Plants contain dietary proteins resistant to gut proteolysis, such as lectins, protease inhibitors and 2S albumin storage proteins(Reference Moreno and Clemente1). Some of them have well-documented effects on human health as gut, metabolic, immunological and hormonal regulators but also as naturally occurring chemotherapeutic agents(Reference Pusztai, Bardocz, Martin-Cabrejas, Muzquiz, Hill, Cuadrado, Pedrosa and Burbano2). In particular, plant protease inhibitors of the Bowman–Birk type, a major protease inhibitor family in legume seeds, have been shown to be capable of preventing or suppressing carcinogenic processes in a wide range of in vitro and in vivo models(Reference Kennedy3–Reference Clemente, Domoney, Govil, Singh and Sharma5). Bowman–Birk inhibitor (BBI) from soyabean has been demonstrated to be structurally and functionally resistant to the challenges (including acidic conditions and the action of proteolytic enzymes) of the gastrointestinal tract (GIT) in vivo; BBI and related proteins can transit through the stomach and small intestine without major degradation and significant amounts reach the colon in their intact form(Reference Clemente, Jiménez, Marín-Manzano and Rubio6). The compact structure of BBI proteins linked to the number and distribution of disulfide bridges seems to be a major contributor to this high stability(Reference Clemente, Vioque, Sanchez-Vioque, Pedroche, Bautista and Millán7). The resistance of BBI proteins to harsh conditions makes these proteins attractive for evaluation as chemopreventive agents, through modulating cell viability and tumour progression, within the GIT(Reference Clemente and Domoney4,Reference Clemente, Domoney, Govil, Singh and Sharma5). BBI-like proteins have been shown to be biologically active in suppressing benzopyrene-induced forestomach carcinogenesis in mice, following oral treatment(Reference Fernandes and Banerji8). Soyabean BBI has been reported to exert a protective effect in dimethylhydrazine-treated rats, reducing the incidence and frequency of colon tumours, without any adverse side-effects on animal growth or organ physiology(Reference Kennedy, Billings, Wan and Newberne9). Other studies have reported health benefits of soyabean BBI and related proteins on GIT health. BBI can reduce inflammation specifically associated with tumour processes(Reference Kennedy3). In a randomised double-blind trial, the treatment of human patients having ulcerative colitis with soyabean BBI was clinically effective, resulting in the regression of disease(Reference Lichtenstein, Deren, Katz, Lewis, Kennedy and Ware10).
Despite the potential clinical relevance of soyabean BBI and related proteins as colorectal chemopreventive agents, no experimental data regarding the potential biotransformation of BBI proteins by gut microbiota have been reported previously. On the other hand, we hypothesised that BBI proteins could exert a modulatory effect on gut microbiota via protease inhibition. Consequently, the aims of the present study were: (1) to evaluate in vitro if soyabean BBI is biotransformed by faecal microbiota resulting in a less efficient or inactive protein, and (2) to investigate whether naturally occurring BBI may or may not affect the growth and composition of faecal microbiota in batch cultures. These data further our knowledge on the gastrointestinal survival of dietary resistant proteins with potential colorectal chemopreventive properties.
Materials and methods
Materials
BBI from soyabean, trypsin (type III) and α-chymotrypsin (type VII) from bovine pancreas, N-α-benzoyl-dl-arginine-p-nitroanilide and N-benzoyl-l-tyrosine ethyl ester were obtained from Sigma (Alcobendas, Madrid, Spain). Raftilose P95, composed of 95 % fructo-oligosaccharides with a degree of polymerisation 3 to 10, was obtained from Orafti (Tienen, Belgium). All other chemicals were of analytical grade.
Animals and faecal samples
Five pigs (20 ± 2 kg mean live body weight) were purchased from Sanchez Romero Carvajal S.A. (Huelva, Spain) and housed individually in 4 m2 pens. They were fed a cereal-based diet without any antimicrobial agent. Faeces from animals were collected in sterile plastic bags immediately after deposition, sealed under anaerobic conditions and stored at − 80°C until inoculum was prepared. All management and experimental procedures, in strict accordance with the guidelines of good practice of laboratory animals of the Spanish Ministry of Agriculture (act no. 1201/2005, 10 October 2005), were implemented by staff trained to carry out such procedures.
In vitro faecal fermentation
Faecal samples were homogenised in 150 mm-NaHCO3 buffer (pH 7·4) (0·2 g faecal sample per ml) by using a masticator (IUL Instruments GmbH, Königswinter, Germany) for 2 min. Coarse particles were removed from these homogenates by filtration through Miracloth (Calbiochem, Nottingham, UK). Under anaerobic conditions, samples (2 ml) were aseptically transferred into 50 ml (28 × 100 mm) polypropylene sterile tubes containing soyabean BBI at a final concentration of 93 μm. Control tubes received no BBI. The fructo-oligosaccharide Raftilose P95 was used as a positive prebiotic control at a final concentration of 10 mg/ml. Samples were incubated at 37°C with periodic mixing for 24 h.
Effect of faecal microbiota on Bowman–Birk inhibitor electrophoretic pattern and inhibitory activities
Fermentation samples (0–24 h) containing soyabean BBI at a final concentration of 93 μm were assessed for trypsin inhibitory activity (TIA) and chymotrypsin inhibitory activity (CIA). The fermentation procedure (see above) was carried out with two different faecal samples, using triplicates of each; for every experiment (n 6), samples were taken at 0, 2, 4, 6, 8 and 24 h, and centrifuged (10 000 g; 4°C) for 20 min. The supernatant fractions were stored at − 20°C before determination of their protease inhibitory activities (TIA and CIA), using three technical triplicates. TIA was measured by using a modified small-scale quantitative assay, with N-α-benzoyl-dl-arginine-p-nitroanilide as the specific substrate, using 50 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) (pH 7·5) as the enzyme assay buffer. One trypsin inhibitor unit was defined as that which gives a reduction in absorbance at 410 nm of 0·01, relative to trypsin control reactions, in a defined assay volume of 10 ml(Reference Domoney and Welham11). CIA was measured using N-benzoyl-l-tyrosine ethyl ester as the specific substrate. One chymotrypsin inhibitor unit was defined as that which gives a reduction in absorbance at 256 nm of 0·01, relative to chymotrypsin control reactions, in a defined assay volume of 10 ml, as previously described(Reference Clemente, MacKenzie, Jeenes and Domoney12). The protease inhibitory activities (TIA and CIA) of supernatant fractions from fermentation samples were compared with those initially added to samples (t = 0 h). For SDS-PAGE analysis, 15 μl of fermentation samples were added to an equal volume of 2 × NuPAGE LDS sample buffer (Invitrogen, Paisley, UK). Protein samples were reduced with dithiothreitol and analysed on 4–12 % Bis-Tris precast gels using NuPAGE MES as the running buffer (Invitrogen). Gels were stained using the Colloidal Blue Staining Kit (Invitrogen).
Bacterial enumeration
Fermentation samples were serially diluted with sterile buffered peptone water (Cultimed, Murcia, Spain) and plated in duplicate to be used in colony-counting assays. Total anaerobic bacterial counts were determined on Brain Heart Infusion (BHI) agar supplemented with 0·5 % glucose, 0·5 % yeast extract, 0·25 % l-cysteine, vitamin K1 (10 μg/l) and haemin (0·02 g/l); plates were incubated anaerobically at 37°C for 48 h. For lactobacilli counts, samples were anaerobically cultured on deMan–Rogosa–Sharpe (MRS) agar plates; in the case of bifidobacteria counting, MRS broth was supplemented with dicloxacillin sodium salt hydrate (0·5 mg/l), LiCl (1 g/l) and l-cysteine hydrochloride (0·5 g/l) at 37°C and incubated for 48 h under anaerobic conditions. Colony-counting assays of enterobacteria and coliforms groups were carried out in 3M PetrifilmTM Enterobacteriaceae and Coliform Count plates, respectively, after incubation at 37°C for 24 h. The bacteroides group was counted in plates of bile aesculin agar (Oxoid, Basingstoke, Hants, UK) after incubation at 37°C for 24 h. Clostridia counts were carried out in reinforced clostridial agar (Oxoid) with polymixin B (20 μg/ml; Sigma) after incubation at 37°C for 48 h. In all cases, numbers of bacteria were expressed as log colony-forming units/g faecal sample.
Statistics
Protease inhibitory activities and bacterial enumeration data were subjected to one-way ANOVA using every bacterial group as a factor with three treatments (control, soyabean BBI and Raftilose). Statistical analysis was performed using Statgraphics Plus 5.1 software (StatPoint Inc., Herndon, VA, USA). Bonferroni's test was used to compare means and statistical significance was set at P < 0·01.
Results
Homogenates of faecal samples from pigs were used to evaluate the effect of microbiota on protease (trypsin- and chymotrypsin-like) inhibitory activities of soyabean BBI during fermentation assays (0–24 h) (Fig. 1). Although a slight decrease (10–15 %) of BBI inhibitory activities (TIA and CIA) at the initial stage (0–6 h) of the fermentation assays was observed, neither TIA nor CIA was significantly influenced by the enzymic or metabolic activity of the faecal microbiota after 24 h. The electrophoretic pattern of BBI proteins was also monitored during the incubation period (Fig. 2). Before incubation with swine faecal inoculum, analysis of soyabean BBI by SDS-PAGE showed a single electrophoretic band of appropriate mass (8 kDa); this pattern was not affected during the incubation period (24 h at 37°C) in the absence of faecal sample. Up to three electrophoretic bands in the range 6–8 kDa were observed when soyabean BBI was incubated with faecal microbiota; this pattern was observed from the earliest stage of fermentation (2 h) and remained constant until the end of the fermentation period (24 h). Soyabean BBI did not affect the bacterial counts of total culturable anaerobic bacteria, lactobacilli, bifidobacteria, enterobacteria, coliforms, bacteroides and clostridia after fermentation (24 h) (Table 1). On the contrary, the use of the commercial fermentable substrate Raftilose P95 resulted in a significant (P < 0·01) increase in the numbers of lactobacilli and bifidobacteria, without affecting the counts of the other groups studied.
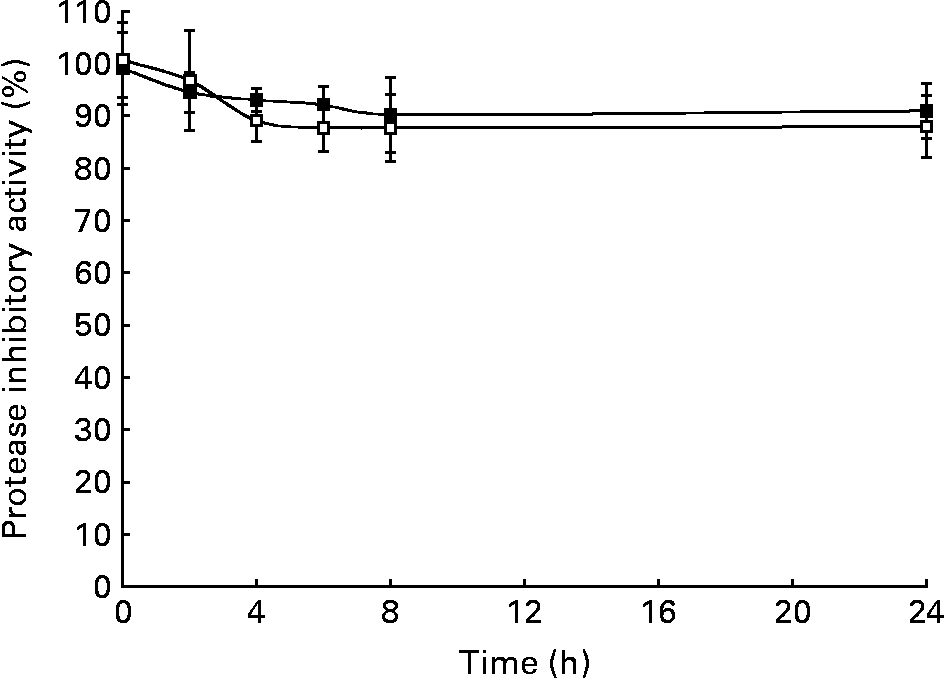
Fig. 1 Effect of faecal microbiota on trypsin inhibitory activity (TIA; –■–) and chymotrypsin inhibitory activity (CIA; –□–) of soyabean Bowman–Birk inhibitor (BBI). Homogenates of faecal samples from pigs were supplemented with soyabean BBI at a final concentration of 93 μm. TIA and CIA of supernatant fractions from fermentation samples at 0, 2, 4, 6, 8 and 24 h were compared with those initially added to samples (t = 0 h). Negative controls received no inhibitor. Data are the means of at least two independent experiments, each having three technical triplicates; bars represent standard deviations.

Fig. 2 Effect of faecal fermentation on SDS-PAGE pattern of soyabean Bowman–Birk inhibitor (BBI). Lane 1, molecular-weight (MW) markers; lane 2, fermentation control (without soyabean BBI) after 24 h; lanes 3–8, fermentation samples with soyabean BBI (93 μm) at 0, 2, 4, 6, 8 and 24 h, respectively; lane 9, soyabean BBI incubated in fermentation media without faeces; lane 10, soyabean BBI.
Table 1 Bacterial numbers (log10 colony-forming units/g faecal sample) in samples from batch cultures incubated at 37°C for 24 h without any additive (control), with soyabean Bowman–Birk inhibitor (BBI) (93 μm) or the prebiotic Raftilose (10 mg/ml)
(Mean values with their standard errors)
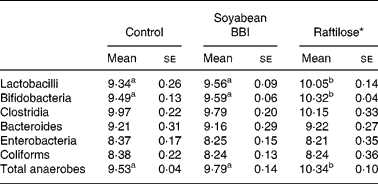
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·01; Bonferroni's test).
* Orafti, Tienen, Belgium.
Discussion
BBI and related proteins have been demonstrated to be colorectal chemopreventive agents within the GIT(Reference Kennedy3, Reference Clemente and Domoney4). In a previous study, we concluded that significant amounts of functionally and biologically active BBI-related proteins from chickpea-based diets reach the large intestine of the pig(Reference Clemente, Jiménez, Marín-Manzano and Rubio6), which is generally held to be a suitable model for human digestive physiology(Reference Miller and Ullrey13). In order to exert any health benefits on the GIT, BBI should be resistant at least in part to the enzymic and metabolic activity of gut microbiota. To our knowledge, data regarding the influence of gut microbiota on functional properties of BBI and related proteins have not been reported previously. In the present study, we have demonstrated that soyabean BBI remains fully active in the presence of faecal microbiota for a period of 24 h (Fig. 1). During the fermentation period, the derivation of up to three electrophoretic bands (of molecular weight in the range 6–8 kDa) from native BBI suggests that either or both of the N- and C-terminal regions of BBI may be particularly accessible to proteolytic cleavage by faecal microbiota (Fig. 2). Such limited proteolysis of BBI-like proteins naturally occurs in legume seeds where multiple post-translationally processed isoforms derived from primary gene products have been described(Reference Domoney, Welham, Sidebottom and Firmin14). The rigid structure of BBI-like proteins consisting of a well-conserved skeleton of cysteine residues, which form seven disulfide bridges, is thought to play a major role in maintaining the structural stability of these resistant proteins(Reference Clemente, Vioque, Sanchez-Vioque, Pedroche, Bautista and Millán7, Reference Ramasarma, Appu Rao and Rao15), precluding further protein hydrolysis. The presumed proteolytic cleavage during the initial stage of fermentation (0–6 h) appeared to diminish the protease inhibitory activities of soyabean BBI to a limited extent. Electrophoretic and functional data for BBI obtained from fermentation samples support the idea that the majority of inhibitory activity is derived from a ‘protein core’ containing both inhibitory domains.
The CIA, specifically linked to the anticarcinogenic properties of BBI-like proteins(Reference Kennedy, Billings, Maki and Newberne16, Reference Clemente, Gee, Johnson, MacKenzie and Domoney17), was almost unaffected by faecal microbiota (Fig. 1). Nonetheless, the relevance of the trypsin inhibitory domains of BBI proteins on health benefits has not been studied deeply and trypsin-like proteases involved in carcinogenesis should be considered also as potential targets of BBI-like proteins(Reference Clemente and Domoney4). Therefore, it is predicted that the survival of significant amounts of BBI and related proteins that are active against trypsin- and chymotrypsin-like proteases could exert a cancer-chemopreventive role in the colon. In agreement with this, in vivo studies have demonstrated that soyabean BBI can prevent or suppress cancer development in many different animal models, including dimethylhydrazine-induced colon and anal gland tumours in mice(Reference St Clair, Billings, Carew, Keller-McGandy, Newberne and Kennedy18). Soyabean BBI has been reported to be effective when used at concentrations as low as 10 mg/100 g diet, using the dimethylhydrazine rat model, in reducing the incidence or frequency of colorectal tumours when compared with animals treated with dimethylhydrazine alone(Reference Kennedy, Billings, Wan and Newberne9). BBI-related proteins from peas at concentrations as low as 20 μm were shown to have a significant suppressive effect on the growth of human colon adenocarcinoma HT29 cells in vitro (Reference Clemente, Gee, Johnson, MacKenzie and Domoney17).
Aberrant functioning of certain serine proteases has been linked to tumour cell invasion and metastasis and, more recently, to angiogenesis and tumour growth(Reference Darmoul, Gratio, Devaud, Lehy and Laburthe19). Several mechanisms whereby BBI can inhibit carcinogenesis via protease inhibition have been hypothesised(Reference Kennedy3, Reference Kennedy20); however, these are not well understood and the precise target(s) of BBI proteins remain unknown. The inhibitory activity of soyabean BBI against proteolytic activities in pre-malignant cells and tissues has been evaluated in order to identify and characterise certain proteases as potential targets. One such candidate is matriptase (MT-SP1), a member of the class of type II transmembrane serine proteases, which exhibits trypsin-like protease activity and has been described in a variety of epithelial cancer cell lines(Reference Bhatt, Takeuchi, Ylstra, Ginzinger, Albertson, Shuman and Craik21). MT-SP1 is implicated in the selective degradation of extracellular matrix proteins, and in the activation of cellular regulatory proteins, such as urokinase-plasminogen activator, hepatocyte-growth factor/scatter factor and protease-activated receptor. Although the ability of soyabean BBI to inhibit the hydrolytic activity of MT-SP1 has been demonstrated(Reference Yamasaki, Satomi, Murai, Tsuzuki and Fushiki22), the clinical relevance of this inhibition has not yet been proven. Chymase, a chymotrypsin-like protease, which is stored in mast cell granules and released upon degranulation, has also been reported to be susceptible to inhibition by soyabean BBI(Reference Ware, Wan, Schechter and Kennedy23); however, no clear correlation between the inhibition of this enzyme and the anti-carcinogenic properties associated with BBI has been reported. Soyabean BBI appears to be internalised by epithelial cells in vitro, and it has been suggested that such internalisation could facilitate the inhibition of intracellular target proteases associated with the transformation of normal to malignant cells(Reference Billings, Brandon and Habres24). It has also been suggested that the anti-carcinogenic activity of soyabean BBI is due to its ability to suppress the induction and expression of proto-oncogenes in carcinogen-treated or radiation-exposed cells and animals(Reference St Clair, Billings, Carew, Keller-McGandy, Newberne and Kennedy18); additional effects of BBI proteins on hormonal modulation and anti-inflammatory properties could be involved. Finally, it has been demonstrated that soyabean BBI abates the proteasomal chymotrypsin-like activity in vitro and in vivo in MCF-7 breast cancer cells accompanied by down-regulation of cyclin D1 and cyclin E(Reference Chen, Huang, Lin-Shiau and Lin25). These recent findings suggest a novel mechanism for BBI in controlling cell proliferation processes and cell death.
There is a growing interest in the colonic microbiota and in the way its metabolic activities impact on host wellbeing and health. In this context, resistant proteins and bioactive peptides may play a role in functional foods and nutraceuticals(Reference Duranti26). Since BBI and related proteins have the ability to inhibit serine proteases as well as an extraordinary resistance to the harsh conditions within the GIT(Reference Clemente, Jiménez, Marín-Manzano and Rubio6), we evaluated the potential influence of these dietary proteins on the growth of different bacterial groups that contribute to host health and wellbeing. In the present paper, we have demonstrated clearly that BBI proteins did not affect the in vitro growth and composition of the different bacterial groups evaluated (Table 1), and overgrowth of undesirable bacterial groups such as coliforms and enterobacteria was not observed. The selective media approach used in the present study is considered adequate to establish how dietary compounds may enrich defined ‘desirable’ organisms and deplete ‘undesirable’ organisms(Reference Roberfroid27); however, recent evidence indicates that the cultivable faecal bacteria represent only a fraction of the bacteria actually present in the gut(Reference Blaut and Clavel28). Further experiments through molecular-based microbiological techniques will be necessary in order to gain definitive information on the effects of these bioactive compounds on full flora diversity(Reference Costabile, Klinder, Fava, Napolitano, Fogliano, Leonard, Gibson and Tuohy29).
In conclusion, the biological activity of BBI, that appears to be linked to chemopreventive properties in the colon, is largely unaffected by microbiota metabolism. The results of the present study, together with previously reported data on the survival of biologically active BBI-related proteins during gastrointestinal digestion in vivo (Reference Clemente, Jiménez, Marín-Manzano and Rubio6), are relevant to further pharmacological and pre-clinical studies of their role in preventing colon carcinogenesis.
Acknowledgements
The present study was carried out with financial support from grants AGL 2004-03 260 and AGR2006-00 706 from Spanish CICYT and Junta de Andalucía, respectively. R. R. and A. C. are recipients of an I3P-CSIC and a Ramon and Cajal contract, respectively. We are grateful to Dr C. Domoney (John Innes Centre, Norwich, Norfolk, UK) for her constructive comments on this paper. The study is not subject to any conflicts of interest. A. C. and L. A. R. designed and revised the full manuscript. M. C. M.-M. and R. R. carried out the microbiological analyses. E. J. carried out the enzymic kinetic experiments.







