Chronic kidney disease (CKD) is a prevalent complex disease and is fast becoming a growing public health problem, leading to premature mortality or poor quality of life, exposing a heavy burden on healthcare systems( Reference Xu, Ärnlöv and Banerjee 1 , Reference Jha, Garcia-Garcia and Iseki 2 ). Evidence available reveals that the global prevalence of CKD has a sharply increasing trend, affecting 10–15 % of general populations( Reference Hakemi 3 , Reference Levin, Tonelli and Bonventre 4 ). Ageing, hypertension, diabetes, hyperlipidaemia, smoking and poor dietary intake have been identified as risk factors for CKD occurrence( Reference Tohidi, Hasheminia and Mohebi 5 , Reference Kazancioglu 6 ). Higher dietary intake of plant protein, vitamin C, Mg, K, n-6 fatty acids and lower intake of animal protein and Na are documented as the potential factors for prevention of CKD occurrence( Reference Farhadnejad, Asghari and Mirmiran 7 , Reference Yuzbashian, Asghari and Mirmiran 8 ). Yet, dietary guidelines have not been developed for the prevention of kidney dysfunction.
Although a great deal of research has been conducted on the importance of dietary fibre intake in the prevention of CVD, diabetes and cancer, encouraging healthcare organisations to recommend dietary fibre intake( Reference King 9 , Reference Lattimer and Haub 10 ), findings on the dietary fibre and kidney function are limited and inconsistent( Reference Gopinath, Harris and Flood 11 – Reference Sorensen, Hsi and Chi 13 ). One cohort study indicates that subjects in the highest quartile of dietary cereal fibre, compared with those in the lowest, had a 50 % decreased risk for incidence of CKD; however, total fibre intake was not associated with the occurrence of CKD( Reference Gopinath, Harris and Flood 11 ). Xu et al.( Reference Xu, Huang and Riserus 12 ) showed that higher total fibre intake was positively associated with estimated glomerular filtration rate (eGFR) among elderly men. In addition, greater dietary total fibre intake was associated with reduced risk of incident kidney stones in postmenopausal women( Reference Sorensen, Hsi and Chi 13 ).
Previous studies on dietary fibre intake and kidney function have been conducted in developed countries. The Tehran Lipid and Glucose Study (TLGS) is a large prospective cohort study with different dietary habits required for substantiating and persuasion of preventive dietary recommendations. Current TLGS analyses showed that plant-based diets rich in fibre were related to a decrease in incident CKD( Reference Asghari, Farhadnejad and Mirmiran 14 , Reference Asghari, Yuzbashian and Mirmiran 15 ); total dietary fibre intake was also associated with reduced CVD risk( Reference Mirmiran, Bahadoran and Khalili Moghadam 16 ), and fruit fibre was associated with decreased the metabolic syndrome( Reference Hosseinpour-Niazi, Mirmiran and Mirzaei 17 ).
The purpose of this study was primarily to evaluate the association of total fibre intake with the risk of incident CKD. We also evaluated the association of dietary fibre from fruits, vegetables, cereals and legumes with the incidence of CKD in a population-based prospective study.
Methods
Study population
This study was conducted within the framework of the TLGS, an ongoing community-based prospective investigation aimed at preventing non-communicable diseases (NCD) by developing programmes promoting healthy lifestyles and reducing NCD risk factors in a sample of residents under coverage of three medical health centres in District No. 13 of Tehran, the capital city of Iran( Reference Azizi, Ghanbarian and Momenan 18 ). The baseline survey was a cross-sectional study conducted from 1999 to 2001, and surveys II (2002–2005), III (2006–2008), IV (2009–2011) and V (2012–2015) are prospective follow-up surveys.
From among the 12 523 participants examined in the third survey of the TLGS, 3462 were randomly selected for dietary assessment, of whom 2417 participants were aged ≥27 years. Subjects with a history of myocardial infarction or stroke due to the possibility of major changes in diet were excluded (n 34). In addition, subjects (n 113) who under- or over-reported energy intakes (<3347 kJ/d (<800 kcal/d) or >17 573 kJ/d (>4200 kcal/d), respectively) and those with missing data on covariates (n 52) were excluded; some individuals fell into more than one exclusion category. To evaluate the incidence, we also excluded subjects who had CKD at baseline (n 360). Finally, 1630 participants were followed up until survey V (response rate: 87 %), with a median duration of 6·1 years (25–75 interquartile range: 5·6–6·5; Fig. 1).
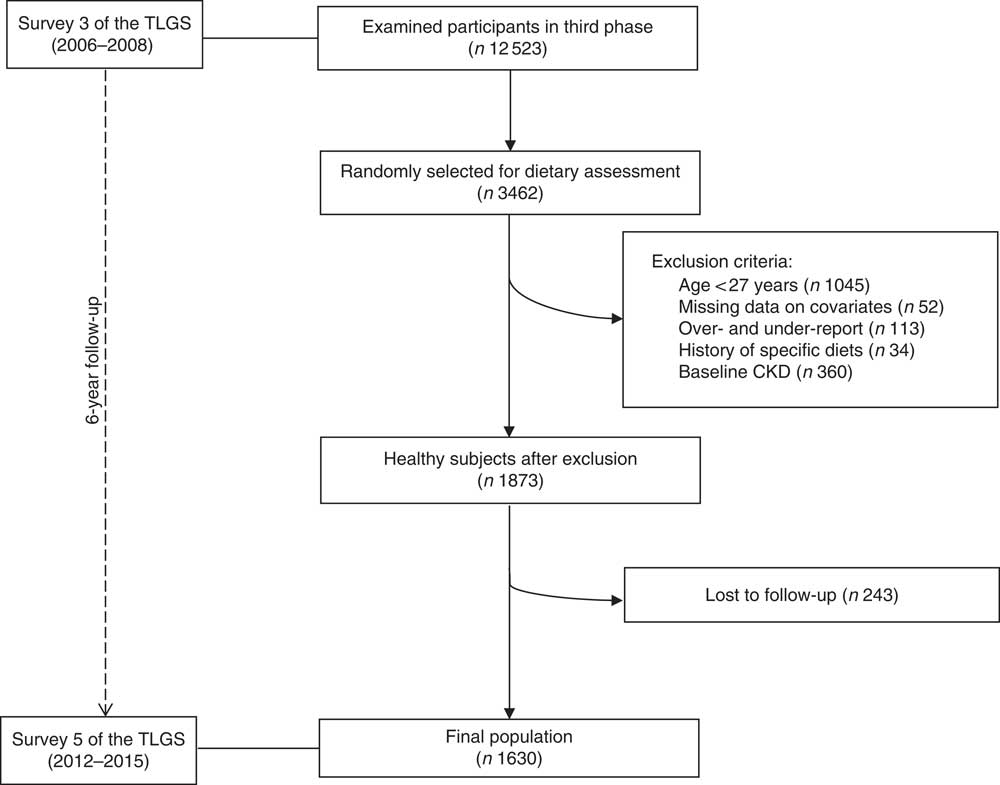
Fig. 1 Flow chart of the Tehran Lipid and Glucose Study (TLGS) participants. CKD, chronic kidney disease.
The ethics committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences approved the study protocol and written informed consent was obtained from all participants.
Measurements
Dietary measurements
Habitual dietary intakes were assessed using a valid and reliable semi-quantitative FFQ by expert interviewers( Reference Asghari, Rezazadeh and Hosseini-Esfahani 19 – Reference Mirmiran, Esfahani and Mehrabi 21 ). Trained dietitians, during face-to-face interviews, asked participants to designate their consumption frequency for each food item consumed during the previous year on a daily, weekly and monthly basis. As the Iranian food composition table (FCT) is incomplete, the United States Department of Agriculture (USDA) FCT was used. For national foods not listed in the USDA FCT, the Iranian FCT was the alternative. We calculated total dietary fibre, as well as fibre contribution from cereals, legumes, vegetables and fruits.
The reliability and validity of the FFQ, which were evaluated against twelve 24-h dietary recalls and two FFQ in a previous study, indicated that the FFQ provides reasonably valid measures of the average long-term dietary fibre intake( Reference Asghari, Rezazadeh and Hosseini-Esfahani 19 – Reference Mirmiran, Esfahani and Mehrabi 21 ).
Measurement of covariates
Information on physical activity was collected by using the modifiable activity questionnaire (MAQ) to calculate metabolic equivalent task (MET)-minutes per week. High reliability (98 %) and moderate validity (47 %) were found for the Persian translation of MAQ( Reference Momenan, Delshad and Sarbazi 22 ). Low levels of physical activity were considered as MET <600 min/week. Weight was recorded in light clothing to the nearest 0·1 kg on a SECA digital weighing scale (Seca 707; Seca Corporation; range 0·1–150 kg) and height was measured without shoes to the nearest 0·1 cm. BMI was calculated as weight (kg) divided by square of height (m2). Arterial blood pressure was measured manually, using a mercury sphygmomanometer with a suitable cuff size for each participant after a 15-min rest in the supine position. Systolic blood pressure (SBP) was determined by the onset of the tapping Korotkoff sound, whereas diastolic blood pressure (DBP) was determined as the disappearance of the Korotkoff sound. Blood pressure was measured twice and the average was considered as the participant’s blood pressure.
Blood samples were taken from all participants at the TLGS research laboratory after an overnight fast of 12–14 h. Fasting plasma glucose (FPG) and 2-h plasma glucose (equivalent to 75 g anhydrous glucose; Cerestar EP) were assayed by enzymatic colorimetric method using glucose oxidase, with both inter- and intra-assay CV being <2 %. Serum creatinine was measured according to the standard colorimetric Jaffe_Kinetic reaction method at baseline (2006–2008) and after 6 years of follow-up (2012–2015). Both intra- and inter-assay CV were below 3·1 %; all analyses were performed using commercial kits (Pars Azmoon Inc.).
Definitions
Hypertension was defined as SBP/DBP≥140/90 mmHg or current therapy for a definite diagnosis of hypertension( Reference Lenfant, Chobanian and Jones 23 ). Diabetes was defined according to the criteria of the American Diabetes Association as FPG≥7·0mmol/l or 2-h post 75-g glucose load≥11·1 mmol/l or current therapy for a definite diagnosis of diabetes( 24 ). We used the Modification of Diet in Renal Disease (MDRD) equation formula to express eGFR in ml/min per 1·73 m2 of body surface area( Reference Levey, Bosch and Lewis 25 ). The abbreviated MDRD study equation is as follows:
eGFR=186×(serum creatinine)−1·154×(age)−0·203×(0·742 if female)×(1·210 if African-American).
Patients were classified based on their eGFR levels by the National Kidney Foundation guidelines( 26 ); eGFR≥60 ml/min per 1·73 m2 as not having CKD and eGFR<60 ml/min per 1·73 m2 as having CKD.
Statistical analysis
All data were analysed using the Statistical Package for the Social Sciences program (SPSS) (version 15.0; SPSS Inc.) and P values<0·05 were considered statistically significant. Total dietary intake of fibre was categorised into the tertile cut-off points as ≤17·7, 17·8–26·0 and >26·1 g/d; dietary fibre from fruits, vegetables, cereals and legumes was categorised into three groups according to the tertiles of the distribution among the total population. Continuous variables were reported as a mean ± standard deviation and categorical variables as percentages. Tests of a trend for continuous variables across tertiles of the total fibre intake were conducted using ANOVA and for categorical variables χ 2 test was used.
The OR and 95 % CI for the incidence of CKD according to tertiles of dietary exposure was assessed with multivariable logistic regression models. In this analysis, the first tertile of dietary exposure was considered as the reference category. To calculate the trend of OR across increasing tertiles of dietary exposure, the median values of each fibre category were considered as continuous variables. Three models were considered to adjust potential confounders (model 1, crude (without any covariate); model 2, age (continuous), sex, smoking (yes/no), total energy intake (continuous), physical activity (low, moderate, heavy); and model 3, diabetes (yes/no) and using angiotensin-converting-enzyme inhibitor (yes/no)). As a further adjustment for intakes of dietary fat, n-3 fatty acids, K, Mg and hypertension did not change the relations substantially, these covariates were not considered in the final models.
Results
We recorded 220 (13·5 %) cases of incident CKD, with a range of eGFR between 29 and 59 ml/min per 1·73 m2, after 6·1 years of follow-up. Mean age and total fibre intake of participants was 42·8 (sd 11·2) years and 23·8 (sd 11·7) g/d. General characteristics of study participants across tertiles of total fibre intake are presented in Table 1. Participants in the highest, compared with the lowest, tertile of total fibre intake were less likely to be women (P<0·05). No significant differences were found by means of age, BMI and eGFR and prevalence of low physical activity, current smoker, diabetes, hypertension and antihypertensive drug across tertiles of total fibre intake.
Table 1 Baseline characteristics of participants according to tertiles (T) of total fibre intake (Mean values and standard deviations; percentages)
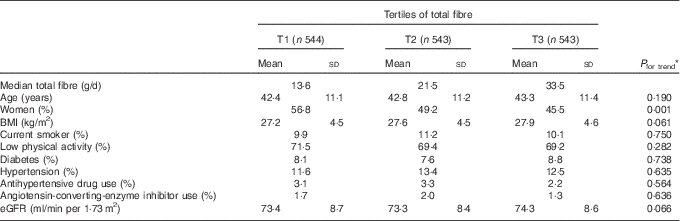
eGFR, estimated glomerular filtration rate.
* Tests of trend for continuous variables across tertiles of total fibre intake were conducted using ANOVA, and for categorical variables χ 2 test was used.
Dietary intakes of participants across tertile categories of total fibre intake are shown in Table 2. Participants in the top tertile of total fibre intake had higher consumption of protein, plant protein, carbohydrate and K than those in the bottom tertile (P<0·05); however, consumption of animal protein, total and SFA tended to decrease across tertiles of total fibre intake (P<0·05).
Table 2 Baseline dietary intakes of participants, according to tertiles (T) of total fibre intake
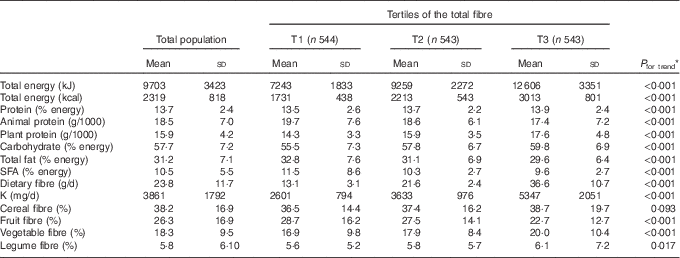
* Test of trend across tertiles of total fibre intake was conducted using ANOVA.
In the crude model, total fibre intake was inversely associated with an incidence of CKD (Table 3). After adjustment for age, sex, smoking, total energy intake, physical activity, diabetes and using angiotensin-converting-enzyme inhibitor, the OR for subjects in the highest compared with the lowest tertile of total fibre intake was 0·47 (95 % CI 0·27, 0·86). A significant decreasing linear trend was noted across tertiles of total fibre intake for risk of incident CKD (P for trend<0·001). In addition, the risk of incident CKD decreased by 11 % for every 5 g/d increase in total fibre intake.
Table 3 Incident chronic kidney disease according to the dietary fibre intake and per 5-g increase in intake among participants of the Tehran Lipid and Glucose Study (Odds ratios and 95 % confidence intervals)
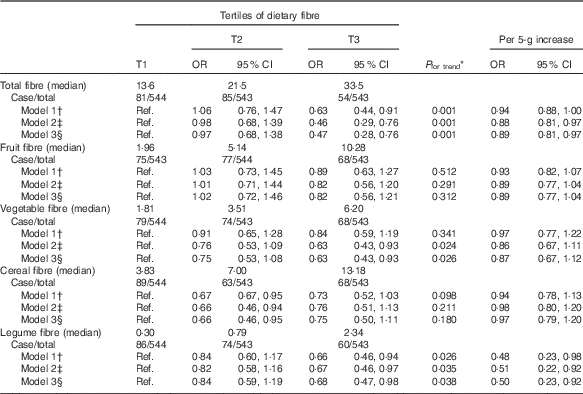
Ref., referent values.
The tertile range for each category was ≤17·7, 17·8–26·0 and >26·1 g/d for total fibre; ≤3·5, 3·6–7·2 and >7·3 g/d for fruit fibre; ≤2·6, 2·7–4·5 and >4·6 g/d for vegetable fibre; ≤5·4, 5·5–9·4 and >9·5 g/d for cereal fibre; and ≤0·5, 0·6–1·2 and >1·3 g/d for legume fibre.
* P for trend across tertiles calculated with the exposure modelled as a continuous variable.
† Model 1: crude.
‡ Model 2: adjusted for age, sex, smoking, total energy intake, physical activity.
§ Model 3: additionally adjusted for diabetes and using angiotensin-converting-enzyme inhibitor.
We also examined the association between various sources of dietary fibre and incidence of CKD. After adjusting for potential confounders, OR for participants in the highest compared with the lowest tertile of fibre from vegetables was 0·63 (95 %CI 0·43, 0·93) and from legumes it was 0·68 (95 % CI 0·47, 0·98). In addition, a significant decreasing linear trend was noted across tertiles of dietary fibre from vegetables and legumes for the risk of incident CKD (P for trend<0·05). The risk of incident CKD decreased 50 % for every 5 g/d increase in dietary fibre from legumes. Furthermore, there was no significant association of fibre from fruits and cereals with the risk of incident CKD.
Discussion
This population-based prospective study showed that high total fibre intake was related to lower incidence of CKD after 6·1 years of follow-up. Subjects who consumed more than 26·0 g/d of fibre had 50 % lower risk of CKD occurrence compared with those who consumed ≤17·7 g/d. We also observed an 11 % lower risk of incident CKD per 5-g/d increase in total fibre intake. Protective associations of specific sources of fibre with the risk of incident CKD were observed for vegetable and legume fibre. No association was observed between the risk of CKD and the cereal or fruit fibre intake.
Relatively limited studies investigated the association of total fibre intake with CKD( Reference Gopinath, Harris and Flood 11 , Reference Xu, Huang and Riserus 12 , Reference Diaz-Lopez, Bullo and Basora 27 , Reference Metcalf, Baker and Scragg 28 ). Data of 2600 participants aged ≥50 years from Blue Mountains Eye Study showed no significant association of fibre intake with the prevalence of moderate CKD( Reference Gopinath, Harris and Flood 11 ). However, among 1110 participants aged 70–71 years, higher intake of total fibre had desirable association with eGFR( Reference Xu, Huang and Riserus 12 ). In a cross-sectional study of non-diabetic individuals from the PREDIMED study, among subjects in the highest quartile of fibre intake the risk of incident CKD decreased by 42 %( Reference Diaz-Lopez, Bullo and Basora 27 ). In addition, in 5416 subjects, aged >40 years, dietary fibre consumption was found to be associated with a reduced risk of albuminuria( Reference Metcalf, Baker and Scragg 28 ). Besides studies investigating the association of dietary fibre on CKD, there are studies that show other desirable effects of dietary fibre on kidney-related disorders( Reference Sorensen, Hsi and Chi 13 , Reference Huang, Ding and Chen 29 ). A recent meta-analysis showed that total fibre intake was associated with 26 % lower risk of renal cell carcinoma( Reference Huang, Ding and Chen 29 ). In addition, women with no history of kidney stones in the highest compared with the lowest categories of dietary fibre intake had a 22 % decreased risk of stone formation( Reference Sorensen, Hsi and Chi 13 ).
In our study, participants in the highest tertile of dietary fibre intake showed lower consumption of animal protein and saturated fat and higher consumption of plant protein and K in comparison with those in the lowest tertile. Indeed, higher fibre intake was accompanied by healthier dietary behaviour that may affect kidney function; however, there were no differences between participants in the tertiles of fibre intake according to the BMI, current smoker, physical activity and anti-hypertensive drug use. In a previous study, we observed that independent of hypertension and diabetes, higher intakes of plant protein and PUFA had a decreasing effect on risk of CKD, whereas animal protein increased the risk of CKD( Reference Yuzbashian, Asghari and Mirmiran 8 ). Furthermore, the foods rich in fibre are the sources of K as well. K can bind to organic anions and metabolised to bicarbonate, so that the net rate of endogenous acid production in comparison with the rate of acid production from animal foods decreased( Reference Phisitkul, Khanna and Simoni 30 ). Hence, plant-based diet by providing alkali might decrease the risk of CKD( Reference Mirmiran, Yuzbashian and Bahadoran 31 ).
According to the present study, fibre from vegetables and legumes, but not from fruits and cereals, was significantly associated with incident CKD. We also found that per 5-g/d increase in intake of fibre from legumes led to 50 % lower risk of incident CKD. Gopinath et al.( Reference Gopinath, Harris and Flood 11 ) observed that participants in the highest compared with those in the lowest quartile of dietary cereal fibre intake had a 50 % reduced risk of incident CKD; however, there were no significant findings between fibre from fruits and vegetables and the risk of CKD. Our results on kidney function are consistent with a previous meta-analysis that showed that the risk of renal cell carcinoma was inversely associated with legume and vegetable fibre intake, but not with fibre intake from fruits and cereals( Reference Huang, Ding and Chen 29 ). Dietary intake of legumes decreased the risk of incident CKD by 17 %, after 23 years of follow-up( Reference Haring, Selvin and Liang 32 ). People with higher scores of the diet rich in vegetables and legumes such as the Mediterranean diet had 51 % less risk of developing CKD( Reference Asghari, Farhadnejad and Mirmiran 14 ). Therefore, on the basis of the findings from our study and others, health benefits of fibre intake on kidney function may depend on the food source of dietary fibre. A previous report on cardiovascular outcomes has similarly shown the effects of fibre from different food sources( Reference Kan, Stevens and Heiss 33 ).
In our study, we observed that, contrary to the dietary fibre from vegetables and legumes that simultaneously increased with total dietary fibre, intakes of fibre from cereals and fruits did not have a similar trend with that of total dietary fibre intake. Therefore, we found no significant relation of cereals and fruits with the incidence of CKD.
The beneficial effects of dietary fibre from legumes and vegetables on kidney function can be explained by several potential factors: first, dietary fibre from legumes may decrease the glycaemic index of consumer foods leading to a delay in the postprandial glycaemia( Reference Trinidad, Mallillin and Loyola 34 ). Second, dietary fibre sources (vegetables and legumes) are also rich in antioxidants and vitamins. Third, vegetable and legume fibre consumption can decrease the risk of incident CKD by attenuating known risk factors such as diabetes, hypertension, hyperlipidaemia and low-grade inflammation( Reference Ma, Hebert and Li 35 – Reference Levey, Coresh and Balk 37 ). Higher dietary fibre intake was inversely associated with improved glycaemic control, increased insulin sensitivity and reduced risk of type 2 diabetes, all of which are well known for their harmful effects on kidney function( Reference Yao, Fang and Xu 38 , Reference Fujii, Iwase and Ohkuma 39 ). Another potential risk factor for CKD is hypertension. The blood pressure-lowering effect of dietary fibre was demonstrated in a recent meta-analysis( Reference Whelton, Hyre and Pedersen 40 ).
There are some limitations to this study that need to be mentioned. First, as in most epidemiological studies, our definition of CKD is based on a limited number of isolated creatinine measurements that were not repeated within 3 months to confirm a chronic reduction in GFR. Second, despite controlling for various confounders in our analysis, residual confounding due to unknown or unmeasured confounders cannot be excluded.
Of the study’s noteworthy strengths are the use of a complex validated 168-question FFQ and a prospective cohort design with high-quality data and low loss to follow-up. In addition, unlike previous studies, the present study provided data based on habitual dietary intakes in a population-based sample of participants, thereby increasing the generalisability of its results.
We observed inverse associations between total fibre intake and risk of incident CKD – results that show that high fibre intake, mainly from legumes and vegetables, may reduce the occurrence of CKD. This study provides important insight into the beneficial effects of the nutritional management for prevention of CKD and the desirable effects of dietary fibre intake in a Middle-Eastern population that is distinctly different from developed countries. Prospective studies in other populations are needed to confirm these associations and provide the evidence needed to translate these findings into clinical results.
Acknowledgements
The authors express their appreciation to the participants of the Tehran Lipid and Glucose Study for their enthusiastic support, and the staff of the Tehran Lipid and Glucose Study Unit of the Research Institute for Endocrine Sciences for their valuable help. The authors would like to acknowledge Ms Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
This work was funded by a grant from the Research Institute for Endocrine Sciences, Shadid Beheshti University of Medical Sciences, Tehran, Iran.
G. A., S. S and E. Y. designed the research; E. Y. analysed and interpreted data; and G. A., S. S. and E. Y. drafted the initial manuscript. P. M. and F. A. supervised the project and approved the final version of the manuscript to be submitted. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.









