Mg is one of the important ions that maintain nerve, muscle and heart stress. Mg plays a key role in a wide range of cellular functions known to affect many aspects of the endocrine system(Reference De Baaij, Hoenderop and Bindels1–Reference Gröber, Schmidt and Kisters4). Many studies showed that lower serum or urinary Mg was associated with increased risk of ischaemic stroke(Reference Joosten, Gansevoort and Mukamal5), CHD and cardiovascular mortality(Reference Del Gobbo, Imamura and Wu6,Reference Qu, Jin and Hao7) . The main cause of hypomagnesaemia in humans is usually inadequate dietary intake. Dietary Mg is mainly absorbed by the small intestine via passive cell-side transport, which is driven by an electrochemical gradient and solvent drag(Reference Graham, Caesar and Burgen8). Low Mg intakes raise inflammatory and CVD risks and increasing dietary Mg intake is associated with a reduced risk of stroke, heart failure, diabetes and all-cause mortality(Reference King, Mainous and Geesey9–Reference Dong, Xun and He11). Some studies have shown that serum Mg concentrations was negatively associated with type 2 diabetes and indices of glycaemic control and insulin resistance(Reference Bertinato, Wang and Hayward12). There is also a literature view that serum Mg concentration is positively correlated with diabetes and glycaemic control index and insulin resistance(Reference Guerrero-Romero and Rodríguez-Morán13–Reference Huerta, Roemmich and Kington15).
Blood cell count is a commonly used and widely available test method and is also associated with type 2 diabetes or obesity to some extent(Reference Twig, Afek and Shamiss16–Reference Barazzoni, Gortan Cappellari and Semolic19). In addition, in many studies, the use of Mg and serum Mg for disease treatment has been explored based on the relationship between Mg and blood cells(Reference Van Orden, Eggett and Franz20–Reference Than, Soe and Palaniappan23). There is data suggesting that magnesium valproate is beneficial as an anti-diabetic agent in type 2 diabetes mellitus and also prevents its cardiac complications(Reference Bhoomika, Raghunathan and Porwal24). Study indicates that type 2 diabetic patients have intracellular Mg defieiency and that oral Mg supplementation can increase the intracellular levels towards normal. In addition, Mg supplementation markedly reduces platelet (PLT) aggregation in response to known agonists of PLT aggregation(Reference Nadler, Shaw and Malayan25). There was no significant correlation between serum Mg and PLT counts in maintenance haemodialysis patients(Reference Rafieian-Kopaie and Nasri26). However, studies on the relationship between serum Mg and human blood cell counts, especially for type 2 diabetes and central obesity, are rare.
The aim of our study was to assess changes in the relationship between serum Mg and blood cell count in China adults with type 2 diabetes or central obesity. Using the Metabolic, Life-style and Nutrition Assessment in adults survey – China Health and Nutrition Survey (CHNS), we report that an elevated Hb, erythroctye and PLT count increased across progressive Mg groups in some subgroups.
Research design and methods
Study design
The CHNS is a large-scale, national cross-sectional survey that was designed to investigate the health and nutritional status among Chinese residents. Currently, data are available for 2009. A stratified multistage cluster random process was used to draw samples from nine provinces of China, which included Shandong, Henan, Liaoning, Hunan, Heilongjiang, Jiangsu, Hubei, Guangxi and Guizhou. All participants voluntarily joined the present study with informed consent and the study was approved by institutional review board from the University of North Carolina at Chapel Hill and Chinese Center for Disease Control and Prevention.
Study population
Participants included in the present study were aged 18 years or older. Information on age, sex, region, activity level, dietary behaviour was collected. There were 8163 participants included in the survey organised in 2009. This sample is varied, with variation found in a wide range of related biochemical markers, healthy factors, nutritional and demographic measures(Reference Popkin, Du and Zhai27). In addition, blood counts included leucocyte count, erythrocyte count and PLT count.
The present study is a cross-sectional analysis with complete information for the determination of type 2 diabetes, central obesity, blood cell count and blood Mg, as described later. Every subject underwent same examinations during this survey.
Measurements and definitions
Testing of blood cell counts was completed in local laboratories of each site in accordance with consensus guidelines of the CHNS. Testing of glycosylated Hb (HbA1c) and blood Mg was completed only in the provincial laboratories, which meets all requirements for accurate measurement and testing. The method of routine blood testing was the 3 or 5 classification automated haematology analyser. (Blood samples were analysed using the Sysmex XE-2100D automated haematology analyser. For the method of the specific machine, follow its standard operating procedure.)
Weight was measured to the nearest 0⋅1 kg with lightweight clothing on a calibrated beam scale and height was measured to the nearest 0⋅1 cm without shoes using a portable stadiometer. BMI was calculated as weight in kilograms divided by the square of height in metres. Waist circumference (WC) was measured at a point midway between the lowest rib and the iliac crest in a horizontal plane using non-elastic tape. Height, weight and WC were measured by trained examiners following a standard protocol from the WHO(Reference Alberti, Zimmet and Shaw28). Height, weight and WC measurements were made at the same location and followed the same protocol at each study visit.
Central obesity was defined by WC >90 cm for men and >80 cm for women(Reference Alberti, Zimmet and Shaw28). Laboratory data were obtained within 2 months of the questionnaire visit.
Subjects were initially classified with type 2 diabetes (yes/no) and central obesity, and then further classified according to the blood Mg (below 0⋅65 mmol/l, or 0⋅66–0⋅94 mmol/l or above 0⋅95 mmol/l) in accordance with classification of the CHNS.
Additional information based on interviews and the physical examination of patients at the time of the visit and data from clinical records included age, sex, weight, height, BMI, dyslipidemia, dietary factors and use of anti-diabetic drugs.
Statistical methods
Differences between Mg groups in socio-demographic and clinical characteristics were evaluated by ANOVA for continuous variables and the χ2 test for categorical variables. In particular, we calculated and tested the differences in blood cell count among the three Mg groups: in the total sample, type 2 diabetes (yes/no) and central obesity (yes/no) subgroups.
A generalised linear model of the association between blood cell count (leucocytes, erythrocytes, PLT) and Mg (three groups) was built adjusting for age (continuous), sex, BMI (continuous), WC (continuous), type 2 diabetes (yes/no), anti-diabetic drugs treatment (yes/no), insulin injection (yes/no), blood pressure (continuous), energy intake (continuous), fat intake (continuous), protein intake (continuous), carbohydrate intake (continuous), HbA1c (%), urea (continuous), uric acid (continuous), apo A-1 (continuous), apo B (continuous), lipoprotein (continuous), creatinine (continuous), HDL-cholesterol (continuous), LDL-cholesterol (continuous) and insulin (continuous).
Separate adjusted models were also built for subjects with and without type 2 diabetes. As a sensitivity analysis, we performed more parsimonious models, excluding adjustment for some variables dealing with possible collinearity among some covariates. P < 0⋅05 were considered to be statistically significant.
Results
A total of 8163 subjects (with mean age of 50⋅9 years and 46⋅7 % males) were included in this analysis. Most of them (91⋅7 %) were without type 2 diabetes and 38⋅4 % had central obesity. The mean clinic blood cell count (leucocytes, erythrocytes, PLT) were 6⋅3 (×109/l), 4⋅7 (×109/ml) and 213⋅0 (×109/l) and the mean blood Mg was 0⋅94 mmol/l.
In participants with type 2 diabetes, shown in Table 1, 52⋅2 % had normal Mg, 1 % had low Mg (≤0⋅65 mmol/l) and 46.8 % had high Mg (≥0⋅95 mmol/l). Subjects with high Mg were characterised by significantly older age (P < 0⋅0001), higher blood pressure (P < 0⋅0001) and higher WC (in male) (P < 0⋅0001), but these differences were not clinically meaningful (P > 0⋅05).
Table 1. Sample characteristics according to magnesium status (Mean values and standard deviations; numbers of participants and percentages)

HbA1c, glycated Hb.
* To convert energy in kcal to kJ, multiply by 4⋅184. To convert uric acid in mg/dl to μmol/l, multiply by 59⋅48. To convert lipoprotein in mg/dl to μmol/l, multiply by 0⋅0357. To convert insulin in μIU/ml to pmol/l, multiply by 6⋅945.
The presence of Mg was accompanied by a significantly higher prevalence in various biochemical indicators (P < 0⋅01). These biochemical indicators include all the analytical values in Table 1 except for lipoprotein and HDL-cholesterol values.
Table 2 shows blood cell count values in the whole group according to the central obesity category and Mg status. A significant increase for blood cell counts, with the exception of leucocyte and PLT count, across progressive Mg groups (from low Mg ≤ 0⋅65 mmol/l, normal Mg 0⋅66–0⋅94 mmol/l to high Mg ≥ 0⋅95 mmol/l) in all subjects were noted (P < 0⋅05). In addition, when compared with the group without central obesity, the mean increment in PLT among subjects with central obesity was 211⋅1 (×109/l) in the group with Mg ≤ 0⋅65 mmol/l, 216⋅8 (×109/l) in the group with Mg 0⋅66–0⋅94 mmol/l and 217⋅2 (×109/l) in the group with Mg ≥ 0⋅95 mmol/l. Unlike the whole subjects and central obesity groups, a significant increase for PLT across progressive Mg groups in the subjects without central obesity group was noted (P < 0⋅01). HbAc1, leucocytes and PLT were significantly higher among subjects with central obesity than without central obesity (P < 0⋅05). HbAc1, leucocytes and erythrocytes were significantly higher among subjects with type 2 diabetes than without (P < 0⋅05).
Table 2. Blood cell count and Hb values according to central obesity and magnesium status (Mean values and standard deviations)
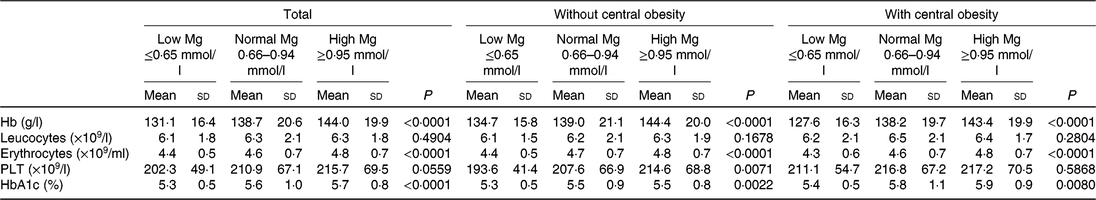
PLT, platelets; HbA1c, glycated Hb.
Hb, erythrocytes, but not PLT and leucocytes, were significantly higher among subjects with higher Mg than in subjects with lower Mg (P < 0⋅05) both in central obesity (yes/no) subgroups.
Data for subjects with and without type 2 diabetes are presented in Table 3. As depicted in Table 3, a significant increase for Hb, erythrocytes, PLT across progressive Mg groups was observed in subjects without type 2 diabetes (P < 0⋅05). However, no significant changes for Hb, leucocytes, erythrocytes, PLT across progressive Mg groups were observed in subjects with type 2 diabetes and without central obesity (P > 0⋅05).
Table 3. Blood cell count, Hb and glycated Hb (HbA1c) values according to central obesity and magnesium status in patients without type 2 diabetes and in patients with diabetes (Mean values and standard deviations)
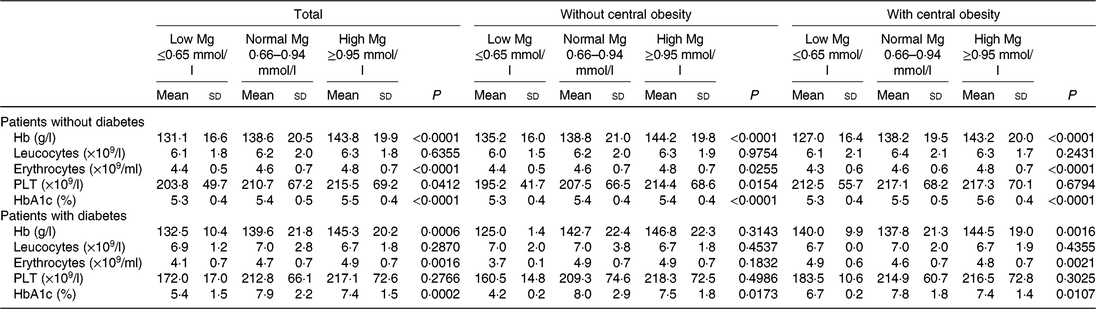
PLT, platelets.
Multivariable analysis of blood cell count with magnesium
The generalised linear model showed that after full adjustment for demographic characteristics, lifestyles, dietary factors and clinic variables (including diabetes, blood glucose, level, anti-diabetic drugs treatment, insulin injection, blood pressure, urea, uric acid, apo A-1, apo B, lipoprotein, creatinine, HDL-cholesterol, LDL-cholesterol, insulin), Hb, erythrocytes, PLT, but not leucocytes, were significantly higher among subjects with higher Mg than in subjects with lower Mg (P < 0⋅05). Multivariable models for leucocytes failed to attain statistical significance. When the parsimonious model was used, the aforementioned general pattern was quite similar (Table 4). And also, this pattern was noted as quite similar among subjects without type 2 diabetes by using a generalised linear model or parsimonious model. However, both multivariable models for PLT failed to attain statistical significance among subjects with type 2 diabetes, different from subjects without type 2 diabetes.
Table 4. Generalised linear model of the association between blood cell count, Hb and magnesium according to diabetes status* (Mean values and 95 % confidence intervals)
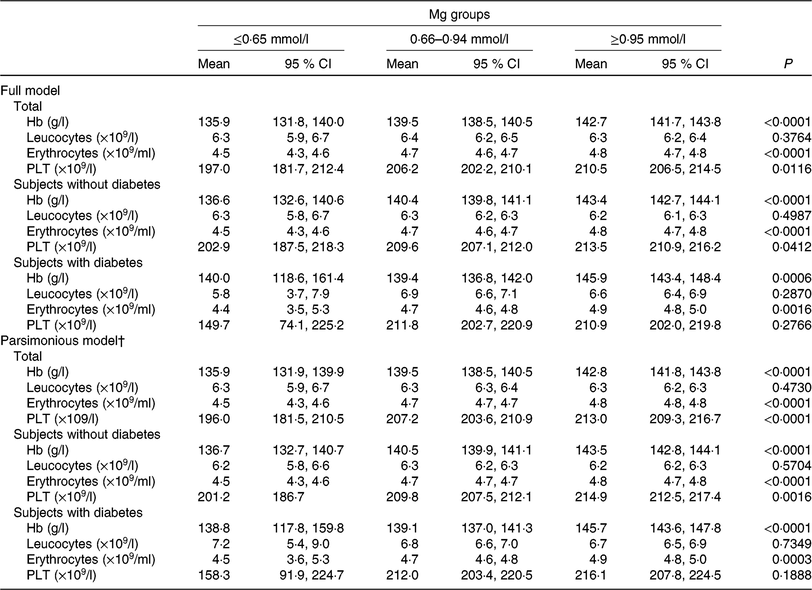
PLT, platelets.
* Models were adjusted for demographic characteristics, lifestyles, dietary factors and clinic variables (including diabetes, blood glucose level, antidiabetic drugs treatment, insulin injection, blood pressure, urea, uric acid, apo A-1, apo B, lipoprotein, creatinine, HDL-cholesterol, LDL-cholesterol, insulin).
† The parsimonious model adjusted for the same variables as the full model except for some variables dealing with possible collinearity among some covariates.
Discussion
The previous study shows that central obesity increased the risk of type 2 diabetes in Chinese people and was associated with increasing glucose. In central obesity, diet-induced weight loss reduces PLT activation(Reference Russo, Traversa and Bonomo29). In our study, central obesity disturbed the positive association between PLT count and serum Mg. However, this positive association was still noted in subjects without central obesity.
These data demonstrate that the presence of higher serum Mg is not associated with significant and substantial increases or decrease in leucocyte count, irrespective of central obesity and existence of type 2 diabetes. Erythrocytes, PLT and Hb are significantly and substantially higher in the presence of higher serum Mg in patients without type 2 diabetes and without central obesity. On the contrary, PLT is not associated with serum Mg among patients with type 2 diabetes or central obesity. Even after full adjustment for demographic characteristics, lifestyles, dietary factors and biochemical markers, increasing trends in Hb and erythrocytes were noted along with the increase of serum Mg in all subjects and PLT in subjects without type 2 diabetes and without central obesity.
There was no significant correlation between serum Mg and blood count and Hb in type 2 diabetes without central obesity. In the study of Scibior et al. (Reference Scibior, Zaporowska and Ostrowski30), a statistically significant increase in Hb level along with the increase of serum Mg concentration was demonstrated. Therefore, metabolism between Hb and serum Mg, in subjects with diabetes but without central obesity, was disturbed and somewhat special.
Significant increase (P < 0⋅05) in total leucocyte count was observed in diabetic untreated rats compared with diabetic rats with oral Mg treatment. Erythrocyte count was increased (P < 0⋅05) in diabetic rats (with oral Mg treatment) compared with the diabetic untreated group(Reference Ige and Adewoye31). But in our study, there was no correlation between serum Mg and leucocyte count in adults without oral Mg treatment in any subgroup. On the other hand, increased level of leucocyte count was associated with incidence of type 2 diabetes(Reference Zhang, Yang and Zhang32). Our study supported this view in every subgroup (P < 0⋅05), according to Table 3.
Recent evidence attests that erythropoiesis disorder is commonplace in human body disorders such as CVD, venous thromboembolism, cancer, type 2 diabetes, community-acquired pneumonia, chronic obstructive pulmonary disease, liver and kidney failure as well as in other acute or chronic conditions(Reference Salvagno, Sanchis-Gomar and Picanza33). Among the biological Mg surrogates, an association was found between serum Mg and erythrocyte Mg. Serum Mg was involved in the erythrocyte metabolism(Reference Czernichow, Zarebska and Preziosi34).
In the paper by Sanchez-Morito et al. (Reference Sanchez-Morito, Planells and Aranda35), Mg deficiency led to increased intestinal absorption of Fe and decreased erythrocyte counts. The structural and functional changes in the erythrocyte caused by Mg deficiency probably account for the decrease in erythrocyte and Hb concentrations(Reference Sanchez-Morito, Planells and Aranda35). In our study, subjects’ erythrocyte counts increased with increasing serum Mg, except for type 2 diabetes. Thus, serum Mg increase might be a positive factor in raising erythrocyte count. However, diabetes may disrupt the metabolism between Mg and erythrocytes.
In the previous study, serum Mg was found to be positively correlated with PLT count (P < 0⋅001)(Reference Scibior, Zaporowska and Ostrowski30). Also, there was significant reduction in HBA1c in groups with given Mg supplementation(Reference Shahbah, Hassan and Morsy36). Similar results can be found in our study: serum HbA1c of subjects with type 2 diabetes in high Mg group was lower than serum HbA1c of subjects in normal Mg group. But in the low Mg group, there was a strange phenomenon where subjects showed lower level of HbA1c. Compared with subjects without type 2 diabetes, type 2 diabetes caused metabolism disorder in blood sugar and serum Mg.
Immature PLT fraction is elevated in patients with type 2 diabetes and associated with poor glycaemic control(Reference Lee, Kim and Song37). The Mg level was inversely correlated with leucocyte (P = 0⋅028) and PLT (P = 0⋅016) counts on patients with haemodialysis(Reference Stolic, Jovanovic and Trajkovic38). However, serum Mg was inversely associated with thrombocytopenia in healthy adults(Reference Lu, Zhan and Yu39). Our study partly supported this view in subjects without type 2 diabetes and central obesity. But in subjects with type 2 diabetes or central obesity, there was no correlation between PLT and serum Mg, accounting for disturbed metabolism by diseases. And there was no significant difference between subjects with type 2 diabetes and without type 2 diabetes in the PLT count. The low numbers in the low Mg group is one limitation of the study. We will pay attention to this issue in future research.
In summary, blood cell count, with the exception of leucocytes, was associated with serum Mg, but this association is somehow disturbed by type 2 diabetes or central obesity.
Acknowledgements
This research used data from the CHNS. We thank the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924, T32 HD007168), the University of North Carolina at Chapel Hill, the National Institutes of Health (NIH) (R01-HD30880, DK056350, R24 HD050924 and R01-HD38700) and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) for financial support for the CHNS data collection and analysis files from 1989 to 2015 and future surveys, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009 and Beijing Municipal Center for Disease Prevention and Control since 2011.
The present study was funded by NIH (R01-HD30880, DK056350, R24 HD050924 and R01-HD38700) and the NIH Fogarty International Center (D43 TW009077, D43 TW007709). However, no fund provider has any role in the design, analysis or writing of this article. The design, analysis and writing of this paper received no specific grant from any funding agency, commercial or not-for-profit sectors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
D. L., L. Y. and S. L. contributed to the study concept, design and supervision; the analysis and interpretation of the data; and the drafting and critical revision of the manuscript for important intellectual content. Q. Z., L. Z. and Q. L. contributed to the analysis and interpretation of the data and to critical revision of the manuscript for important intellectual content. H. L. and J. Z. contributed to administrative, technical and material support for the study; to the analysis and interpretation of the data; and to critical revision of the manuscript for important intellectual content.
The authors have no potential relevant conflicts of interest to disclose.









