Necrotising enterocolitis (NEC) is a devastating disease of prematurity and a leading cause of death from gastrointestinal disease in the neonatal intensive care units of hospitals around the world(Reference Alganabi, Lee and Bindi1–Reference Alsaied, Islam and Thalib3). One of the most devastating complications of NEC is the development of lung injury(Reference Patel and Denning4), which is more severe than the lung injury which occurs in premature infants who do not develop NEC(Reference Laughon, O’Shea and Allred5,Reference Ganapathy, Hay and Kim6) . In seeking to understand the causes of NEC and its associated lung injury, a growing body of research(Reference Hackam7) has shown that NEC typically develops in the premature infant in the setting of an abnormal microbiome that is enriched in gram negative bacteria(Reference Neu8), often after the administration of formula feeds(Reference Eaton, Rees and Hall9). These findings suggest that the interplay between formula administration and gut immaturity combines in the pathogenesis of this disease(Reference Nino, Sodhi and Hackam10). Prior studies – including our own – have focused on the first of these factors, showing that the premature intestine expresses high levels of the bacterial receptor, toll-like receptor 4 (TLR4), which becomes activated by the abnormal microbiome of the premature host, leading to barrier injury and NEC(Reference Hackam, Sodhi and Good11,Reference Kovler, Sodhi and Hackam12) . There has been less focus on the role of infant formula in NEC development, which represents a significant gap in our knowledge. This gap is particularly important given that the solid link between formula administration and NEC development reveals that specific components of the infant formula may contribute to both NEC development and the presence of NEC-induced lung injury(Reference Sodhi, Fulton and Good13). The precise components of formula that induce NEC, and strategies to manipulate these components as a therapeutic approach for NEC and NEC-induced lung injury, thus remain of great interest.
In seeking to understand the mechanisms leading to the development of NEC-induced lung injury, our laboratory has recently shown that the activation of TLR4 on the intestinal epithelium leads to the release of the damage-associated molecule HMGB1, which can activate TLR4 on the pulmonary epithelium, resulting in NEC(Reference Jia, Sodhi and Yamaguchi14). Mice lacking HMGB1 on the intestinal epithelium and mice lacking TLR4 in the lung epithelium were both protected from NEC, indicating that a link between the gut and the lung leads to disease development. Moreover, the presence of greater lung immaturity and impaired lung development could also contribute to the presence of lung injury in NEC(Reference Bæk, Cilieborg and Nguyen15,Reference Drucker, Jensen and Te Winkel16) . In seeking to understand how nutritional strategies could influence NEC development, we recently developed a modified fat nutritional formula which is enriched in fats that do not require lipase for their digestion, an approach that was based upon the known deficiency of lipase in the premature host(Reference Sodhi, Fulton and Good13). Administration of formula containing this pre-digested fat (which we called the ‘PDF’ system) in place of standard formula reduced experimental NEC in mice, through the reduction of reactive oxygen species (ROS) within the intestinal mucosa(Reference Sodhi, Fulton and Good13). We now seek to extend these studies to test whether administration of an formulas that is enriched in ‘PDF system’ could prevent the development of NEC-associated lung injury in neonatal mice, and if so, whether it could do so by enhancing lung development.
In order to test the potential role of the ‘PDF system’ in the protection against NEC-associated lung injury, we turned to a well-established and clinically validated model of NEC in 7-d-old neonatal mice. To induce NEC, we used a clinically relevant model that mimics the disease in humans, in which neonatal mice that were exposed to twice daily hypoxia were gavaged with infant formula for 4 d along with enteric bacteria from a patient that had suffered severe NEC(Reference Lu, Yamaguchi and Fulton17). At the end of the 4-d model, mice develop patchy intestinal necrosis that resembles human NEC, and upregulate pro-inflammatory cytokines within the intestinal epithelium that are also seen in the human disease(Reference Zhou, Nino and Yamaguchi18). In testing the role of PDF-containing formula on NEC-associated lung development, we studied mice at postnatal age 7–8 years, which is an age similar to premature human infants at approximately 28 weeks gestation, when clinical NEC is seen(Reference Stanford, Gong and Noonan19). Neonatal mice of this age also express high levels of TLR4 in the intestine, just as in the human condition(Reference Gribar, Sodhi and Richardson20), rendering them highly susceptible to developing human NEC-like disease(Reference Kovler, Sodhi and Hackam12). The current experimental system therefore allows us to evaluate the role of PDF in infant formula in the prevention of NEC-induced lung injury and to also shed light on key potential mechanisms involved.
Methods
Animal study approval
All mice experiments described in this study were carried out following the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by Johns Hopkins University protocol, according to the ‘NC3R’S The ARRIVE Guidelines. C57BL/6J mice were purchased from the Jackson Laboratory and bred in the pathogen-free facility at Johns Hopkins University for multiple generations to stabilise intestinal microbiota. All mice were given ad libitum access to water, food and housed in a temperature-controlled room (22°C) with 12 h’ light and dark cycles. All mice used in the study were euthanised humanely using isoflurane anaesthesia by inhalation (∼3–4 % isoflurane) followed by cervical dislocation.
Induction of necrotising enterocolitis in neonatal mice
Experimental NEC was induced in 7–8-d-old (∼3 g body weight) neonatal mouse pups as previously described(Reference Lu, Yamaguchi and Fulton17,Reference Zhou, Nino and Yamaguchi18,Reference Sodhi, Wipf and Yamaguchi21–Reference Jia, Sodhi and Yamaguchi23) . In brief, mouse pups of both sexes were separated from dams, and then randomly allocated into control (non-NEC) or NEC groups. NEC-group mice were gavage fed (40 μl/g) five times/d (07.00–19.00) with formula containing either ‘standard fat’ or ‘PDF’, supplemented with bacterial stock that had been cultured from the stool of an infant with severe NEC (12·5 μl stool slurry in 1 ml formula). The formula (50 µl/g of mouse body weight) was administered using a 24-French angiocatheter placed into the mouse oesophagus. Mice were also exposed to hypoxia (5 % O2, 95 % N2) for 10 min in a chamber (Billups-Rothenberg INC.), twice daily for 4 d. Where indicated, non-NEC control mice were reared on standard fat or PDF formulas for 4 d without NEC stool and no hypoxia. The breastfed (control) animals remained with their mothers and received breast milk ad libitum. Assignments of pups to each group were made based on the available pups from each litter, after reaching the required number to reach significance according to the power analysis (below).
Nutritional formulas
Nutritional formulas were manufactured by and obtained from Abbott Nutrition. Their detailed nutritional profiles and ingredients have been previously published(Reference Sodhi, Fulton and Good13). Briefly, the standard fat formula oil system was composed entirely of Triacylglyceride (TAG) oils, a mixture of 39 % (by weight) high oleic safflower oil, 29 % (by weight) soya oil, 27·9 % (by weight) coconut oil and 4·1 % (by weight) oils associated with vitamins and proteins. The PDF-enriched formula is composed of a mixture of soyabean Non-esterified fatty acids (NEFA) (17·5 %), monoglycerides (20 %) and phospholipid lecithin (10·3 %) along with TAG oils, high oleic safflower oil (34·8 %) and coconut oil (14·8 %). In the manufacture of the PDF-enriched fat formula, soyabean NEFA were sourced from crude soya oil and then were physically distilled by combining oil and water hydrolysis at a high temperature. Formulas also contained monoglycerides and phospholipid lecithins, small molecular emulsifiers that are widely utilised in various nutritional products and are derived from fatty acids. Soyabean NEFA was added into the formula in the Calcium salt form, where the NEFA was first mixed with calcium hydroxide during manufacturing. Both standard and PDF-containing formulas were subjected to the same manufacturing conditions, including equal emulsification, homogenisation and final thermal treatment. All formulas are representative of commercial ready-to-feed shelf-stable human infant formulas.
Necrotising enterocolitis severity assessment
NEC severity was determined based upon a validated scoring system that was applied to de-identified, paraformaldehyde fixed/paraffin embedded/haematoxylin and eosin-stained intestinal sections from distal small intestine (ileum). Slide reviewers were blinded to the group allocation. Histological NEC severity score was assigned as described previously(Reference Werts, Fulton and Ladd24), 0 (no injury), 1 (minor – submucosal, lamina propria separation), 2 (moderate separation of submucosa, lamina propria and oedema in submucosal and muscular layers) and 3 (severe separation of submucosa, lamina propria, severe oedema and villous sloughing or loss of villi).
Immunohistochemistry and haematoxylin and eosin staining
Immunofluorescent staining of lung tissues was performed on 4 % paraformaldehyde-fixed 5 μM thick paraffin sections. The sections were first warmed to 56°C in a vacuum incubator (Isotemp Vacuum Oven, Fisher Scientific), then washed immediately in xylene, gradually re-dehydrated in ethanol (100 %, 95 %, 70 %, water) and then processed for antigen retrieval in citrate buffer (10 mM pH 6·0)/microwave (1000 watt, 6 min). Samples were then washed with Phosphat Buffered Saline (PBS), blocked with 1 % BSA/5 % donkey-serum (1 h, room temperature), and then incubated overnight at 4°C with primary antibodies (1:200 dilutions in 0·5 % BSA), washed 3 times with PBS, incubated with appropriate fluorescent-labelled secondary antibodies (1:1000 dilution in 0·5 % BSA, Life Technologies Inc) and the nuclear marker, 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Cat # D9542, Sigma). The primary antibodies used were anti-3′-nitrotyrosine (3′-NT, Cat # ab61392, Abcam), anti-myeloperoxidase (Cat # RB373A, Thermo Scientific), anti-surfactant A (SP-A, Cat # ab49566, Abcam), anti-surfactant D (SP-D, Cat # ab168366, Abcam) and anti-ZO-1 (Cat # 40–2200, ZYMED). In Situ Death Detection Kit, Fluorescein (TUNEL, Cat # 11684795910, Roche) was stained as per manufacture instructions. For myeloperoxidase colour development, ABC DAB staining kit (Vector labs, Cat# SK-4100) was used as per manufacturer’s instructions. All immunohistochemistry and haematoxylin and eosin stained slides were mounted using Gelvatol solution prior to imaging using a Nikon confocal microscope (Nikon, Inc). Images were quantified for fluorescent intensity using ImageJ software.
Quantitative real-time PCR
Total RNA was isolated from the snap-frozen, full thickness small intestine (ileum) using RNeasy® kit (Cat # 74106, Qiagen), and checked first for RNA purity and concentration on a SpectraMax® microplate reader (Molecular Devices). 0·5 μg of total RNA was reverse transcribed for cDNA synthesis using the QuantiTect® Reverse Transcription kit (Cat # 205313, Qiagen). Quantitative real-time PCR was then performed on a Bio-Rad CFX96 Real-Time System (Bio-Rad Labs) using Sybr green mix (Bio-Rad Labs), forward and reverse primers (custom designed using NCBI Primer-BLAST online program and ordered from Integrated DNA Technologies) (Table 1). The mRNA expression relative to the housekeeping gene ribosomal protein large P0 (Rplp0) was calculated using the 2–△△CT method as described(Reference Zhou, Nino and Yamaguchi18).
Table 1. Primers

Nasal instillation of N-acetylcysteine
Some NEC group mice on the standard fat formula were given 20 μl of N-acetylcysteine (NAC) (Cat # A9165, Sigma, 25 mg/ml stock in saline) via direct nasal instillation, once daily for 4 d of the NEC model. NAC was intranasally delivered dropwise to the nares using a p20 pipette while the mouse was in a nose-up supine position as described previously(Reference Southam, Dolovich and O’Byrne25).
Induction of Lipopolysaccharide (LPS)-induced lung injury in neonatal mice
Age and body weight-matched C57BL/6 at postnatal day 7(p7) mice of both sexes were separated from the lactating dams, randomly divided into two groups and housed in a 32°C paediatric incubator. Mice were then reared on formulas containing either ‘standard fat formula’ or the ‘PDF-containing formula’ (40 μl ml/g) and were fed using a 24-French angiocatheter placed into the mouse oesophagus five times per day for 4 d. On day 5, both standard fat and PDF-containing fat groups were further subdivided into two groups, that is, ‘LPS’ and ‘control’ groups. The ‘LPS group’ was given LPS via the intraperitoneal route (5 mg/kg, i.p) or intranasal (20 μg/mice in 20 μl volume from 1 mg/ml LPS stock). Mice in the ‘control group’ received saline via the same route as the ‘LPS group’. All mice were killed 6 h later, and lungs were harvested for analysis.
Lung maturation and wet/dry mass studies
Age and body weight-matched C57BL/6 at postnatal 7(p7) mice of both sexes were separated from the lactating dams, randomly divided into two different groups and housed in a 32°C paediatric incubator (Air Shields Isolette C-400, Hill-Rom). Mice were either orally gavaged with ‘standard fat formula’ or ‘PDF system’ 40 μl ml/g, five times/d, using a 24-French angiocatheter placed into the mouse oesophagus for 4 d. On day 5, mice were euthanised using CO2 euthanasia, and lungs were harvested for total RNA or fixed with 4 % paraformaldehyde for the histological studies. For wet/dry mass studies, the whole lungs were briefly dried on the absorbent paper, transferred to pre-weighed aluminium foil, measured for the wet lung weight, dried for 48 h in 58°C incubator re-measured for the dried lung weight.
Statistical analysis
All data were analysed using Graph Pad Prism 9 (GraphPad Software). A power analysis was performed in order to calculate sample size, using an effect size of 2-fold for gene expression, NEC severity, expression of surfactant protein, ZO-1, 3′-NT and a difference of 20 % for dry lung weight and a difference of 10 % for wet/dry lung weight between groups. The standard deviation for each of these parameters was based upon our previously published studies(Reference Lu, Yamaguchi and Fulton17,Reference Zhou, Nino and Yamaguchi18,Reference Sodhi, Wipf and Yamaguchi21–Reference Jia, Sodhi and Yamaguchi23) , and we accepted significance (type 1 error) of 5 % (P = 0·05), the probability of finding an effect of 80 % and an attrition rate based on prior studies of 10 %. The corrected sample size (including attrition) was then determined according to Charan et al.(Reference Donovan, Liu and Shen26). Experiments were thus performed with at least three mice per group, as indicated in each figure where a dot represents an individual mouse, and within the figure legends.
Data were analysed for statistical significance by ordinary one-way ANOVA followed by Tukey’s multiple comparison test. The pups of both sexes were randomised to the treatment group, and blinded analyses.
Results
Administration of infant formula containing ‘pre-digested fat’ prevents lung injury in neonatal mice with necrotising enterocolitis
To evaluate the effects of the fat component of infant formula on the development of NEC-induced lung injury, we subjected neonatal mice to our well-established experimental NEC model, which includes formula feeding, hypoxia and the administration of stool bacteria from an infant with severe NEC, and then altered the fat composition, from ‘standard fat’ to ‘PDF’. As shown in Fig. 1(a)–(e), the induction of NEC in mice using formula containing ‘standard fat’ resulted in inflammation in the small intestine that was characterised by disruption of the mucosa, loss of the intact villus architecture (Fig. 1(d)) and the expression of the pro-inflammatory cytokines Tnf-α (Fig. 1(k)) and Lcn-2 (Fig. 1(l)). Moreover, the inflammation that developed in the intestine was also associated with the development of inflammation in the corresponding lungs of these mice, as manifested by an influx of inflammatory cells, as well as a loss of airway architecture and vascular extravasation (Fig. 1(l)), and increase in pro-inflammatory cytokines, Tnf-α (Fig. 1(m)) and Lcn-2 (Fig. 1(n)). Importantly, mice that were fed formula that contained the ‘pre-digested system’ which was enriched in pre-digested TAG (i.e. NEFA and monoglycerides) that are not dependent on the activity of endogenous lipases did not get the NEC, as revealed by normal histology and reduced pro-inflammatory cytokine response both in the intestine (Fig. 1(e), (k), (l)) and lungs (Fig. 1(e), (j), (m), (n)). In non-NEC control studies, mice that were reared on either the ‘standard fat’ or the ‘PDF system’ alone did not develop any inflammatory response or histological abnormalities in either the intestine (Fig. 1(b), (c), (k), (l)) or the lungs (Fig. 1(g), (h), (m), (n)), excluding a toxic effect of the formula alone.
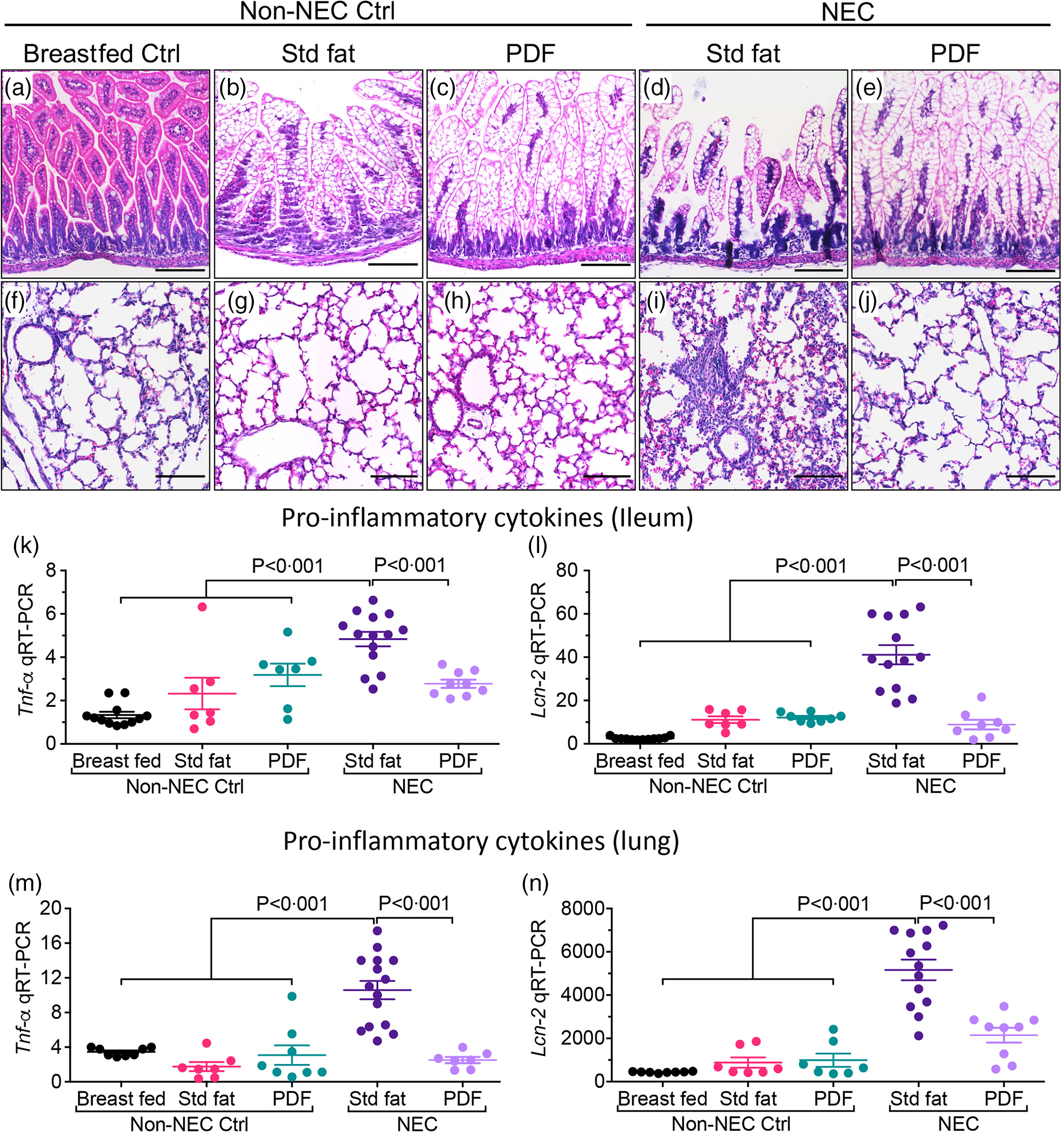
Fig. 1. Administration of formula containing pre-digested fat prevents NEC-induced lung injury in neonatal mice. (a)–(n) Representative photomicrographs of haematoxylin and eosin (H&E) stained small showing histology and gene expression of pro-inflammatory cytokines, in non-NEC control and NEC mice. (a)–(e) Histology of the small intestine (ileum). (f)–(j) Histology of the lungs. (k)–(L) qRT-PCR expression of pro-inflammatory cytokines in the small intestine (ileum), (k) Tnf-α and (l) Lcn-2. (m)–(n) qRT-PCR expression of pro-inflammatory cytokines in the lungs, (m) Tnf-α and (n) Lcn-2. Each dot in scatter-dot plots represents data from an individual mouse, (n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 100 μm.
Two additional endpoints were evaluated in order to determine the effects of either the ‘standard fat’ or ‘PDF system’ on the extent of lung injury. As shown in Fig. 2, neonatal mice that were induced to develop NEC with ‘standard fat’-containing infant formula developed extensive apoptosis within the lung, seen as brightly stained TUNEL positive lung cells in lung sections (Fig. 2(d)i–ii and (f)) when compared with the lung sections from non-NEC control mice (Fig. 2(a)–(c)i, (a)–(c)ii, (f)). In contrast, mice fed with modified fat formula containing ‘PDF’ NEC showed little apoptosis (Fig. 2(e)i–ii and (f)). The immunohistochemical findings were corroborated by increased expression levels within the lungs of the pro-apoptosis gene PUMA (the p53 up-regulated modulator of apoptosis), which were significantly higher in ‘standard fat’ NEC formula mice compared with breastfed control and NEC mice fed ‘PDF’ v. ‘standard fed NEC’(Fig. 2(g), P < 0·001). In parallel, we measured the extent of polymorphonuclear neutrophil (PMN) infiltration into the lungs of mice induced to develop NEC using the above fat-containing formulas. As shown in Fig. 3, in mice administered standard fat-containing formula, NEC induction resulted in marked accumulation of PMN in the airways, as characterised as an intense immunostaining of the PMN marker myeloperoxidase (Fig. 3(d)i–ii and (f)). The immunostaining findings were confirmed by the presence of increased mRNA expression of neutrophil-specific enzyme ELANE (elastase of neutrophils) (Fig. 3(g)). Importantly, the induction of NEC using formula containing the ‘PDF’ system resulted in minimal PMN accumulation in the lungs (Fig. 3(e)i–ii and (f)), and a corresponding reduction in the expression ELANE mRNA levels (Fig. 3(g)), which were both similar to non-NEC control mice (Fig. 3(a)–(c)i–ii, (f), (g)). Taken together, these findings reveal the beneficial effect of modifying the fat composition of infant formula as a strategy to prevent NEC-induced lung injury.
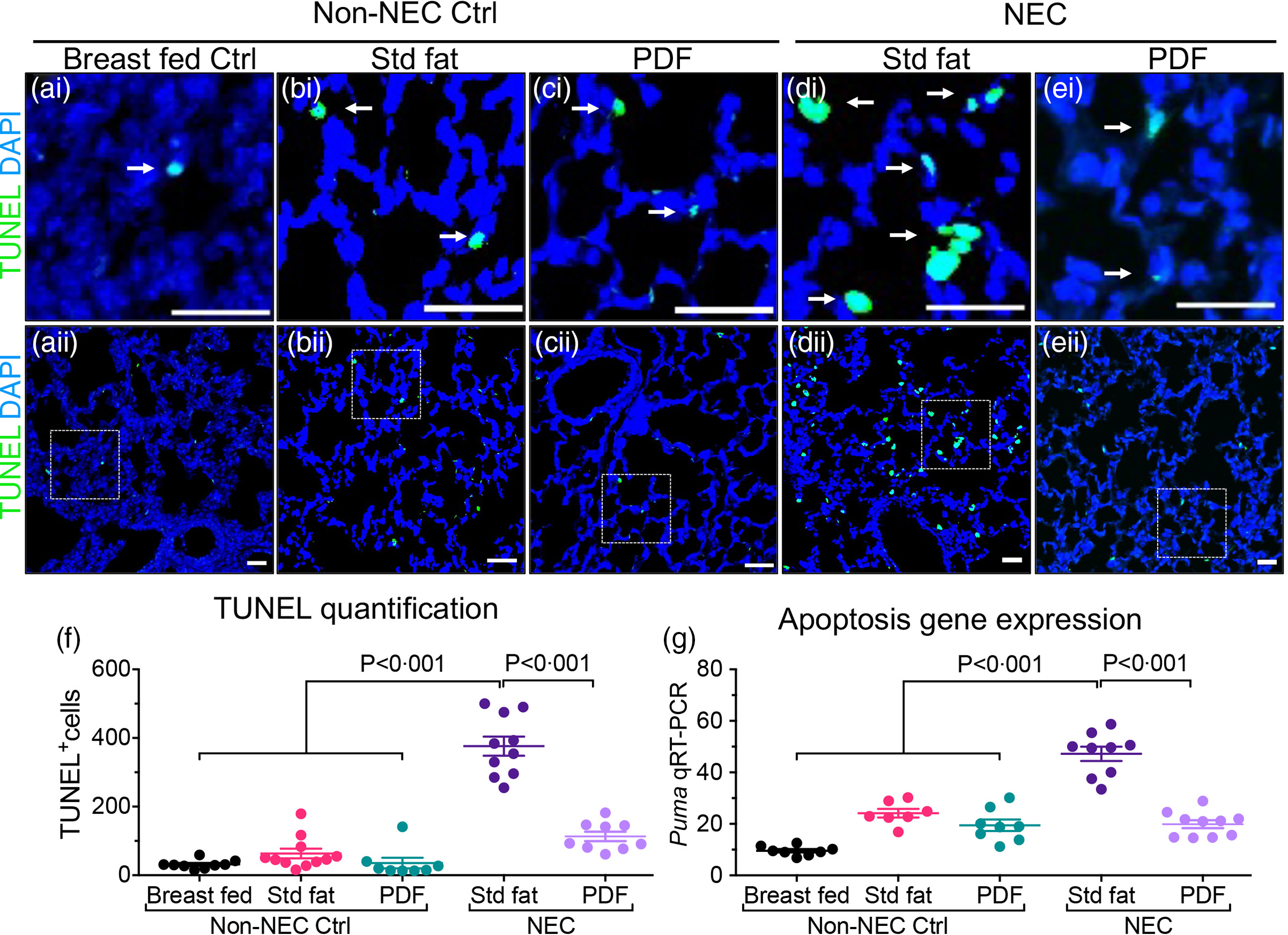
Fig. 2. Administration of formula containing pre-digested fat prevents apoptosis of lung epithelial cells in necrotising enterocolitis. (a)–(e)i–ii Representative immunofluorescence images of TUNEL staining (TUNEL green, DAPI nuclei blue), showing apoptosis (white arrows), in lungs of non-NEC control (a)–(c)i–ii and NEC mice (d), (e)i–ii. (f) Quantification of TUNEL positive cells, using ImageJ software (two or more separate areas from each mouse lung section were imaged and quantified, total mice n ≥ 4 mice/group). (g) qRT-PCR of Apoptosis gene Puma (P53 Up-Regulated Modulator Of Apoptosis), each dot in scatter-dot plot represents data from an individual mouse (n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 25 μm.

Fig. 3. Administration of formula containing pre-digested fat reduces polymorphonuclear neutrophil (PMN) accumulation into the neonatal lung. (a)–(e)i–ii Representative photomicrographs of myeloperoxidase (MPO) stained images with DAB staining showing accumulation of PMN (DAB stained brown cells, black arrows) in lungs of non-NEC controls (a)–(c)i–ii and NEC (d–E)i–ii mice. (f), (g) qRT-PCR expression of PMN marker genes, (g) Mpo and (h) neutrophil expressed neutrophil (ELANE) (each dot in scatter-dot plots represents data from an individual mouse, n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 20 μm.
Administration of modified fat formula containing ‘pre-digested fat system’ prevents loss of lung surfactant proteins and tight junctions in mice with necrotising enterocolitis
After establishing that the ‘PDF system’ containing formula is protective against NEC-induced lung injury, we next determine the effects on lung surfactant proteins secreted by the epithelial type II cells into the lung space, which are critical components of the protective barrier of the lung(Reference King and Chen27). As shown in Fig. 4, the induction of NEC with formula containing ‘standard fat’ resulted in a marked decrease in the expression by immunostaining within the lung of surfactant protein SP-A (Fig. 4(d) and (k)) and surfactant protein SP-D (Fig. 4(i) and (l)). Importantly, both SP-A and SP-D were restored to baseline levels in the lungs of mice that had been administered the ‘PDF system’ containing formula (Fig. 4(d), (i), (k), (l)). Interestingly, both surfactant A and D were significantly higher in the non-NEC control mice fed with the ‘PDF system’ (Fig. 4(c) and (h)) when compared with the ‘standard fat’ non-NEC control mice (Fig. 4(b), (g), (k), (l), P < 0·05). These findings demonstrate that administration of the ‘PDF system’ results in the development of lungs with significantly higher surfactant proteins as compared with control mice.

Fig. 4. Administration of formula containing pre-digested fat prevents loss of lung surfactant proteins in the lungs in neonatal mice with NEC. (a)–(j) Representative confocal images of surfactant proteins in lungs of non-NEC control ((a)–(c), (f)–(h)) and NEC ((d), (e), (i), (j)) mice. (a)–(e) Immunostaining of surfactant protein A (SP-A, green staining, white arrowheads). (F–J) Immunostaining of surfactant protein D (SP-D, red staining, white arrowheads). (k), (l) quantification of immunofluorescence intensity of SP-A (k) and SP-D (l) using ImageJ software (each dot in scatter-dot plots represents data from an individual area, two or more areas from each mouse lung section IHC were imaged and quantified, n ≥ 4 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 20 μm.
We next evaluated the effects of the PDF-containing formula on the expression of tight junctions within the lungs of mice with NEC. As shown in Fig. 5, the administration of standard fat-containing formula resulted in a marked decrease in the expression of the tight junction proteins ZO-1 by immunostaining (Fig. 5(d)i–ii and (f)) and quantitative real-time PCR (Fig. 5(g), and these were restored in the presence of PDF (Fig. 5(e)i–ii, (f), (g)). It is again noteworthy that ZO-1 staining and mRNA expression were both significantly higher in non-NEC mice that were reared on the ‘PDF system’ compared with the ‘ standard fat’ (Fig. 5(b), (c)i–ii, (f), P < 0·01), suggesting again that the alveolar epithelial cells are more differentiated with PDF nutrition. Taken together, these findings illustrate that PDF within formula can reduce lung injury and enhance lung differentiation. We next focused on the mechanisms involved and examined the potential effects of ROS-mediated damage to the newborn lung.

Fig. 5. Administration of formula containing pre-digested fat prevents the loss of tight junctions in the neonatal lungs of mice with NEC. (a)–(e)i–ii Representative confocal images of tight junction protein Zona-Occludin (ZO-1, red staining, white arrowheads) in lungs of non-NEC control (a)–(c)i–ii and NEC (d)–(e)i–ii mice. (f) Quantification of ZO-1 immunofluorescence intenstity measured using ImageJ software (each dot represents a different area under focus, 1–2 areas/section/mice, n ≥ 7 mice/group). (g) qRT-PCR expression ZO-1 (each dot represents data from an individual mouse, n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 25 μm.
Administration of modified fat formula containing ‘pre-digested fat’ reduces oxidative stress in the lungs of mice induced to develop necrotising enterocolitis
Neonatal lung disease is thought to develop in response to the development of ROS, which causes significant cellular injury, leading to pneumocyte death and alveolar injury. Lung injury in the setting of other inflammatory processes involves the accumulation of ROS(Reference Marseglia, D’Angelo and Granese28). We therefore next investigated whether NEC-induced lung injury was associated with the accumulation ROS, and whether these were reduced by the administration of formula containing PDF. As shown in Fig. 6, the expression levels of 3′-nitrotyrosine (3′-NT), which is a marker of cell damage and a product of the oxidation of proteins by superoxide (O2-) and peroxynitrite (ONOO-), were elevated in the lungs of mice that were fed ‘standard-fat’ formula (Fig. 6(c)i–ii and (f)). Importantly, the levels of 3′-NT were significantly reduced in the presence of ‘PDF system’ enriched formula (Fig. 6(e)i–ii, (f), P < 0·001), suggesting a protective, antioxidant effect of the PDF diet. Additionally, the transcripts levels of superoxide-generating enzyme NADPH oxidase 2 (NOX2) also known as cytochrome b (558) subunit beta or Cytochrome b-245 heavy chain(Reference Diebold, Smith and Li29) that produce ROS are significantly increased in lungs of ‘standard formula’ fed NEC mice and were reduced in the presence of ‘PDF enriched formula fed’ NEC mice versus. ‘standard formula fed’ NEC (Fig. 6(g), P < 0·01). No significant changes in 3’-NT staining or NOX2 expression were identified among non-NEC control mice (Fig. 6(a)–(c)i–ii, (f), (g)). Based upon these findings, we next explore whether a reduction in ROS could reduce lung injury, in order to gain greater insights into the protective mechanisms of PDF diet on NEC-induced lung injury.

Fig. 6. Administration of formula containing pre-digested fat prevents NEC-induced nitrosylation in the lung. (a)–(e)i–ii Representative confocal images of 3′-nitrotyrosine (3′-NT, green staining, white arrows) in lungs of non-NEC control (a)–(c)i–ii and NEC (d)–(e)i–ii mice. (f) Quantification of 3′-NT immunofluorescence intenstity measured using ImageJ software (each dot represents a different area under focus, 1–2 areas/section/mice, n ≥ 4 mice/group). (g) qRT-PCR of superoxide radical, that is, ROS generation enzyme NADPH oxidase 2 (Nox2) (each dot represents data from an individual mouse, n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software. Scale bars: 25 μm.
Administration of the reactive oxygen species scavenger N-acetylcysteine preserves the morphology of lung epithelial cells and reduces necrotising enterocolitis-induced lung injury
We next evaluate whether inhibition of ROS directly could achieve lung protection in the setting of NEC. To do so, we treated the neonatal mice induced to develop NEC using the ‘standard fat’ formula with the redox scavenger NAC via direct nasal instillation. NAC treatment to the lungs did not protect against NEC development, as evident from NEC histology (Fig. 7(a)i–ii) and pro-inflammatory cytokine Tnf-α expression (Fig. 7(a)iii). However, the lungs of NAC-treated NEC mice were protected against NEC-induced lung injury, as revealed by a lack of inflammation on lung histology (Fig. 7(b)i–ii), reduced expression of pro-inflammatory cytokine Tnf-a (Fig. 7(b)iii), reduced PMN influx (Fig. 7(c)i–ii) and expression of neutrophil elastase (Fig. 7(c)iii), reduced apoptosis (Fig. 7(d)i–iii), reduced 3′-NT (Fig. 7(e)i–iii) and restored levels of SP-A (Fig. 7(f)i–iii), SP-D (Fig. 7(g)i–iii) and ZO-1 (Fig. 7(h)i–iii). Taken together, these results indicate that NEC-induced lung injury can be prevented by ROS inhibition and provide insights into the mechanisms by which PDF reduces NEC-induced lung injury.
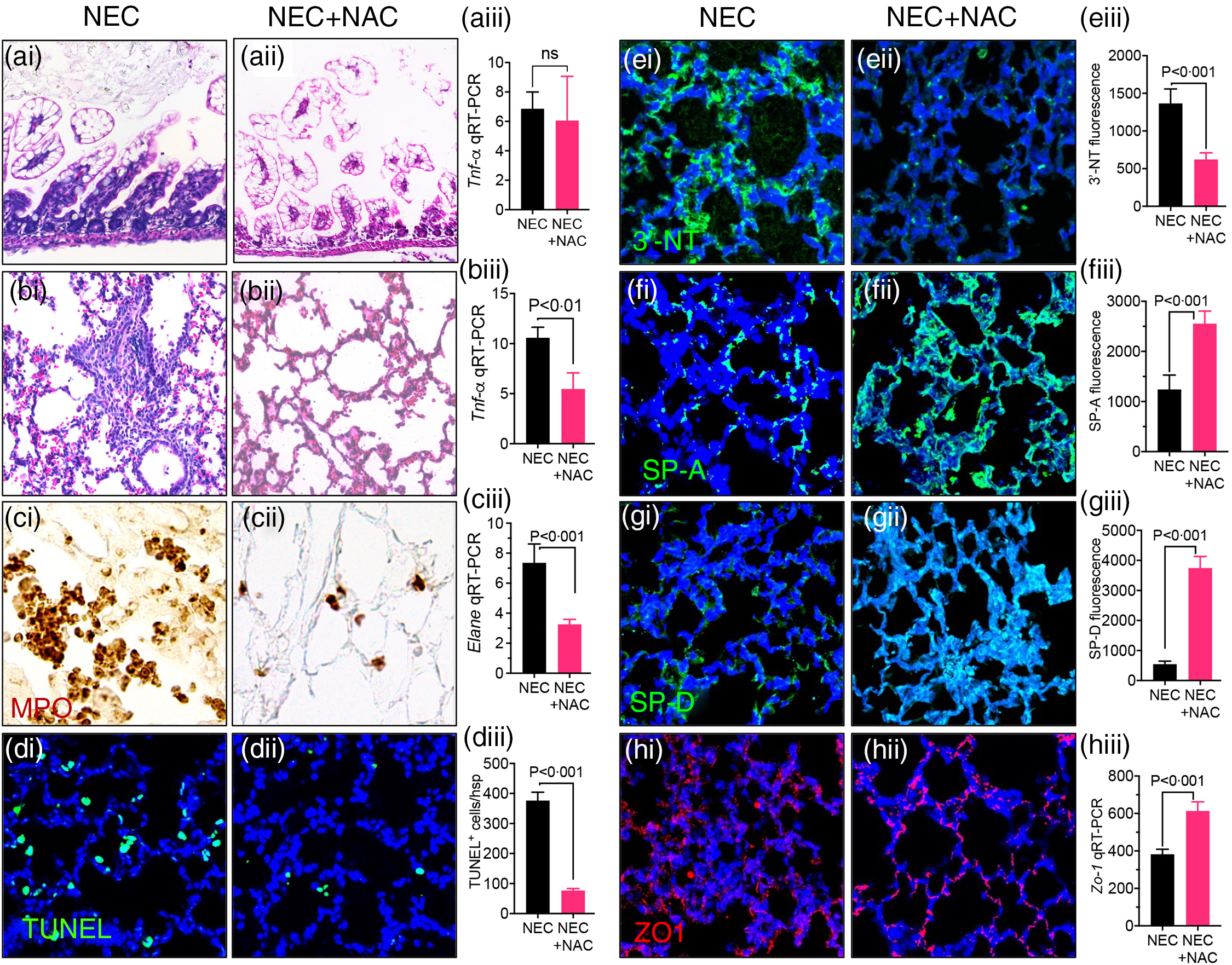
Fig. 7. Intranasal administration of the reactive oxygen species (ROS) scavenger N-acetylcysteine (NAC) prevents NEC and NEC-induced lung injury. (a)–(b)i–iii Assessment of NEC histology as shown by representative photomicrographs of H&E stained histological sections of small intestine (ileum) (a)i–ii, lung (b)i–ii, and qRT-PCR expression of pro-inflammatory cytokine Tnf-a in the small intestine. (a)iii and lung (b)iii in NEC mice either given intranasal saline (NEC) or NAC (NEC + NAC). (c)i–iii Assessment of PMN infiltration lungs as shown by representative images of myeloperoxidase (MPO) staining (DAB stained brown cells) (c)i–ii and qRT-PCR expression of neutrophils marker ELANE (c)iii. (d)i–ii Assessments of apoptosis as shown by representative confocal images of TUNEL staining (d)i–ii) and TUNEL quantification (d)iii. (e)i–iii Assessment of oxidative injury as shown by representative confocal images of 3′-NT staining (e)i–ii and quantification using image J software (e)iii. (f)i–ii, (g)i–ii Assessment of surfactant protein levels as shown by representative confocal images of surfactant proteins SP-A (f)i–ii and SP-D (g)i–ii along with immunofluorescence intenstity quantification of SP-A (f)iii and SP-D (g)iii using image J software. (h)i–iii Assessment of tight junction proteins as measured by representative confocal images Zona-Occludin (ZO-1) staining (h)i–ii and qRT-PCR expression of ZO-1 (h)iii. Each bar on the bar graphs represents data from 6 to 8 mice with their standard error of the mean. Statistical significance was determined by student’s t test using GraphPad prism 9 software. Scale bars: (a)i–(b)i 100 μm (c)i–(h)ii, 25 μm.
Administration of modified fat formula containing ‘pre-digested fat’ protects against LPS-induced lung injury
We next studied if the mice reared on the PDF system are resistant to LPS-induced lung injury. To do so, we exposed the lungs to LPS via either the systemic route, that is, intraperitoneal administration of LPS or by direct exposure using intranasal inhalation. The administration of LPS resulted in a significant induction in pro-inflammatory cytokines Il-6 and Lcn-2, and neutrophil elastase in mice that were reared on ‘standard fat’ formula when LPS was administered either systemically (Fig. 8(a)–(c)) or via direct intranasal inhalation (Fig. 8(d)–(f), while the mice reared on the ‘PDF system’ were significantly protected (Fig. 8(a)–(f)) from LPS-induced inflammation. These findings further support the notion that that mice’s lungs reared on the ‘PDF system’ are more resilient to injury and inflammation.

Fig. 8. Administration of formula containing pre-digested fat prevents protects against LPS-induced lung injury. Dot plot charts of qRT-PCR expressions of pro-inflammatory cytokines Il-6 (a), (d), Lcn2 (b), (e), and neutrophils enzyme ELANE (c), (f), in the lungs of mice reared on the ‘standard fat’ (std fat) and the ‘pre-digested fat system’(PDF), and treated with LPS via interaperitoneal route (a)–(c) and intranasal route (d)–(f). Each dot in scatter-dot plots represents data from an individual mouse (n ≥ 5 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software.
Administration of ‘standard fat’ containing formula induces mild lung oedema v. ‘pre-digested fat system’
In the final series of studies, we assessed the wet to dry lung ratio, an indicator of lung oedema. To do so, we first measured the total body weight, following 4 d of formula feedings, and found no statistical difference between the ‘standard fat’ and the ‘PDF system’ groups (Fig. 9(a)). Similarly, the absolute measurements of the wet lungs were not different between the ‘standard fat’ and the ‘PDF system’ groups (Fig. 9(b)). However, the dry lung weight was significantly increased in mice reared on the ‘PDF system’ (Fig. 9(c), P < 0·05), which corresponded with a significantly reduced wet to dry lung weight ratio in mice reared on the ‘PDF system’ as compared with mice reared on the ‘standard fat formula’ (Fig. 9(d), P < 0·01). These data strongly suggest that even at baseline, in the absence of NEC induction, mice that were reared on the ‘PDF system’ had significantly reduced predisposition to the development of oedema as compared with mice fed a standard formula. Taken in aggregate, these findings reveal that the administration of a formula that is enriched in PDF offers lung protective properties.

Fig. 9. The effects of formula administration on wet/dry lung weights in neonatal mice. Dot plot charts of total body weight (a), wet lung weight (b), dry lung weight (c) and wet/dry lung weight ratio (d), in mice reared on the ‘standard fat or the ‘pre-digested fat system’ over a period of 4 d from postnatal 7 to p11. Each dot in scatter-dot plots represents data from an individual mouse (n ≥ 7 mice/group). Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparisons tests using GraphPad prism 9 software.
Discussion
Prematurity is defined as a birth that occurs before 37 completed weeks of gestation(Reference Shapiro-Mendoza, Barfield and Henderson30). Approximately 10 % of births in the USA are premature, resulting in approximately 500 000 premature infants born in the USA each year(Reference Blencowe, Cousens and Oestergaard31). Globally, there are over 15 million babies born prematurely every year, 60 % of whom are born in Africa and South Asia, with over 3·5 million premature babies born in India and 1·1 million premature babies born in China(32). The survival rate of a premature infant is largely determined by the degree of prematurity, such that infants born before 22 weeks gestation have a mortality rate of over 90 %, while the survival increases to over 90 % for infants born at 27 weeks gestational age(Reference Myrhaug, Brurberg and Hov33). The incidence of long-term complications of prematurity includes retinopathy of prematurity, bronchopulmonary dysplasia, NEC, intraventricular haemorrhage and periventricular leukomalacia resulting in long-term health problems that include vision impairment, lung dysfunction, intestinal disability and neurological dysfunction(Reference Stoll, Hansen and Bell34,Reference Kelly and Tobias35) . The degree of morbidity of each of these complications of prematurity is particularly severe when NEC co-exists, especially in the case of lung dysfunction(Reference Hintz, Kendrick and Stoll36).
The current study focuses on strategies to prevent the development of NEC-induced lung injury, which remains a major cause of morbidity and mortality in patients with NEC, and for which there are few effective treatment strategies(Reference Howlett, Ohlsson and Plakkal37). Prior investigators have shown that the development of lung injury in NEC involves a coordinated pro-inflammatory response within the lung parenchyma that results in impaired healing within the pulmonary epithelium(Reference Wang, Yu and Li38). We have previously shown that NEC-induced lung injury results in part from activation of the bacterial receptor TLR4 on the pulmonary epithelium which results in an influx of inflammatory neutrophils and a destruction of the alveolar membrane(Reference Jia, Sodhi and Yamaguchi14). In a follow-up study, we found that the adoptive transfer of CD4 T cells from lungs of mice with NEC could induce lung injury in immune incompetent mice, indicating the importance of T cells in the pathogenesis of NEC-induced lung injury(Reference Jia, Sodhi and Yamaguchi23). We further showed that TLR4 signalling on the surfactant associated protein C-1 (Sftpc1) positive pulmonary epithelial cells resulted in the induction of pro-inflammatory Th17 lymphocytes into the lung, and that aerosolised inhibition of either TLR4 or the Th17 recruiting chemokine CCL25 significantly protected the lungs in the setting of NEC(Reference Jia, Sodhi and Yamaguchi23). Taken in aggregate, these findings illustrate that strategies that can directly inhibit either the pro-inflammatory response in the lung or the ability of pro-inflammatory cells to reach the lung can reduce the development of NEC-induced lung injury. The current studies extend those prior findings by now revealing that by modifying the fat components of the infant diet, we can also directly reduce the extent of NEC-induced lung injury. These findings therefore have potential translational significance, by providing the possibility of lung protection in infants at risk for lung injury in the setting of NEC through the use of dietary modifications alone.
Standard of care for NEC-induced lung injury involves supporting the infant’s requirements for gas exchange (i.e. delivering oxygen to the tissues and removing carbon dioxide) including the use of advanced ventilatory strategies(Reference Gibbs, Jensen and Alexiou39), the selective administration of medical therapies including corticosteroids(Reference Cuna, Lewis and Dai40), nitric oxide and vitamin A(Reference Pasha, Chen and Zhou41,Reference Kalikkot Thekkeveedu, Guaman and Shivanna42) , and minimisation of ventilator-induced lung damage(Reference Hennelly, Greenberg and Aleem43). These fundamental therapeutic principles are similar to those applied in the management of bronchopulmonary dysplasia which is a major pulmonary condition that affects premature infants, except that NEC-induced lung injury is more severe and takes longer to treat than other forms of neonatal lung disease(Reference Willis and Ambalavanan44). There is no specific treatment for NEC-induced lung injury, and so the current approach of instituting a diet that can reduce lung injury in the setting of NEC offers the potential for new avenues for care.
We now offer additional insights into the mechanisms by which a diet rich in PDF can protect the lung. Specifically, we reveal that mice fed a diet rich in pre-digested TAG (NEFA and monoglycerides) displayed greater overall lung differentiation, as revealed by increased surfactant protein A and D expression, increased tight junction ZO-1 expression and reduced lung-weight ratio at baseline. It stands to reason that a more differentiated lung would be less prone to inflammatory-mediated injury than a relatively undifferentiated lung, thus perhaps explaining why effects on lung differentiation may be positively associated with reduced injury. Moreover, the finding that the administration of a diet rich in pre-digested TAG is protective against injury in the setting of increased surfactant expression in the lung is consistent with clinical strategies in which surfactant administration effectively treats lung injury in premature infants(Reference Venkataraman, Kamaluddeen and Hasan45). While the precise mechanisms by which dietary factors could modulate lung development in the newborn remain unclear, prior studies in other models support this conclusion. For instance, a diet rich in EPA and γ-linolenic acid has been shown to be beneficial in patients with acute lung injury(Reference Singer, Theilla and Fisher46), although more recent pooled meta-analyses have questioned the efficacy of this approach(Reference Li, Bo and Liu47). The potential beneficial effects of dietary modulation for the prevention of lung injury have been evaluated in mothers who have been administered DHA supplementation(Reference Marc, Piedboeuf and Lacaze-Masmonteil48), and in infants who have been administered n-3 long chain PUFA, especially DHA by meta-analysis(Reference Wang, Zhou and Cui49), and have failed to show a significant benefit to mothers significantly. These findings suggest that additional strategies to protect the lung are warranted, in particular those that focus on enhancing lung development, as in the current approach.
One of the important features of the current studies relates to the mouse model used, and its potential relevance to the population of premature infants in which NEC-induced lung injury develops. The neonatal mouse model of NEC has been clinically validated by many laboratories and replicates many of the clinical and physiological features of the intestinal manifestations of the disease(Reference Kovler, Salazar and Fulton50). In particular, the model induces the development of patchy intestinal necrosis and the expression of pro-inflammatory cytokines that are seen in the human disease(Reference Sampah and Hackam51). The model begins in mice on day 7, as this time point is reflective of the physiological and anatomic characteristics of premature infants, with respect to the elevated expression of TLR4, a relative lack of Paneth cells, an enrichment of pro-inflammatory lymphocytes in the intestinal mucosa and a relative paucity of goblet cells(Reference Stanford, Gong and Noonan19,Reference Lu, Sodhi and Jia52,Reference Cho, Rudloff and Lao53) . Moreover, the model also replicates the key features of human NEC-induced lung disease, including alveolar injury, leukocyte infiltration and epithelial damage(Reference Jia, Sodhi and Yamaguchi14,Reference Jia, Sodhi and Yamaguchi23) . That said, the mouse model of NEC also has several limitations. First, it is virtually impossible to recreate the unique environment of the neonatal intensive care unit, the natural site at which premature infants receive care and also develop NEC, in the animal facility. Environmental microbes and other stressors may have important roles in NEC pathogenesis that are simply not accounted for in animals. To this end, Peng et al. have provided evidence linking environmental stressors in the neonatal intensive care unit to biobehavioural responses in preterm infants(Reference Peng, Bachman and Jenkins54). Further, differences between the intestinal microbiota of mouse and humans could have unanticipated physiological relevance to the presence of inflammation in both the gut and the lung in the current model(Reference Donovan, Liu and Shen26), despite our attempts to reduce this variable through the administration of human intestinal microbiota in the current model. Finally, as with all mouse models, there are limitations regarding body size, weight, litter size and additional genomic differences that together can limit the applicability of the current model. Other models of premature lung development have been developed in piglets(Reference Chandra, Davis and Drexler55), rabbits(Reference Salaets, Aertgeerts and Gie56), baboons(Reference Albertine57) and lambs(Reference Kramer, Ikegami and Moss58), and each offers relative advantages and disadvantages in balancing cost, translatability and ease of model development, and each could potentially be adapted to the study of NEC-induced lung injury. Taken in aggregate, the current mouse NEC model has been widely adopted by many laboratories around the world and is now a standard research tool for the study of this complex disease.
One of the possible interpretations of the current studies is that because protection against NEC-induced lung injury is a natural benefit of preventing NEC, perhaps the best way to protect the lungs is to prevent NEC development in the first place. While the current data do not refute this conclusion outright, we submit that such a conclusion provides an incomplete assessment of the current findings. Indeed, there are many patients who develop and then recover from NEC, only to go on to develop significant morbidity from lung injury; in these patients, the presence of persistent lung injury can be the major limiting factor in their ultimate recovery, and a significant number of patients who survive NEC have limited exercise tolerance(Reference Kurscheid and Holschneider59), as well as ongoing lung disease(Reference Bazacliu and Neu60). These observations suggest that the lungs of premature infants, which are particularly susceptible to the development of bronchopulmonary dysplasia, are at additional risk of lung injury in the setting of NEC, which can persist long after NEC is resolved. These findings support a strategy of dietary modification in order to protect the lungs, in a way that may confer additional benefits to that obtained through simple NEC prevention alone.
Further studies are required to understand how the PDF is metabolised during the neonatal period, and what metabolic pathways if any are altered in the presence of fatty acid administration, and how the gut-lung axis could impact neonatal development in general. It should be noted that the oil composition was enriched in soyabean oil. This oil was chosen as it is widely used in infant formula along with other plant oils such as coconut and high oleic safflower oils. Moreover, the fatty acid profile of soyabean oil is favourable in designing infant formula so as to mimic human milk’s fatty acid profile, as it contains fatty acids such as palmitic, oleic, linoleic and α-linolenic acids(Reference Teichert and Akoh61,Reference Dai, Sun and Li62) , and was thus selected in order to maintain these important fatty acids within the formula at physiologically relevant levels. Additional studies could be performed in order to address whether other pre-digested oils could be more or less beneficial in preventing NEC-associated lung injury.
All aspects of the study design have been designed to maximise the translatability of the results to humans. The underlying pathophysiology between the mouse model and the human disease is similar, and the biomarkers that are used to measure disease severity are also similar between humans and mice. That said, differences between mouse pathophysiology and human heterogeneity will impact the degree of translatability. We therefore assessed the degree of translatability according to the criteria of Wendler et al (Reference Wendler and Wehling63). Based upon the strong in vitro and in vivo evidence for the role of exaggerated immunity in the pathogenesis of NEC, the validity of the current animal disease model, past data from both mouse and piglets in NEC reduction, as well as the effect on biomarkers (IL-6) that we used to predict disease severity after the administration of the PDF-containing diet, the estimated degree of translatability of the current studies is estimated to be high(Reference Wendler and Wehling63).
In summary, we have shown that a formula containing PDF can protect the lungs in the setting of NEC, through a mechanism that involves a reduction in inflammation and an increase in lung differentiation. These findings indicate that nutritional therapies may have previously unrecognised roles in the prevention of NEC, and the mitigation of its long-term complications, including the development of NEC-associated lung injury.
Acknowledgements
The authors acknowledge Mr. Raheel Ahmad for his help with the creation of NEC models. D. J. H. was supported by grants (RO1 DK117186, RO1DK121824 and R35GM141956) from the National Institutes of Health, and this research was funded in part by a Sponsored Research Grant from Abbott Nutrition. There was no role by Abbott Nutrition in the design of the study, collation and analysis of data or decision to publish.
C. P. S. and D. J. H designed and/or performed experiments, analysed results, wrote and edited the manuscript. A. J. S., M. K., W. B. F., Y.Y., A. I., S.W., T. P., H. J. and P. L. designed and performed the experiments. M. F and T. D. provided funding helped in writing and editing and also in the initial concept of the study.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521004104














