There is evidence that the inclusion of fibre in diets leads to adaptational changes in the gastrointestinal tract (GIT) when dietary fibre is consumed over prolonged periods of time( Reference Longland, Low and Quelch 1 – Reference Zhao, Wang and Liu 9 ). These adaptational changes include, but are not restricted to, changes in the hindgut microbial population( Reference Zhao, Wang and Liu 9 – Reference Kleessen, Stoof and Proll 12 ), SCFA concentrations in hindgut digesta and faeces( Reference Montoya, Saigeman and Rutherfurd 7 , Reference Tulung, Rémésy and Demigné 13 , Reference Walter, Eastwood and Brydon 14 ), the anatomy and histology of both the small and large intestine( Reference Brunsgaard and Eggum 4 , Reference Tulung, Rémésy and Demigné 13 , Reference Brunsgaard and Eggum 15 ), apparent faecal digestibility of NSP( Reference Longland, Carruthers and Low 16 , Reference Jonathan, Souza da Silva and Bosch 17 ) and mineral absorption( Reference Tulung, Rémésy and Demigné 13 ). This response to dietary fibre varies over time. In pigs fed diets containing either sugar beet pulp or wheat bran as dietary fibre sources, it was found that the Lactobacilli–Enterobacteria ratio in the proximal colon changed throughout the experimental period of 42 d, while the activity of certain microbial enzymes was detected only at specific times (e.g. cellulase activity was detected only at 42 d)( Reference Castillo, Martín-Orúe and Anguita 6 ). Moreover, the adaptational response to dietary fibre intake for some characteristics such as total tract nutrient digestibility appears to differ for different types and amount of fibre( Reference Longland, Low and Quelch 1 , Reference Brunsgaard, Bach Knudsen and Eggum 2 , Reference Walter, Eastwood and Brydon 14 , Reference Longland, Carruthers and Low 16 , Reference Jonathan, Souza da Silva and Bosch 17 ). This suggests that the adaptational responses, at least for some parameters, are controlled by factors other than just the time of adaptation.
Previous findings have shown that increased dietary fibre leads to increases in the amounts of non-dietary materials (e.g. mucins) at the terminal ileum( Reference Ito, Satsukawa and Arai 18 – Reference Satchithanandam, Vargofcak-Apker and Calvert 21 ), which means a higher amount of non-dietary material entering the hindgut and thus available for fermentation. Recently, it has been shown by our group( Reference Montoya, Rutherfurd and Moughan 22 ) that non-dietary materials (e.g. mucins and microbial bodies) in the ileal digesta collected from pigs fed diets containing kiwi fruit, a high fibre source, are a major if not the main substrate for SCFA production in the hindgut (65 % of the total predicted SCFA). In that study, the concentration of kiwi fruit in the diet also influenced the predicted hindgut production of SCFA. Kiwi fruit was used here as a model dietary fibre source, as it has both a similar content of fibre and a similar soluble to insoluble fibre ratio to other fruits such as apple( Reference Sims and Monro 23 ). The soluble fraction (5·2 % on a DM basis) contains mainly pectic polysaccharides, while the insoluble fraction (6·2 % on a DM basis) contains mainly cellulose and hemicellulose( Reference Sims and Monro 23 – Reference Redgwell, Melton and Brasch 25 ), the most common fibre constituents in a human diet. Considering that SCFA production is related to several factors in the hindgut, such as changes in the pH and microbial population, it could be expected that fibre-related physiological changes in the upper gut may also affect lower GIT adaptational changes (e.g. fermentative capacity).
In the presently described study, effects of the amount of dietary kiwi fruit on adaptation of intestinal fermentation over time were investigated using the pig as an animal model for the adult human upper gut digestion (mouth to the terminal ileum)( Reference Miller and Ullrey 26 , Reference Moughan, Cranwell and Darragh 27 ), combined with an in vitro fermentation assay where ileal digesta from the pig were incubated with a human faecal inoculum to model human hindgut fermentation( Reference Coles, Moughan and Awati 28 ). The pig was not used as an animal model for the adult human lower gut, as the pig possesses a greater caecum that may increase the retention time of digesta in the lower gut and therefore increase the extent of fermentation. The study had two distinct lines of investigation. First, it was hypothesised that the foregut (mouth to terminal ileum) adapts differently over time to diets containing different amounts of fibre. This hypothesis was tested by determining in pigs fed two dietary levels of kiwi fruit the changes over time in (i) the 16S ribosomal RNA (rRNA) gene copy number of the total bacteria, Enterobacteria and Lactobacillus in the terminal ileal digesta, (ii) the nutrient composition of the ileal digesta, (iii) the amounts of nutrients entering the hindgut from the terminal ileum and (iv) the fermentative potential of ileal digesta (hindgut fermentability of ileal digesta organic matter, OM, and predicted hindgut production of SCFA) and predicted hindgut absorption of SCFA derived from the fermentation with a standard human faecal inoculum of ileal digesta collected at different times over a 6-week period after kiwi fruit feeding. Second, it was hypothesised that the hindgut adapts differently over time to diets containing different amounts of fibre and this was tested by determining in pigs fed two dietary levels of kiwi fruit the changes over time in (i) the 16S rRNA gene copy number of the total bacteria, Enterobacteria and Lactobacillus in faeces and (ii) the fermentative capacity of the faecal bacteria (in vitro fermentation of a standard purified fibre source using pig faeces collected at different times over a 6-week period after kiwi fruit feeding).
Methods
Animals and dietary treatments
Ethics approval for the animal trial was obtained from the Animal Ethics Committee, Massey University, Palmerston North, New Zealand. A total of fourteen entire male pigs of approximately 12 weeks of age (Hampshire×(Landrace×Large white), 41·4 (sem 2·98) kg mean body weight) were surgically modified with the implantation of a simple T ileal cannula at the terminal ileum, and housed individually in pens (1·5×1·5 m) in a room maintained at 21±2°C with a 10 h/14 h light/dark cycle as described in detail elsewhere( Reference Montoya, Saigeman and Rutherfurd 7 , Reference Montoya, Rutherfurd and Moughan 29 ) for a different part of the same study.
The DM and total dietary fibre contents of the kiwi fruit were 17 and 18·8 % (on a DM basis), respectively. A fibre-free diet and two diets containing either 133 or 266 g/kg kiwi fruit (Actinidia deliciosa cv. Hayward) on a DM basis (i.e. 782 and 1565 g fresh kiwi fruit per kg diet DM, respectively), as the sole dietary fibre source (28 or 48 g total dietary fibre/kg DM, respectively), were formulated to meet the nutrient requirements of growing pig as prescribed by the National Research Council( 30 ) (online Supplementary Table S1). The diets were formulated to have similar crude protein and gross energy contents. Titanium dioxide (TiO2) was included in the diets as an indigestible marker. The amounts of kiwi fruit in the diets correspond to amounts of dietary fibre found in a typical Western diet (3–7 %)( Reference Baer, Rumpler and Miles 31 ), and were comparable to the amounts achieved by ingestion of one or two kiwi fruit by a human at each meal. The fibre-free diet was given to pigs to minimise the effects of dietary fibre consumed before the start of the study (baseline).
The daily ration, adjusted weekly, was calculated as 90 g of DM/kg of metabolic body weight (BW0 · 75) and was fed to pigs as two meals given at 09.00 and 16.00 hours. Kiwi fruit was added to the synthetic experimental diets, freshly peeled and crushed, just before each meal. There were no food refusals during the study.
Experimental design
In vivo assay
Pigs (n 14) were adapted to the pens and change in diet (from a commercial diet to a semisynthetic diet) for 1 week before surgery. During this period and the post-surgery period (9 d), pigs received the adaptation diet. Pigs were then allocated at random to two dietary regimens. A total of seven ileal cannulated pigs were fed the fibre-free diet for 7 d followed by the low kiwi fruit-containing diet for a further 44 d (i.e. 51 d in total), while another seven ileal cannulated pigs were given the fibre-free diet for 7 d followed by the high kiwi fruit-containing diet for a further 44 d. The last days of the fibre-free period were regarded as baseline (point zero of the study, Fig. 1). Faecal and ileal digesta samples were collected for two consecutive days. Collection of faecal grab samples was started on days –3, 4, 14, 28 and 42 and that of ileal digesta was started on days –2, 5, 15, 29 and 43. Faecal samples were collected before ileal digesta to avoid interference with the flow of material through the hindgut for faecal collection. Fresh faecal samples were collected (within 5 min) after anal stimulation when pigs were eating the earlier meal. Ileal digesta were continuously collected from each pig on each sampling day over a 6 h period, starting 1 h post-feeding( Reference Montoya, Saigeman and Rutherfurd 7 ). The ileal digesta and faecal samples collected for SCFA and microbial analyses were immediately frozen and stored at –20 and –80°C, respectively, until analysis. The ileal digesta and faeces collected for nutrient composition analysis and determination of the fermentative potential of ileal digesta were frozen at –20°C and freeze-dried before analysis. The faeces to be used to determine hindgut microbiota fermentative capacity were collected fresh into isolated containers flushed with CO2 at 37°C.
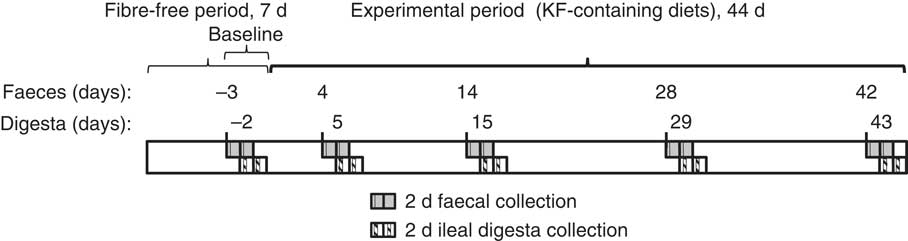
Fig. 1 Experimental timeline and timeline of sample collection. The bars inside each collection day represent the faecal collection after anal stimulation and the 6 h ileal digesta collection. There were seven pigs allocated to this time regimen for the low-kiwi fruit (KF) diet and seven pigs for the high-KF diet.
In vitro fermentation assays
Fermentative potential of ileal digesta
A human faecal inoculum-based in vitro hindgut fermentation assay was used to determine the effect of the amount of kiwi fruit consumed over an extended period of time on the fermentative potential of the material exiting the small intestine (ileal digesta). Thus, the results (e.g. hindgut OM fermentability of ileal digesta, predicted hindgut SCFA production and SCFA absorption) were assumed to represent the fermentative potential of ileal digesta by the large intestinal microbiota of an adult human.
Ethics approval was obtained from the Human Ethics Committee, Massey University, Palmerston North, New Zealand. Fermentation of ileal digesta, collected on days 5, 15, 29 and 43 (kiwi fruit-feeding period), was performed as previously described( Reference Coles, Moughan and Awati 28 , Reference Coles, Moughan and Awati 32 , Reference Montoya, Rutherfurd and Moughan 33 ) using a standard pooled human faecal inoculum. Fresh faeces were collected from three healthy adult human volunteers directly into isolated containers flushed with CO2 at 37°C as required for each assay day in an attempt to have a more representative microbial population than that obtained for only one volunteer. During the study, the volunteers had eaten an unspecific Western diet and did not consume any medication. They had not been treated with antibiotics for 3 months. The same volunteers were used each time and maintained a regular (three meals per d, coffee in the morning and a serving of fruit in the afternoon) and a similar (meat/fish, vegetables/fruits and starchy food) diet throughout the experimental period. The same amount of fresh faecal material per participant was used to prepare fresh pooled inoculum. Fresh inoculum rather than frozen inoculum was used to avoid any reduction in fermentative capacity due to storage. It was assumed that the fermentative capacity of the pooled faeces was similar over the time of the study. Samples of freeze-dried ileal digesta (0·1g) and blank samples were fermented with the fresh pooled human faecal inoculum for 24 h at 37°C( Reference Coles, Moughan and Awati 28 ). Ileal digesta and faeces collected during the fibre-free-feeding period were used to compare the baseline values for some selected characteristics between pigs assigned to the diets containing the two levels of kiwi fruit.
Hindgut microbiota fermentative capacity
A pig-based in vitro hindgut fermentation assay was used to determine the change in fermentative capacity of the hindgut microbiota as a result of the amount of kiwi fruit intake over an extended period of time. A standard fibre mixture (1:1:1, pectin–inulin–wheat bran) was prepared as a purified fibre substrate for the faecal microbiota. The purified substrate (0·1g) was then fermented with a pig faecal inoculum freshly prepared from fresh faeces collected from each pig at each time point (i.e. days –3, 4, 14, 28 and 42). Fermentations were performed for 24 h at 37°C following a standard validated procedure( Reference Coles, Moughan and Awati 28 ). For each pig at each time point, blank samples (inoculum only) were also fermented.
Chemical analyses and bacterial quantification
The diets and ileal digesta were analysed in duplicate for DM, ash, starch (Kit AA/AMG; Megazyme), crude protein (N×6·25; using an elemental analyser LECO)( 34 ), soluble and insoluble dietary fibre( Reference Prosky, Asp and Schweizer 35 ) and TiO2 ( Reference Short, Gorton and Wiseman 36 ). Diets were analysed for gross energy (LECO AC-350 Automatic Calorimeter) and diethyl ether extract (using Soxhlet apparatus and petroleum ether extraction)( 34 ), and faeces for DM, ash, and TiO2. DM and ash content were determined for the material remaining after the in vitro fermentation.
SCFA (acetic, propionic, butyric and valeric acids) were determined in supernatants obtained from faeces, ileal digesta, and after in vitro fermentation of both ileal digesta and purified fibre, using GC( Reference Montoya, Saigeman and Rutherfurd 7 , Reference Montoya, Rutherfurd and Moughan 33 ).
The ileal digesta and faecal samples were analysed for total bacteria, Enterobacteria (Enterobacteriaceae family) and Lactobacillus using quantitative PCR as described in detail previously( Reference Han, Balan and Molist Gasa 37 ). Enterobacteria and Lactobacillus were selected for study as they represent groups of potentially harmful and beneficial bacteria, respectively, and they have shown adaptational changes over time in growing pigs( Reference Castillo, Martín-Orúe and Anguita 6 ). Bacteria were expressed as the 16S rRNA gene copy number.
Calculations
Due to the effect of the main factors (kiwi fruit level or time) or their interaction on the DM of faeces and the amount of DM entering and exiting the hindgut (online Supplementary Table S2), data were normalised appropriately for food DM intake based on the amounts of indigestible marker (i.e. data were expressed based on a kg diet DM intake). Normalising of parameters allowed comparing within and between response variables, as they were expressed in the same unit, and controlled for differences over time in food DM intake.
The amount of DM entering or exiting the hindgut normalised for a kg of diet DM intake was calculated as follows:
DM entering (ileal digesta) or exiting (faeces) the hindgut (kg DMileal digesta or faeces/kg diet DM intake)=(TiO2 diet/TiO2 ileal digesta or faeces)
Normalisation of the concentrations of SCFA (mmol/kg DM ileal digesta or faeces) and bacteria (16S rRNA gene copy number/kg DM ileal digesta or faeces) in the terminal ileal digesta and faeces and concentrations of nutrients (g/kg DM ileal digesta) in ileal digesta were obtained as follows (using SCFA as an example):
Ileal or faecal SCFA concentration normalised for food DM intake (mmol/kg diet DM intake)=SCFA concentration (mmol/kg DM ileal digesta or faeces)×DM entering or exiting the hindgut (kg DM ileal digesta or faeces/kg diet DM intake)
The calculations used to determine hindgut OM fermentability of ileal digesta or fibre substrate, SCFA production from the in vitro fermentation, predicted total tract OM digestibility and predicted SCFA production and absorption values are as given below. The calculations have been fully described by Montoya et al. ( Reference Montoya, Rutherfurd and Moughan 33 )
Hindgut OM fermentability of ileal digesta or fibre substrate in vitro (%) = ((OMb–(OMa–((OMblank initial+OMblank final)/2)))/OMb)×100
SCFA production in vitro (mmol/kg ileal digesta or fibre substrate DM incubated)=(SCFAafter in vitro fermentation of ileal digesta–SCFAbefore in vitro fermentation of ileal digesta–((SCFAblank initial+SCFAblank final)/2))×kg DMileal digesta or fibre substrate
Predicted total tract OM digestibility in vivo/in vitro (%) = ((OMD–(((100–hindgut OM fermentability of ileal digesta in vitro )/100)×OM entering the hindgut))/OMD)×100
Predicted hindgut SCFA production normalised for food DM intake in vivo/in vitro (mmol/kg diet DM intake)=SCFA production in vitro fermentation of ileal digesta×DM entering the hindgut
Predicted hindgut SCFA absorption normalised for food DM intake in vivo/in vitro (mmol/kg diet DM intake)=predicted hindgut SCFA production in vitro +normalised ileal SCFA concentration–normalised faecal SCFA concentration
where OMb and OMa are the amounts of OM in the substrate (ileal digesta or fibre source) either before or after in vitro fermentation. OMblank initial, OMblank final, SCFAblank initial and SCFAblank final are the amounts of OM, and the concentration of SCFA in the blank bottle (which contained inoculum but no substrate (ileal digesta or fibre source)) before (initial) and after (final) in vitro fermentation, respectively( Reference Coles, Moughan and Awati 32 ). OMD is the OM content in the diet.
The hindgut OM fermentability and production of SCFA of the fibre source could be affected by the 16S rRNA gene copy number of bacteria present in faeces. Thus, to determine the fermentative capacity of the faecal microbiota both measures were expressed by a 16S rRNA gene copy number. The total number of bacteria was not determined, but an estimation of bacterial unit was based on the normalised total faecal bacteria (16S rRNA gene copy number/kg diet DM intake). In addition, a factor 1011 was applied for ease of expression. Thus, the OM fermentability and production of SCFA for the purified fibre substrate were calculated as follows (using OM fermentability as an example):
Normalised hindgut OM fermentability of the model fibre substrate (% per 16S rRNA gene copy number of total faecal bacteria/kg diet DM intake/1011)=hindgut OM fermentability of fibre substrate in vitro /(normalised amount of 16S rRNA gene copy number of total faecal bacteria/1011)
Statistical analysis
For each experiment, the number of replications required to detect a statistical difference (5 %) between treatments was estimated, with a power >80 % at a two-tail 5 % significance level, based on estimates of variance (sd 2·5 %) and means found in previous studies( Reference Coles, Moughan and Awati 28 , Reference Noblet, Fortune and Dupire 38 , Reference Libao-Mercado, Yin and van Eys 39 ). A total of six replicates per treatment was deemed to be satisfactory. However, in this study, seven replicates per treatment were used to consider the possibility of loss of an animal during the study due to cannula malfunction.
Statistical analyses were performed using the MIXED model procedure of SAS (SAS/STAT version 9.4; SAS Institute Inc.). Linear mixed models were performed for all variables. The full statistical model included the effect of dietary inclusion level of kiwi fruit, time (as either a categorical or a numerical variable up to cubic order) and their interaction as fixed effects, and the pig as a random effect. When the interaction effect was not statistically significant, it was removed from the final model. The most appropriate covariance structure (simple, compound symmetry, autoregressive, ante-dependence, unstructured, heterogeneous compound symmetry, Toeplitz or heterogeneous autoregressive) of the mixed model for each response variable was selected after fitting the models by the restricted maximum likelihood method and comparing them using the log-likelihood ratio test. The selected polynomial model for each response variable was chosen after comparing higher order models with reduced order models (i.e. removing predictors that did not affect the response variable) using the log-likelihood ratio test. Finally, the best model describing each response variable was chosen after comparing the selected models having time as either a categorical or a numerical covariate using the log-likelihood ratio test. For all of the response variables, the best model had time as a numerical variable.
The model diagnostics for each response variable were tested after combining the PROC UNIVARIATE and the ODS GRAPHICS procedures of SAS before comparing the means. When a response variable did not fulfil the model assumptions of normality and homoscedasticity, a transformation of its raw data was conducted. A predictor effect was considered significant when P≤0·05, and a trend when 0·05<P≤0·10.
An independent Student’s t test was performed to compare some of the studied parameters at baseline (i.e. fibre-free period) between the pigs assigned to the diets containing two levels of kiwi fruit.
The PROC CORR of SAS was used to determine different correlations. Correlation analysis (n 56) was conducted between nutrient contents of ileal digesta and the fermentative potential of ileal digesta (hindgut OM fermentability and production of SCFA) determined after in vitro fermentation of ileal digesta collected from pigs with a pooled human faecal inoculum and expressed as either per kg DM ileal digesta or per kg diet DM intake. This analysis aimed to determine whether the fermentative potential of ileal digesta was potentially influenced by the nutrient contents in the substrate.
Correlation analysis was also conducted between the faecal bacteria with: amounts of OM, nutrients, SCFA, and total ileal bacteria entering the hindgut and the fermentative potential of ileal digesta expressed as per kg diet DM intake. For ileal digesta, further correlations were determined between normalised concentrations of nutrients and total bacteria. The correlation analysis only considered the dataset of the kiwi fruit-feeding period (i.e. from 4 to 43 d).
Results
All measures were similar between both groups of pigs at baseline (P>0·05; online Supplementary Table S3).
In this section, the tables provide the results where there was no statistically significant interaction between kiwi fruit level and time, while the figures show the results with a statistically significant interaction.
Foregut adaptation
Bacteria in pig ileal digesta
There was no interaction (P>0·05) between kiwi fruit level and time for the total bacteria in ileal digesta (Table 1). The total bacteria (16S rRNA gene copy number/kg diet DM intake) in the ileal digesta of the pigs fed the diet containing the high amount of kiwi fruit was 1·5-fold higher (P<0·05) compared with pigs fed the diet with the low amount of kiwi fruit, while the Enterobacteria and Lactobacillus were similar (P>0·05). There was also an effect (P<0·05) of time on Enterobacteria and Lactobacillus and a tendency (P<0·1) for an effect of time on total ileal bacteria and the Lactobacillus–Enterobacteria ratio. The total bacteria and Enterobacteria in the ileal digesta for both diets decreased at a daily rate of 11 and 13×109 16S rRNA gene copy number/kg diet DM intake, respectively. The Lactobacillus decreased at a daily rate of 0·14×109 over the first 4 weeks and thereafter increased at a daily rate of 0·17×109 (P<0·05), while the Lactobacillus–Enterobacteria ratio increased linearly (P<0·05).
Table 1 Ileal bacteria (normalised for diet DM intake) for pigs fed diets containing two amounts of kiwi fruit (KF) over 44 dFootnote * (Mean values with their pooled standard errors of the mean, n 28 for the KF level effect and n 14 for the time effect)

* The interaction between diet and time was not significant (P>0·05). Therefore, the term was removed from the final model.
† A logarithm base 10 transformation of the raw data (16S rRNA gene copy number/kg diet DM intake) was required to achieve the model assumptions of normality and homoscedasticity.
‡ Values represent the mean bacteria (1011 16S rRNA gene copy number/kg diet DM intake) after back transformation.
§ L or Q, linear or quadratic effect for the time factor, respectively.
|| A logarithm base 10 transformation of the raw data was required to achieve the model assumptions of normality and homoscedasticity.
¶ Values represent the ratio after back transformation.
Nutrient composition of the ileal digesta, in vitro hindgut fermentability of ileal digesta organic matter and in vitro hindgut production of ileal digesta SCFA
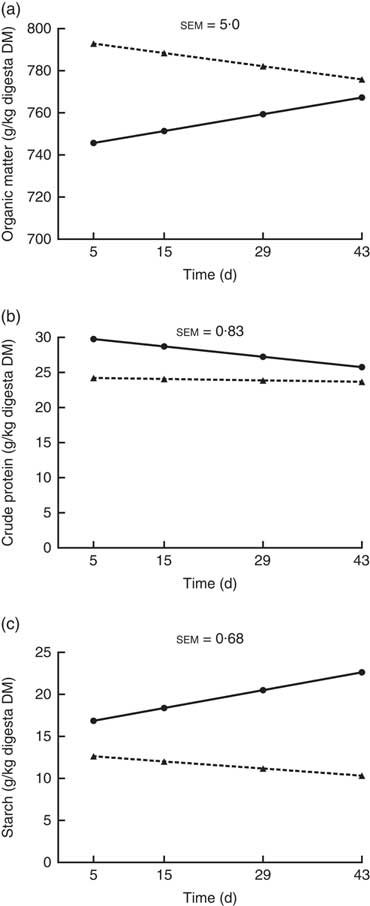
The OM, crude protein and starch contents of the ileal digesta (g/kg digesta DM) showed a significant interaction (P<0·05; Fig. 2) between kiwi fruit level and time. For the other nutrients analysed (i.e. soluble and insoluble dietary fibre), no interaction (P>0·05) was observed between kiwi fruit level and time. The OM content increased over time (0·57 g/kg digesta DM per d; P<0·05) for the diet containing the low kiwi fruit concentration but tended to decrease for the diet containing the high amount of kiwi fruit (–0·45 g/kg digesta DM per d; P=0·06). The crude protein content decreased over time (–0·11 g/kg digesta DM per d; P<0·05) for the diet with the low kiwi fruit concentration but was constant for the diet containing the high amount of kiwi fruit (P=0·55). The starch content increased over time (0·15 g/kg digesta DM per d; P<0·05) for the diet with the low amount of kiwi fruit but was constant for the diet containing the high amount of kiwi fruit (P=0·12). The pigs fed the diet containing the high amount of kiwi fruit had higher (P<0·01) soluble (70·5 v. 62·4 g/kg DM digesta) and insoluble (388 v. 289 g/kg DM digesta) dietary fibre concentrations in ileal digesta, but these values were not influenced by time (P>0·05; data not shown).
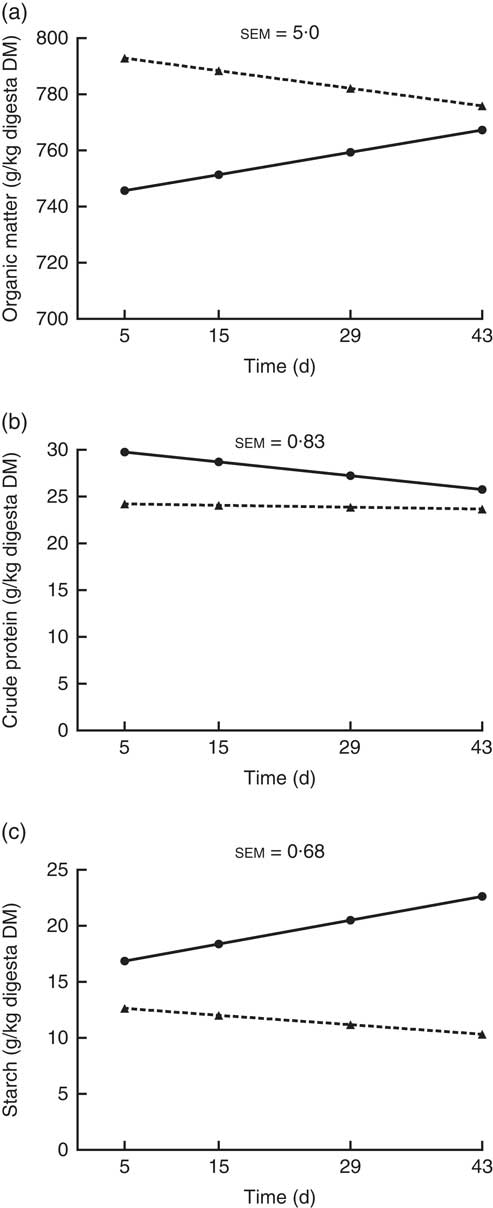
Fig. 2 Organic matter (OM) (a), crude protein (b) and starch (c) contents of ileal digesta of pigs fed diets containing two levels of kiwi fruit (KF) over an extended period of time. The P-values for diet, time and their interaction effects for OM were P<0·001, P=0·72 and P=0·004, respectively, for crude protein were P<0·001, P=0·001 and P=0·013, respectively, and for starch were P=0·033, P=0·099 and P=0·001, respectively. sem, pooled standard error of the mean, n 7. ![]() , 133 g/kg DM of KF;
, 133 g/kg DM of KF; ![]() , 266 g/kg DM of KF.
, 266 g/kg DM of KF.
The in vitro hindgut OM fermentability of ileal digesta increased linearly over time (0·11 % units/d; P<0·05; Table 2) for both diets, but it was not influenced by the dietary kiwi fruit level (P>0·05).
Table 2 Hindgut organic matter (OM) fermentability of ileal digesta and SCFA produced after in vitro fermentation (human inoculum) of the ileal digesta of pigs given diets containing two levels of kiwi fruit (KF) over 44 dFootnote * (Mean values with their pooled standard errors of the mean, n 28 for the KF level effect and n 14 for the time effect)

* The interaction between diet and time was not significant (P>0·05). Therefore, the term was removed from the final model.
† L or Q, linear or quadratic effect for the time factor, respectively.
‡ For the butyric acid production, there was a significant (P<0·001) interaction between KF level and time as detailed in Fig. 3.
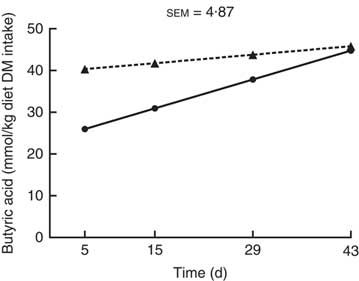
For butyric acid production (mmol/kg DM digesta incubated), an interaction (P<0·001; Fig. 3) between kiwi fruit level and time was found, while no interaction was found in the production of the other SCFA (P>0·05; Table 2). Butyric acid production increased (P<0·001) over time for the diet containing the low amount of kiwi fruit (6·5 mmol/kg DM incubated per d; P<0·001), but it did not change for the diet containing the high amount of kiwi fruit (P=0·40) (Fig. 3). The acetic acid production increased (5·6 mmol/kg DM incubated per d; P<0·05; Table 2) for the first 4 weeks and thereafter decreased (11·7 mmol/kg DM incubated per d). SCFA production was not influenced by the dietary kiwi fruit level (P>0·05).

Fig. 3 Butyric acid production after in vitro fermentation (human faecal inoculum) of ileal digesta for pigs fed diets containing two levels of kiwi fruit (KF) over an extended period of time. The P-values for diet, time and their interaction effects were P=0·358, P<0·001 and P<0·001, respectively. sem, pooled standard error of the mean, n 7. ![]() , 133 g/kg DM of KF;
, 133 g/kg DM of KF; ![]() , 266 g/kg DM of KF.
, 266 g/kg DM of KF.
Nutrients entering the hindgut, predicted total tract organic matter digestibility and predicted production and absorption of SCFA in the hindgut
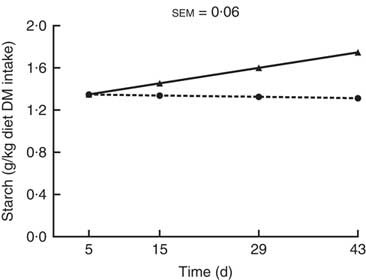
Starch (g/kg diet DM intake) was the only nutrient entering the hindgut for which there was a significant (P<0·001; Fig. 4) interaction between kiwi fruit level and time. The amount of starch entering the hindgut increased over time (0·011 g/kg diet DM intake per d; P<0·05) for the diet containing the low amount of kiwi fruit, while it was constant over time for the diet containing the high amount of kiwi fruit (P=0·78). The amounts of OM, crude protein, soluble and insoluble dietary fibre entering the hindgut were 1·6-, 1·4-, 1·8- and 1·7-fold greater, respectively (P<0·001; Table 3), for the pigs fed the diet containing the high level of kiwi fruit, but only the amounts of crude protein and insoluble dietary fibre entering the hindgut were influenced by time (P<0·05). The amounts of crude protein and insoluble dietary fibre decreased over the first 4 weeks (–0·02 and –0·16 g/kg diet DM intake per d, respectively; P<0·05) and thereafter increased (0·01 and 0·24 g/kg diet DM intake per d, respectively) for both diets.

Fig. 4 Amount of starch entering the hindgut of pigs fed diets containing two levels of kiwi fruit (KF) over an extended period of time. The P-values for diet, time and their interaction effects were P=0·726, P=0·045 and P=0·019, respectively. sem, pooled standard error of the mean, n 7. ![]() , 133 g/kg DM of KF;
, 133 g/kg DM of KF; ![]() , 266 g/kg DM of KF.
, 266 g/kg DM of KF.
Table 3 Amounts of organic matter (OM) and nutrients entering the hindgut of pigs, predicted total tract apparent digestibility of OM and predicted SCFA production and absorption in the hindgut for diets containing two levels of kiwi fruit (KF) fibre consumed over 44 dFootnote * (Mean values with their pooled standard errors of the mean, n 28 for the KF level effect and n 14 for the time effect)

SDF, soluble dietary fibre; IDF, insoluble dietary fibre.
* The interaction between diet and time was not significant (P>0·05). Therefore, the term was removed from the final model.
† The OM or specific nutrients entering the hindgut normalised for the consumption of a kg of diet DM.
‡ For the amount of starch entering the hindgut, there was a significant (P=0·001) interaction between KF level and time as detailed in Fig. 4.
§ L or Q, linear or quadratic effect for the time factor, respectively.
|| The predicted total tract digestibility was estimated based on the determined apparent ileal OM digestibility and the predicted hindgut OM fermentability of ileal digesta based on an in vitro fermentation assay (the in vitro hindgut fermentation values are given in Table 2).
¶ The predicted hindgut SCFA production was estimated based on the SCFA produced after in vitro incubation of ileal digesta with a human faecal inoculum and corrected for the amount of DM entering the hindgut normalised for the consumption of a kg of DM diet.
** For the predicted hindgut valeric acid production, there was a significant (P=0·033) interaction between KF level and time as detailed in Fig. 5.
†† A logarithm base 10 transformation of the raw data was required to achieve the model assumptions of normality and homoscedasticity.
‡‡ Values represent the mean for each response variable after back transformation.
§§ The SCFA absorption in the hindgut was predicted based on the determined ileal and faecal normalised concentrations of SCFA and the hindgut production of SCFA. The estimated values were obtained after summing the SCFA entering (normalised ileal concentrations), and produced in the hindgut (predicted based on an in vitro assay), and then subtracting the excreted SCFA (normalised faecal concentrations).
|||| For the predicted hindgut butyric acid absorption, there was a significant (P=0·05) interaction between KF level and time as detailed in Fig. 6.
The predicted total tract apparent digestibility of OM (%) was lower (–2·8 % units) for the diet containing the high kiwi fruit concentration (P<0·001) compared with the diet containing the low kiwi fruit concentration (Table 3), but there was no time effect.
There was an interaction (P<0·05) between kiwi fruit level and time for the predicted hindgut production (mmol/kg diet DM intake) of valeric acid only (Fig. 5). The production of valeric acid increased over time for the diet containing the low amount of kiwi fruit (0·12 mmol/kg diet DM intake per d; P<0·05), but it did not change for the diet containing the high amount of kiwi fruit (P=0·16). The predicted hindgut production of acetic and propionic acids (Table 3) was higher (on average 1·6-fold higher) for the diet containing the high kiwi fruit concentration (P<0·01), but they were not influenced by time (P>0·05). In contrast, the predicted hindgut production of butyric acid was influenced by time (0·28 mmol/kg DM intake per d; P<0·001), but it was not influenced by the concentration of kiwi fruit in the diet (P>0·05).
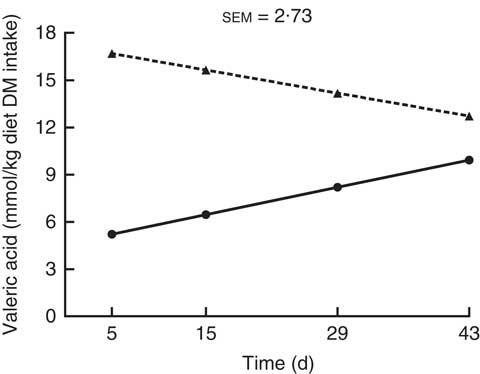
Fig. 5 Predicted hindgut production of valeric acid after in vitro fermentation (human faecal inoculum) of ileal digesta of pigs fed diets containing two levels of kiwi fruit (KF) for 44 d. The P-values for diet, time and their interaction effects were P=0·035, P=0·851 and P=0·033, respectively. sem, pooled standard error of the mean, n 7. ![]() , 133 g/kg DM of KF;
, 133 g/kg DM of KF; ![]() , 266 g/kg DM of KF.
, 266 g/kg DM of KF.
For only the predicted hindgut absorption of butyric acid (mmol/kg diet DM intake), there was an interaction between kiwi fruit level and time (P=0·05; Fig. 6). The predicted hindgut absorption of butyric acid increased over time for the diet containing low amount of kiwi fruit (0·49 mmol/kg diet DM intake per d; P<0·05), but it did not change for the diet containing the high amount of kiwi fruit (P=0·24). The predicted hindgut absorption of acetic and propionic acids (Table 3) was higher (on average 1·6-fold) for the diet containing the high kiwi fruit concentration (P<0·01), but their absorptions were not influenced by time (P>0·05).

Fig. 6 Predicted hindgut absorption of butyric acid after in vitro fermentation (human faecal inoculum) of ileal digesta of pigs fed diets containing two levels of kiwi fruit (KF) for 44 d. The P-values for diet, time and their interaction effects were P=0·118, P=0·002 and P=0·050, respectively. sem, pooled standard error of the mean, n 7. ![]() , 133 g/kg DM of KF;
, 133 g/kg DM of KF; ![]() , 266 g/kg DM of KF.
, 266 g/kg DM of KF.
Hindgut adaptation
Bacteria in pig faeces
There was an effect of dietary kiwi fruit level and time on the total faecal bacteria and Lactobacillus normalised for diet DM intake (16S rRNA gene copy number/kg diet DM intake) (P<0·05; Table 4). The total bacteria and Lactobacillus were 1·8- and 2·2-fold higher (P<0·05), respectively, in the faeces of the pigs fed the diet containing the high level of kiwi fruit. The total bacteria in faeces increased (P<0·05) at a daily rate of 8·9×109 16S rRNA gene copy number/kg diet DM intake for both diets, while Lactobacillus and the Lactobacillus–Enterobacteria ratio decreased (e.g. –25×106 16S rRNA gene copy number of Lactobacillus/kg diet DM intake per d) over the first 28 d and thereafter increased (e.g. 5·4×106 16S rRNA gene copy number of Lactobacillus/kg diet DM intake per d) (P<0·05). Enterobacteria were not affected by the level of kiwi fruit or time (P>0·05).
Table 4 Faecal bacteria (normalised for diet DM intake) for pigs fed diets containing two levels of kiwi fruit (KF) over 44 dFootnote * (Mean values with their pooled standard errors of the mean, n 28 for the KF level effect and n 14 for the time effect)

* The interaction between diet and time was not significant (P>0·05). Therefore, the term was removed from the final model.
† A logarithm base 10 transformation of the raw data (16S rRNA gene copy number/kg diet DM intake) was required to achieve the model assumptions of normality and homoscedasticity.
‡ L or Q, linear or quadratic effect for the time factor, respectively.
§ Values represent the mean bacteria (1011 16S rRNA gene copy number/kg diet DM intake) after back transformation.
|| A logarithm base 10 transformation of the raw data was required to achieve the model assumptions of normality and homoscedasticity.
¶ Values represent the ratio after back transformation.
Hindgut microbiota fermentative capacity
The kiwi fruit concentration influenced (P<0·05; Table 5) the hindgut OM fermentability of fibre substrate (normalised values; %/16S rRNA gene copy number per kg diet DM intake per 1011) and the hindgut production of acetic and propionic acids (normalised; mmol/kg DM incubated per 16S rRNA gene copy number per kg diet DM intake per 1011). For example, the hindgut OM fermentability of fibre substrate was 1·4-fold higher in the pigs fed the diet containing the low level of kiwi fruit.
Table 5 Faecal microbiota fermentative capacityFootnote * (normalised hindgut in vitro organic matter (OM) fermentability and SCFA production) for a model fibre substrate fermented with an inoculum prepared from fresh faeces collected at different times from pigs fed diets containing two levels of kiwi fruit (KF) over 44 dFootnote † (Mean values with their pooled standard errors of the mean, n 27 for the KF level effect and n 12 or 14Footnote ‡ for the time effect)

* The OM fermentability of fibre substrate and SCFA production data were normalised for the number of 16S rRNA gene copies of total faecal bacteria/1011 present in the faecal samples. The purified fibre substrate consisted of a mixture of pectin–inulin–wheat bran (1:1:1).
† The interaction between diet and time was not significant (P>0·05). Therefore, the term was removed from the final model.
‡ n 6 at 4 d for the diets containing 133 and 266 g/kg DM of KF for the SCFA production.
§ A logarithm base 10 transformation of the raw data was required to achieve the model assumptions of normality and homoscedasticity.
|| L, Q or C, linear, quadratic or cubic effect for the time factor, respectively.
¶ Values represent the mean for each response variable after back transformation.
** A square root transformation of the raw data was required to achieve the model assumptions of normality and homoscedasticity.
The normalised hindgut OM fermentability of fibre substrate and SCFA production were influenced by time (P<0·05). The normalised hindgut OM fermentability of fibre substrate and the normalised hindgut production of propionic and valeric acids decreased until day 14 (e.g. 0·85 %/16S rRNA gene copy number per kg diet DM intake per 1011 per d for the normalised hindgut OM fermentability) and thereafter was relatively constant. The normalised hindgut production of acetic and butyric acids decreased over the whole experimental period (0·52 and 0·24 mmol/kg DM incubated per 16S rRNA gene copy number per kg diet DM intake per 1011 per d, respectively) (P<0·05).
Correlations between nutrients in ileal digesta and parameters of hindgut fermentation expressed as both per kg DM ileal digesta and normalised per kg diet DM intake
The starch content of the ileal digesta was correlated with both the hindgut OM fermentability of the ileal digesta and the production of butyric acid (on average r 0·41; P<0·01; online Supplementary Table S4). The crude protein content was correlated with the production of propionic acid (r 0·48; P<0·01), but it tended to be negatively correlated with the production of acetic (P=0·051) and butyric (P=0·09) acids (on average r –0·25). The amount of insoluble dietary fibre was negatively correlated with the production of acetic and propionic acids (on average r –0·33; P<0·05).
The amount of OM entering the hindgut was correlated with the predicted hindgut production of SCFA (r 0·53–0·84; P<0·001; online Supplementary Table S5). An investigation of the nutrient concentrations related to the predicted hindgut production of SCFA showed that the production of acetic, propionic and valeric acids were mainly correlated with crude protein and insoluble and soluble dietary fibre entering the hindgut (r 0·40–0·84; P<0·01), while the production of butyric acid was mainly correlated with starch and soluble dietary fibre entering the hindgut (r 0·41–0·42; P<0·01). Similar correlation results were observed for the predicted hindgut absorption of SCFA (data not shown).
The normalised concentration of the total faecal bacteria was related to the amount of acetic acid both entering the hindgut and predicted to be produced in the hindgut (on average, r 0·29; P<0·05) and also to the predicted hindgut production of butyric (r 0·27; P<0·05) and valeric (r 0·30; P<0·05) acids.
The in vitro hindgut production (per kg DM ileal digesta) of propionic acid was negatively correlated with the production of the remaining SCFA (r –0·27 to –0·46; P<0·05; online Supplementary Table S6), while the in vitro hindgut production of the remaining SCFA was positively correlated with each other (r 0·62–0·69; P<0·05). Similarly, the predicted hindgut production (kg diet DM intake) of all SCFA was positively correlated (r 0·26–0·81; P<0·05) with each other.
Discussion
A combined in vivo/in vitro digestion methodology was applied to determine the hindgut fermentability of ileal digesta OM and to predict the total tract OM digestibility and the production and absorption of SCFA in the hindgut. The approach, its assumptions, advantages and disadvantages have been discussed in detail previously( Reference Montoya, Rutherfurd and Moughan 22 , Reference Montoya, Rutherfurd and Moughan 33 ). Briefly, during the in vitro hindgut fermentation assay (ileal digesta as substrate), it is assumed that the faecal microbial population is similar to the hindgut microbial population and that the fermentative capacity of the faecal microbial population of the pooled human inoculum is similar over time despite inevitable changes in the diets of the volunteers. In addition, the parameters (e.g. incubation time) of the assay are fixed. However, some of the parameters such as transit time in the hindgut (i.e. related to incubation time in the in vitro assay) may differ between dietary treatments, and the microbial population and its fermentative capacity may differ across dietary treatments as observed here. Despite this, it is notable that the predicted total tract OM digestibility values (96 and 93 % for the diets containing 133 and 266 g kiwi fruit/kg DM, respectively) determined using the pooled human faecal inoculum were similar to those obtained directly (in vivo) in the same group of pigs as used here (97 and 94 %, respectively)( Reference Montoya, Saigeman and Rutherfurd 7 ) and with the same statistical conclusions concerning interaction and main effects. For predicting the amounts of SCFA absorbed in the hindgut, in the present work, it was assumed that the amount of SCFA present in pig faeces would be similar to that present in human faeces for the same diet. In support of the latter assumption, similar concentrations of SCFA have been reported in the faeces of humans and pigs( Reference Von Engelhardt, Rönnau and Rechkemmer 40 ), due to the high extent of SCFA absorption (95–99 %) in the hindgut between species( Reference Montoya, Rutherfurd and Moughan 33 , Reference Topping and Clifton 41 , Reference Roy, Kien and Bouthillier 42 ), and the similar rate of SCFA absorption (approximately 9 µmol/cm2 per h for pigs, humans and dogs)( Reference Bugaut 43 , Reference Bergman 44 ). It was also assumed that the ileal concentration of SCFA and their production in the hindgut are similar between growing pigs and adult humans.
Several of the response variables analysed were influenced by time independently of the kiwi fruit level, whereas for a few of them the change over time depended upon the level of kiwi fruit in the diet. In the former case, the change over time (i.e. same rate for both kiwi fruit levels) can be attributed to effects other than the diet, such as age and degree of maturity of the animal. For instance, growing pigs, with similar BW to those used in this study, fed a maize–soyabean-based diet had different total tract OM digestibility over time( Reference Zhao, Wang and Liu 9 , Reference Goff, Milgen and Noblet 45 ). However, the total tract OM digestibility did not change over time in a previous study( Reference Montoya, Saigeman and Rutherfurd 7 ), which could be due to the different diet composition between both studies.
Foregut adaptation
The impact of consuming two dietary levels of kiwi fruit over a 6-week period on the fermentative potential (predicted hindgut SCFA production and OM fermentability) of the ileal digesta entering the hindgut was investigated using the in vitro fermentation assay.
The effects of kiwi fruit level on digestive and fermentative changes over time were mainly observed in the foregut. Changes in the amount of starch entering the hindgut could be used to predict the hindgut production of valeric acid and also the hindgut absorption of butyric acid over time, which differed based on the amounts of kiwi fruit intake. It is important to mention that the predicted hindgut SCFA absorption could represent adaptation in both (or either) the foregut (SCFA entering the hindgut and produced in the hindgut) and the hindgut (SCFA exiting the hindgut). Considering that faecal SCFA concentrations may represent <2 % of the SCFA predicted to be produced in the hindgut( Reference Montoya, Rutherfurd and Moughan 33 ), it is expected that the absorbed SCFA were more influenced by foregut adaptation than by hindgut adaptation.
The effect of time on digesta starch and crude protein contents and the production of butyric acid cannot be strictly considered as an adaptation of the pigs over time, as these parameters were not normalised for food DM intake. Despite this, they do provide useful information relating the different parameters of hindgut fermentation. There was a marked effect of time on the starch and protein contents of the ileal digesta, but the effect of time was only seen for the low-kiwi fruit diet. This is likely due to the amounts of kiwi fruit compounds (e.g. dietary fibre, actinidin) consumed and their effect on nutrient digestion. For instance, actinidin, a cysteine-protease present in green kiwi fruit, helps to improve the small intestinal digestibility of beef muscle protein( Reference Montoya, Cabrera and Zou 46 ). Thus, differences in actinidin level could lead to differences in ileal digesta composition. Starch is a readily fermentable carbohydrate. Thus, the increased starch content in the ileal digesta over time for the low-kiwi fruit diet may explain the higher butyric acid production during hindgut fermentation of the ileal digesta. These parameters were indeed correlated (r 0·42). This study was not designed to elucidate the mechanisms controlling the adaptational changes that were observed. However, several of the correlations found point towards possible causes for the mechanism behind the changes over time.
The increased starch content in the ileal digesta over time for the pigs fed the low kiwi fruit-containing diet also explains the increased amount of starch entering the hindgut for this diet. The change over time for the amount of crude protein and insoluble dietary fibre entering the hindgut for both diets can be explained by the changes in total ileal bacteria. Previous studies have shown that the main source of crude protein in ileal digesta of pigs and humans fed a casein-containing diet is bacterial( Reference Miner-Williams, Deglaire and Benamouzig 47 , Reference Miner-Williams, Moughan and Fuller 48 ). A recent study has shown that ileal bacteria co-fractionated when insoluble dietary fibre is determined using the Prosky method, and it contributed up to 13 % of the analysed insoluble dietary fibre( Reference Montoya, Rutherfurd and Moughan 29 ).
There is evidence here that both the level of kiwi fruit in the diet and time affected the normalised total ileal bacteria. The reduction in the total ileal bacteria and Enterobacteria over time may be explained by the change from a highly digestible (fermentable) fibre-free diet to diets containing dietary fibre. The amount of readily fermentable substrates (e.g. starch, sucrose, fructose) for microbial foregut fermentation was reduced when pigs started receiving the kiwi fruit-containing diets. A change in enzymatic digestion due to the presence of actinidin in the kiwi fruit-containing diets can also be a causative factor. Recently, we developed a combined in vivo/in vitro ileal fermentation assay, which showed that a significant degree of fermentation occurs in the ileum( Reference Montoya, de Hass and Moughan 49 ). Although both groups of pigs in the presently reported study had the same rate of reduction in total bacteria (11×109 16S rRNA gene copy number/kg diet DM intake per d), the pigs fed the diet containing the high amount of kiwi fruit had a greater total ileal bacteria and Enterobacteria. This was probably due to the higher amount of soluble dietary fibre in the diet containing the high level of kiwi fruit. A significant correlation between soluble dietary fibre entering the hindgut and the total ileal bacteria was observed (r 0·30). In summary, the change from the fibre-free diet to the diets containing kiwi fruit reduced the total ileal bacteria, but this reduction was smaller for the diet containing the high level of kiwi fruit, which may be explained by a higher amount of soluble dietary fibre available for microbial fermentation.
The higher daily rate of reduction for Enterobacteria (13×109 16S rRNA gene copy number/kg diet DM intake) compared with the total ileal bacteria (11×109 16S rRNA gene copy number/kg diet DM intake) and Lactobacillus (decrease of 0·11×109 16S rRNA gene copy number/kg diet DM intake over the first 4 weeks and thereafter increase of 0·20×109), suggests that the microbial composition of the ileal digesta changed over time for both diets. This is supported by the increase in the Lactobacillus–Enterobacteria ratio over time, which suggests an improvement in ileal microbiota composition. It is important to note that the low Lactobacillus–Enterobacteria ratio in ileal digesta and faeces was consistent with those reported in growing pigs( Reference Metzler-Zebeli, Hooda and Pieper 50 ) but not in weaned piglets( Reference Metzler-Zebeli, Mann and Schmitz-Esser 51 ).
Only a few significant correlations (e.g. starch and production of butyric acid) were observed when response variables were expressed either on a kg DM ileal digesta or on a kg diet DM intake basis. This suggests that the nutrient content (kg DM ileal digesta) was the main factor leading to the correlation of the response variables expressed per kg diet DM intake. In contrast, other correlations differed for both expressions. For instance, the correlation between insoluble dietary fibre and propionic acid production (kg DM ileal digesta) was significantly negative (r –0·33), but it was significantly positive (r 0·65) when expressed on a kg diet DM intake basis. This suggests that the amount of the nutrients entering the hindgut (kg diet DM intake) was the main factor leading to the correlations found when data were expressed per kg diet DM intake.
The predicted production of butyric acid increased over time for both diets, while the predicted production of valeric acid increased over time but only for the diet containing low amount of kiwi fruit. Based on the correlations observed, these changes may be partly explained by the increasing amounts of starch entering the hindgut but only for the diet containing the low amount of kiwi fruit as the amount of other nutrients oscillated (crude protein and insoluble dietary fibre) or did not change (soluble dietary fibre) over time. Other nutrients (e.g. sugars, organic acids), not analysed in this study, may also have been responsible for the observed changes over time for the predicted hindgut production of butyric acid. It is known( Reference Torres, Munoz and Peters 52 – Reference Torres, Muñoz and Peters 54 ) that the nutrient composition of the sample being fermented impacts the production of SCFA in in vitro fermentation assays. Although it is perhaps surprising that small changes in the amount of starch entering the hindgut influenced the predicted production of butyric and valeric acids as observed, it is important to consider that the total amount of OM entering the hindgut was not affected over time for both diets. An adaptational response in terms of SCFA concentrations in the hindgut has been reported for rats given gum arabic, guar gum, resistant starch or high amylose maize starch as dietary fibre sources( Reference Henningsson, Margareta and Nyman 5 , Reference Tulung, Rémésy and Demigné 13 ). For example, the total caecal concentration of acetic, propionic and butyric acids increased over time for rats given diets containing resistant starch or high amylose maize starch( Reference Henningsson, Margareta and Nyman 5 ). It is important to note, however, that SCFA concentrations in digesta represent only the unabsorbed SCFA. The total production and absorption of SCFA may be quite different. Recently, it was shown that the faecal concentration of SCFA represented only between 0·5 and 1·6 % of the predicted hindgut SCFA production for pigs given diets containing kiwi fruit( Reference Montoya, Rutherfurd and Moughan 33 ).
The predicted absorption of acetic, propionic and valeric acids was not influenced by time, while the predicted absorption of butyric acid increased over time for the diet containing the low amount of kiwi fruit. This was likely a result of changes in the amounts of starch entering the hindgut as described above, which resulted in changes in the predicted hindgut production of butyric acid and lower amount of butyric acid exiting the hindgut for pigs fed the diet containing the low amount of kiwi fruit as well as the reduction in butyric acid exiting the hindgut over time (e.g. 0·82 and 0·28 mmol/kg diet DM intake for day 15 and 43, respectively), as the amount of butyric acid entering the hindgut did not change over time or between diets( Reference Montoya, Saigeman and Rutherfurd 7 ). The lack of changes over time for the predicted absorption of the other SCFA could also be explained by the same factors. Thus, each factor (predicted hindgut SCFA production, SCFA entering the hindgut and SCFA exiting the hindgut) contributes to the amount of SCFA absorbed in the hindgut, but their contribution differs for each SCFA. For instance, the predicted hindgut production of acetic and propionic acids appears to be the most important factor affecting their absorption. In contrast, the predicted absorption of valeric acid, which was not affected by any of the studied effects, appears to be explained mainly by the amount of valeric acid entering the hindgut (kiwi fruit level effect only)( Reference Montoya, Saigeman and Rutherfurd 7 ), valeric acid exiting the hindgut (kiwi fruit level and time effects) and its predicted hindgut production (interaction between kiwi fruit and time effect).
Overall, the results found here for the change over time in the amount of starch entering the hindgut, the predicted hindgut production of valeric acid and the predicted hindgut absorption of butyric acid show that there was an adaptive time response in the foregut that was dependent on the level of kiwi fruit intake.
Hindgut adaptation
The impact of dietary kiwi fruit intake on the fermentative capacity of the pig hindgut microbiota was examined by determining the normalised hindgut fermentability of OM and production of SCFA for a purified fibre substrate after in vitro fermentation with fresh faeces collected from pigs at different times throughout the experimental period. To compare the fermentative capacity of faecal bacteria, the OM fermentability of a fibre substrate and the production of SCFA were normalised for the total bacteria present in each faecal sample.
The same rate of daily increase in normalised total faecal bacteria and changes over time in bacteria composition (Lactobacillus and the ratio between Lactobacillus–Enterobacteria) in the faecal samples for both diets suggest that there was an adaptive response over time, but this was independent of the amount of dietary kiwi fruit. In contrast to the changes in the ileal bacteria, the total faecal bacteria increased, while the Enterobacteria did not change. The increased faecal microbial population over time may be explained by a higher amount of nutrients (e.g. insoluble dietary fibre) released into the hindgut when compared with the fibre-free diet. Thus, the change from the fibre-free diet to the kiwi fruit-containing diets explained the reduction in the total ileal microbial population as described above and the increase in the total faecal microbial population. Similarly, the higher total population of faecal bacteria over time for the pigs fed the diet containing the high amount of kiwi fruit is mainly explained by a higher amount of nutrients entering the hindgut (insoluble dietary fibre, soluble dietary fibre and crude protein). For example, the amount of insoluble dietary fibre was 1·7-fold greater. The different trends in the faecal bacteria suggest that the microbial population composition changed over time. This is confirmed by the fluctuation in the Lactobacillus–Enterobacteria ratio. The method used here assumes that the human faecal inoculum is consistent over time, which is a disadvantage of the combined in vivo/in vitro approach as it does not reflect the adaptational changes in the microbial population as demonstrated here.
It is known that SCFA can modulate the microbial population( Reference Duncan, Holtrop and Lobley 55 ). In this study, the normalised total amount of faecal bacteria was associated with the amount of acetic acid entering the hindgut (kiwi fruit level and time effects) and the predicted hindgut production of acetic, butyric and valeric acids. This suggests that the amount of acetic acid entering the hindgut and the hindgut production of acetic and butyric acids may play a role in shaping the total faecal bacteria, although they were not correlated with the normalised concentration of faecal Enterobacteria and Lactobacillus (data not shown). As SCFA are produced from the fermentation of nutrients from dietary and non-dietary materials( Reference Montoya, Rutherfurd and Moughan 22 ), nutrients are therefore also drivers of the microbial population. In this study, only crude protein and soluble dietary fibre were related statistically to the total amount of ileal bacteria. In another study using growing pigs fed either a soyabean–wheat-based diet alone (control) or a diet supplemented with resistant starch or alginate over a 12-week period it was found that the faecal microbial relative abundance varied between diets over time( Reference Umu, Frank and Fangel 10 ). For example, for the resistant starch-containing diet, the relative abundance of the Lactobacillaceae family was high during the first 2 weeks, but it was reduced afterwards. In contrast, for the alginate diet, the relative abundance of the Lactobacillaceae family increased over time from week 1 to week 7.
The fermentative capacity of the hindgut microbiota was normalised for the number of 16S rRNA gene copies of total bacteria. Feeding the pigs with kiwi fruit-containing diets over a 6-week period markedly changed the normalised fermentative capacity of the hindgut microbiota over time. The level of kiwi fruit consumed also influenced the normalised fermentative capacity. The normalised hindgut OM fermentability of the fibre substrate was reduced equally for both kiwi fruit-containing diets. The reduced normalised hindgut OM fermentability of fibre substrate mainly explains the observed changes in the normalised productions of all SCFA derived from the fermentation of the model substrate over time. A reduction over time in the bacterial fermentative capacity could have been due to several factors such as (i) change in the microbial composition (e.g. Lactobacillus–Enterobacteria ratio), (ii) amount and composition of the substrate used, (iii) reduction in metabolic activity to compensate for the increased normalised total bacteria.
Kiwi fruit is a good model material for investigating fibre fermentation because it has been well characterised and contains equal amounts of soluble (mainly pectin) and insoluble (mainly cellulose and hemicellulose) dietary fibre( Reference Sims and Monro 23 ), the most common fibre constituents in a human diet. However, other foods will contain these fibre types at similar or different ratios (e.g. apples contains similar ratio) and will contain other fibre types (e.g. β-glucan and arabinoxylan in cereals), which may be fermented to a different extent and may affect the adaptation over time in the animal. Thus, the present work needs to be broadened by investigating other sources of dietary fibre at different levels of inclusion in the diet.
In conclusion, the results demonstrate an effect of the amount of dietary kiwi fruit on the ileal and faecal bacteria, amounts of nutrients entering the hindgut, the predicted production and absorption of acetic and propionic acids and the fermentative capacity of the faecal microbiota (e.g. OM hindgut fermentability of the fibre substrate). These parameters in general and the predicted hindgut production of butyric acid changed over time equally for both levels of kiwi fruit. In contrast, other parameters (starch entering the hindgut, predicted hindgut production of valeric acid and predicted hindgut absorption of butyric acid) changed over time but according to the amount of kiwi fruit. This adaptive time response may explain in part the correlations observed between different parameters. The adaptational changes related to the level of kiwi fruit could have been extended to other parameters of intestinal fermentation if the study had lasted beyond the experimental period (44 d). The experimental period was selected based on previous adaptational studies( Reference Longland, Low and Quelch 1 , Reference Brunsgaard, Bach Knudsen and Eggum 2 , Reference Henningsson, Margareta and Nyman 5 , Reference Castillo, Martín-Orúe and Anguita 6 ). A study to determine the effect of the level of kiwi fruit on intestinal fermentation beyond 44 d is warranted.
Acknowledgements
The authors acknowledge Trent Olson and Stuart Saigeman for running the animal trial, Ajitpal Purba for bacterial analysis, Dr Jonathan Godfrey for statistical advice and ZESPRI International Ltd for supplying the kiwi fruit.
This study was funded by ZESPRI International Ltd. However, ZESPRI International Ltd had no role in the design, analysis or writing of this manuscript.
C. A. M., S. M. R. and P. J. M. were responsible for planning the study. S. J. H. and P. Z. helped to conduct the in vitro fermentation study and determined SCFA. C. A. M. was responsible for conducting the experiments, did the statistical analysis and prepared the first draft of the manuscript that was revised by S. J. H., S. M. R. and P. J. M. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003574