopis87,Reference Blas-Garcia, Apostolova and Valls-Belles88) . Therefore, the interaction between mitochondrial β-oxidation and ER stress in the process of regulated lipid metabolism by dietary LC should be studied in the future, which will assist in increasing our understanding of the regulatory mechanism used by dietary LC on lipid metabolism.
Conclusion
Dietary LC can reduce the liver lipid content and improve serum lipid profiles in large yellow croaker fed with HFD. Dietary LC also increased the liver antioxidant capacity to relieve liver damage. These beneficial effects of dietary LC may be caused by the promotion of FA β-oxidation and the biogenesis of mitochondria and peroxisomes. Of note, dietary LC inhibited the ER stress pathway.
This trial is the first to reveal that dietary LC improved lipid metabolism through simultaneously enhancing FA β-oxidation capability and inhibiting the ER stress pathway in vertebrates. Therefore, supplementation with 2·4 ‰ LC in large yellow croaker fed with a HFD would be beneficial for lipid metabolism. LC is also recommended for use as a lipid-lowering additive in farmed fish, especially fish fed with HFD. Furthermore, LC could be used as a target to study the cooperation between FA β-oxidation and ER stress.
Supplementary material
To view supplementary material for this article, please visit http://doi.org/10.1017/S0007114522000101
Acknowledgement
The authors thank Dr Samwel Mchele Limbu, University of Dar es Salaam, Tanzania, and qualified native English-speaking editors at Charlesworth Author Services, for editing the English text of this manuscript.
This research was funded by the National Science Fund for Distinguished Young Scholars of China (31525024), National Natural Science Fund of China (31902379) and the China Agriculture Research System (CARS47-11), National Key Research and Development Program of China (2018YFD0900402), and Key Research and Development Project of Shandong Province (2019JZZY010814).
The authors’ contributions were as follows: Conceptualisation: Q. A. and J. L. (Jiamin Li); Writing – Original Draft Preparation: J. L. (Jiamin Li) and Z. Z.; Writing – review and editing: X. C., K. M. and Q. A; Data Curation: A. K. and J. L. (Jiamin Li); Formal Analysis: W. L. and J. L. (Jiamin Li); Methodology: W. X. and J. L. (Jiamin Li); Software: M. Z. and J. L. (Jiamin Li); Supervision: J. S., J. L. (Jinbao Li) and X. G.; Funding Acquisition: K. M. and Q. A. All authors read and approved the final manuscript.
The authors declare that there are no competing interests associated with the manuscript.












