LEARNING OBJECTIVES
After reading this article you will be able to:
• examine current evidence for the treatment of OCD or OCS in patients receiving treatment for chronic psychotic illness
• describe the prevalence of OCD and OCS in patients with chronic psychotic illness, with associated factors
• describe the available pharmacological strategies for managing OCD or OCS in chronic psychotic illness.
Schizophrenia and related disorders are complex. In addition to the presence of psychotic symptoms, these disorders are acknowledged to be associated with clinically significant mood, anxiety, somatic, cognitive, dissociative and obsessive–compulsive symptoms (van Os Reference Turkcan, Yanbay and Satmis2009; Hwang 2018). These add complexity to severity, diagnosis, treatment, prognosis and patient experience. Where they are present, obsessive–compulsive phenomena may add to the severity such that the criteria for full obsessive–compulsive disorder (OCD) are met.
According to DSM-5, OCD may be diagnosed if the patient experiences intrusive or unwanted recurrent and persistent thoughts, urges and images that they try to suppress, neutralise or ignore. These are obsessions. It may also present with repetitive behaviours or mental acts performed in response to an obsession or according to rigid rules that must be adhered to. These are compulsions. It may also present as a combination of these two symptom types. It is usually time-consuming, costing at least 1 h per day, and is associated with clinically significant distress or impairment in social, occupational or other areas of functioning (American Psychiatric Association 2013).
In the newer ICD-11, OCD is described similarly (World Health Organization 2019).
While OCD on its own can be distressing and debilitating, its coexistence with psychotic conditions adds extra, complex layers of morbidity to both disorders. The comorbidity of these conditions has been noted to be associated with reduced social and occupational functioning (Tonna Reference Szmulewicz, Smith and Valerio2015; El-Shiekh Reference Englisch, Esslinger and Inta2017) and poorer quality of life (Sung-Wan Reference Stryjer, Dambinsky and Timinsky2015). Where present, positive and depressive symptoms of psychosis tend to be more severe (Owashi Reference Owashi, Ota and Otsubo2010; Sung-Wan Reference Stryjer, Dambinsky and Timinsky2015). This comorbidity is associated with higher total scores on the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) (Szmulewicz Reference Swets, Dekker and van Emmerik-van Oortmerssen2015). There are significant positive correlations between total Y-BOCS scores and suicidality (Szmulewicz Reference Swets, Dekker and van Emmerik-van Oortmerssen2015). Comorbidity is also associated with movement disorders (Krüger Reference Krüger, Bräunig and Höffler2000).
It is acknowledged in literature that the presence of obsessive–compulsive symptoms (OCS) that do not meet the diagnostic criteria for OCD also creates a considerable burden. Hence, this comorbidity is an issue of public health importance.
Although this article discusses chronic non-affective psychosis in general, the literature on this subject focuses mainly on schizophrenia and schizoaffective disorders. Therefore these two disorders, occurring in the adult population, will be the central theme on which the discussion will be based.
Historical consideration
The occurrence of obsessive–compulsive features prior to, or along with, psychotic disorders has been recognised for almost 150 years. In 1878, the psychiatrist Carl Westphal theorised that obsessive–compulsive syndrome was prodromal to a psychotic disorder or was a variant of it (Hemrom Reference Hoch and Polatin2009). Eugen Bleuler coined the term schizophrenia in 1911 and posited that obsessive–compulsive symptoms were sometimes prodromal to the psychotic disorder. He felt that such symptoms in schizophrenia were akin to hallucinations. Bleuler theorised that some people with schizophrenia experienced obsessive–compulsive symptoms separate from their psychosis, whereas in others these symptoms were interwoven with the psychosis (Poyurovsky Reference Poyurovsky, Faragian and Kleinman-Balush2013). The American psychiatrist Alfred Gordon thought that obsessions and delusions were part of the same spectrum and that the characteristics of each patient determined the evolution into either obsessive–compulsive neurosis or schizophrenia with obsessive–compulsive symptoms (Gordon Reference Grover, Hazari and Chakrabarti1926). In 1945, the Austrian–British psychiatrist Erwin Stengel observed that when obsessive–compulsive symptoms coexisted with psychosis, the former always preceded the latter (Poyurovsky Reference Poyurovsky, Faragian and Kleinman-Balush2013). Hoch & Polatin (Reference Huber, Gross and Schuttler1949) diagnosed some patients as having a pseudoneurotic form of schizophrenia. Unlike psychoneuroses, pseudoneurotic schizophrenia featured ‘pervading anxiety’, including obsessive–compulsive symptoms, and some patients developed overt psychosis. Ismond Rosen, a British psychiatrist, found that the symptoms of each illness mutually affected the other – which created the novel features of schizo-obsessive disorder (Rosen Reference Rodriguez, Corcoran and Simpson1956).
Huber et al (Reference Huey, Zahn and Krueger1980) examined the results of a study of patients with schizophrenia admitted between 1945 and 1959 to the University Psychiatric Clinic in Bonn, Germany. The authors identified symptoms of impaired functioning that did not resemble schizophrenia. They labelled these ‘basic symptoms’ which differed from the negative symptoms of schizophrenia. The basic symptoms (including obsessional perseveration) were totally subjective and recognised by the patient as unreal and abnormal. The patient's insight into these features – which were prodromal to schizophrenia – was similar to the insight of persons with obsessive–compulsive disorder.
DSM-III, published in 1980, introduced a hierarchy for psychiatric disorders. For example, schizophrenia was considered to be of a higher order than neurotic disorders. If there were obsessive–compulsive features in the presence of psychosis, they were excluded from the diagnosis – and the possibility of co-occurring disorders was not considered (Poyurovsky Reference Poyurovsky, Faragian and Kleinman-Balush2013). This meant that between 1980 and 1994, when DSM-IV was published, diagnosis of comorbid obsessive–compulsive disorder and schizophrenia could not be made using the DSM classification. This probably resulted in underreporting of these comorbid diseases.
Prevalence
Various studies have sought to address the prevalence of OCS and/or OCD in people with psychosis, predominantly schizophrenia, with or without treatment. Beduin et al (Reference Beduin, Swets and Machielsen2012) used a cross-sectional study of people with schizophrenia to show that the prevalence of OCS in those taking antipsychotics was significantly higher than in those not on any antipsychotic (23.4 v. 4.9%). This was also statistically significant. In a sample of Japanese in-patients with schizophrenia, Owashi et al (Reference Owashi, Ota and Otsubo2010) observed comorbid OCD or OCS in 14.1 and 51.1% of the sample respectively. Berman et al (Reference Berman, Merson and Viegner1998) also identified obsessive–compulsive features co-occurring with psychosis in up to 50% of patients.
Fenton & McGlashan (Reference Fonseka, Richter and Müller1986) found that about 13% of patients with schizophrenia exhibited OCS. Rabe-Jablonska (Reference Poyurovsky2001) and Turkcan et al (Reference Tundo, Salvati and Cieri2007) reported prevalences of 13 and 16% in adolescents and in adult in-patients respectively. El Dawla et al (Reference El-Shiekh, Michail and Al-Said2015) found a prevalence of 13.3% in a sample that included both in-patients and out-patients.
Considering people with schizophrenia treated specifically with clozapine or haloperidol, Sa et al (Reference Rosen2009) found that the prevalence of OCD in those taking clozapine was 20%, compared with 10% in those taking haloperidol, although this difference was not statistically significant. However, those taking clozapine showed statistically greater symptom severity than those taking haloperidol. Mukhopadhaya et al (Reference Mukhopadhaya, Krishnaiah and Taye2009) studied people with schizophrenia or schizoaffective disorder who were being treated with clozapine. They found that 14 of 59 individuals (24%) scored positively for OCD. In 65 clozapine-treated patients with schizophrenia, Szmulewicz et al (Reference Swets, Dekker and van Emmerik-van Oortmerssen2015) studied found that the prevalence of OCS was 29.2% (n = 19) and the prevalence of OCD was 13.8% (n = 9).
Seedat et al (Reference Scotti-Muzzi and Saide2007) studied a larger sample of 400 individuals with schizophrenia or schizoaffective disorder. The prevalence of OCD was 10.7% and that of OCS was 2.5%. The combined prevalence for OCD/OCS was 13.2% (n = 53). In Krüger et al (Reference Krüger, Bräunig and Höffler2000), 76 people with schizophrenia were assessed for the presence of OCD. Of these, 12 (15.8%) were identified with the disorder.
In summary, from available evidence, the prevalence of OCS varies widely, ranging from 2.5 to 51%, depending on the population studied. This prevalence increases in more recent cross-sectional studies and in later disease stages. OCD was prevalent in 10–24% of people with severe psychosis.
The implication of these findings is that OCS and OCD are commonly associated with severe psychotic illnesses, specifically schizophrenia and schizoaffective disorders. The prevalence of OCS and OCD is higher in people with schizophrenia than in the general population (Laroche Reference Laroche and Gaillard2016). Longitudinal studies report de novo onset of OCS and OCD with the use of antipsychotics, especially second-generation antipsychotics. Treatment with clozapine is the most commonly implicated antipsychotic. Development of OCS/OCD prior to treatment with antipsychotics was more common in females (Grover Reference Grover, Sahoo and Surendran2015).
Aetiology
The existence and aetiology of OCS/OCD in people with psychosis remains of great interest to clinicians and researchers. Various factors are attributed to the causation of the comorbidity and this could lead to arguments regarding whether both disorders are part of a spectrum.
Evidence suggests a pattern of dysfunction in neurobiology among people with the comorbidity that might be responsible for the co-expression of symptoms (Bottas Reference Cai, Zhang and Yi2005). Neuroimaging findings have identified excessive activation of the frontal cortex and subcortical structures of the brain during symptom provocation in individuals with OCD as well as a reduction in activation with treatment using selective serotonin reuptake inhibitors (SSRIs) or behavioural therapy. This suggests the involvement of serotonin in its aetiology (Nakao Reference Nakao, Okada and Kanba2014).
A link with second-generation (atypical) antipsychotics used in the treatment of schizophrenia has been implicated in the unfolding and worsening of OCS in the comorbid group (Mukhopadhaya Reference Mukhopadhaya, Krishnaiah and Taye2009; Sa Reference Rosen2009; Schirmbeck Reference Schirmbeck and Zink2013). This has been attributed to the prominent antiserotonergic properties of the atypical antipsychotics (Schirmbeck Reference Sahoo, Grover and Nehru2011; Fonseka Reference Fulford, Pearson and Stuart2014). Patients treated with clozapine are deemed to be at high risk of deterioration in comparison with those receiving other antipsychotic medications (Fonseka Reference Fulford, Pearson and Stuart2014). However, it has to be highlighted that the relationship between second-generation antipsychotics and OCS appears to be bidirectional, with evidence pointing to both potential improvement and worsening of OCS in people taking these medications for schizophrenia (Laroche Reference Laroche and Gaillard2016; Zhou 2016).
The evidence on whether there is an association between the emergence of OCS in people with psychosis and the dose of antipsychotic medication is mixed and insufficient. Coskun & Zoroglu (Reference Cunill, Huerta-Ramos and Castells2009) posit a positive relationship between dose and the emergence of OCS, whereas Doyle et al (Reference Dykema2014) debunk any relationship. However, the use of antipsychotics for a minimum of 6 months appears to increase the risk of OCS (Beduin Reference Beduin, Swets and Machielsen2012), although most sample sizes were small. This probable heightened risk is supported by the findings of Grover et al (Reference Grover, Sahoo and Surendran2015) that OCS symptoms emerge within 12 months of commencing clozapine. Fonseka et al (Reference Fulford, Pearson and Stuart2014) argue that there is still a need to elucidate the association between duration of treatment, the impact of genetics and the causing or worsening of OCS by second-generation antipsychotics.
Although the duration of antipsychotic treatment has been in the spotlight as a potential cause of schizo-obsessive disorder, the chronicity of schizophrenia has also been implicated (Swets Reference Sung-Wan, Bo-Ok and Jae-Min2014). This claim, however, does not highlight the effect of treatment in the reported association. It could be argued that the duration of treatment will reflect the chronicity of the illness and therefore both should be considered jointly.
Summary
There appears to be a neurobiological basis for the comorbidity of psychosis and OCS/OCD which favours the significant involvement of serotonin, and this hypothesis is supported by neuroimaging. Second-generation antipsychotics are known to both improve and worsen symptoms. The most implicated antipsychotic is clozapine. Increased duration of illness and the duration of treatment are thought to independently increase the risk of comorbidity.
Pathophysiology
The cellular mechanisms, including neurobiology, genetics and neurocognitive aspects, involved in the pathology of OCS in people with schizophrenia are unclear (Scotti-Muzzi Reference Schirmbeck, Rausch and Englisch2017). Apart from antiserotonergic activity, which is recognised to be involved (El-Shiekh Reference Englisch, Esslinger and Inta2017), altered glutamate neurotransmission has also been implicated. The precise mechanism for the latter is also unclear. Elevated levels of glutamate have been found in the anterior cingulate cortex of people with first-episode psychosis treated with antipsychotic medications but still experiencing persistent psychotic symptoms (Demjaha Reference Devi, Rao and Badamatha2014) and of people with treatment-resistant schizophrenia (Mouchlianitis Reference Mouchlianitis, Bloomfield and Law2016); elevated levels have also been linked to the severity of negative symptoms (Egerton Reference El Dawla, Assad and El Habiby2012).
Genetics studies posit that SLC1A1, GRIN2B and their interactions increase susceptibility to the development of OCS/OCD in psychotic illness (Cai Reference Coskun and Zoroglu2013). A broader range of gene interactions between SLC1A1, BDNF, DLGAP3 and GRIN2B appear to predispose people with schizophrenia to develop OCS (Zink Reference Zink, Knopf and Kuwilsky2014). Again, the mechanism with which the latter develops is unclear.
In stand-alone OCD, the emergence of symptoms is linked with cortico-striato-thalamo-cortical circuitry dysfunction (Huey Reference Hwang and Buckley2008; Pauls Reference Pauls, Abramovitch and Rauch2014). This is thought to result from an imbalance in the feedback loop, which, in turn, leads to increased orbitofrontal–subcortical pathway activity (Pauls Reference Pauls, Abramovitch and Rauch2014). Given that the clinical presentations of both stand-alone OCD illness and OCS in schizophrenia are similar, the activities in the pathophysiological pathways highlighted above could be useful in further understanding OCS in schizophrenia.
Psychopathology
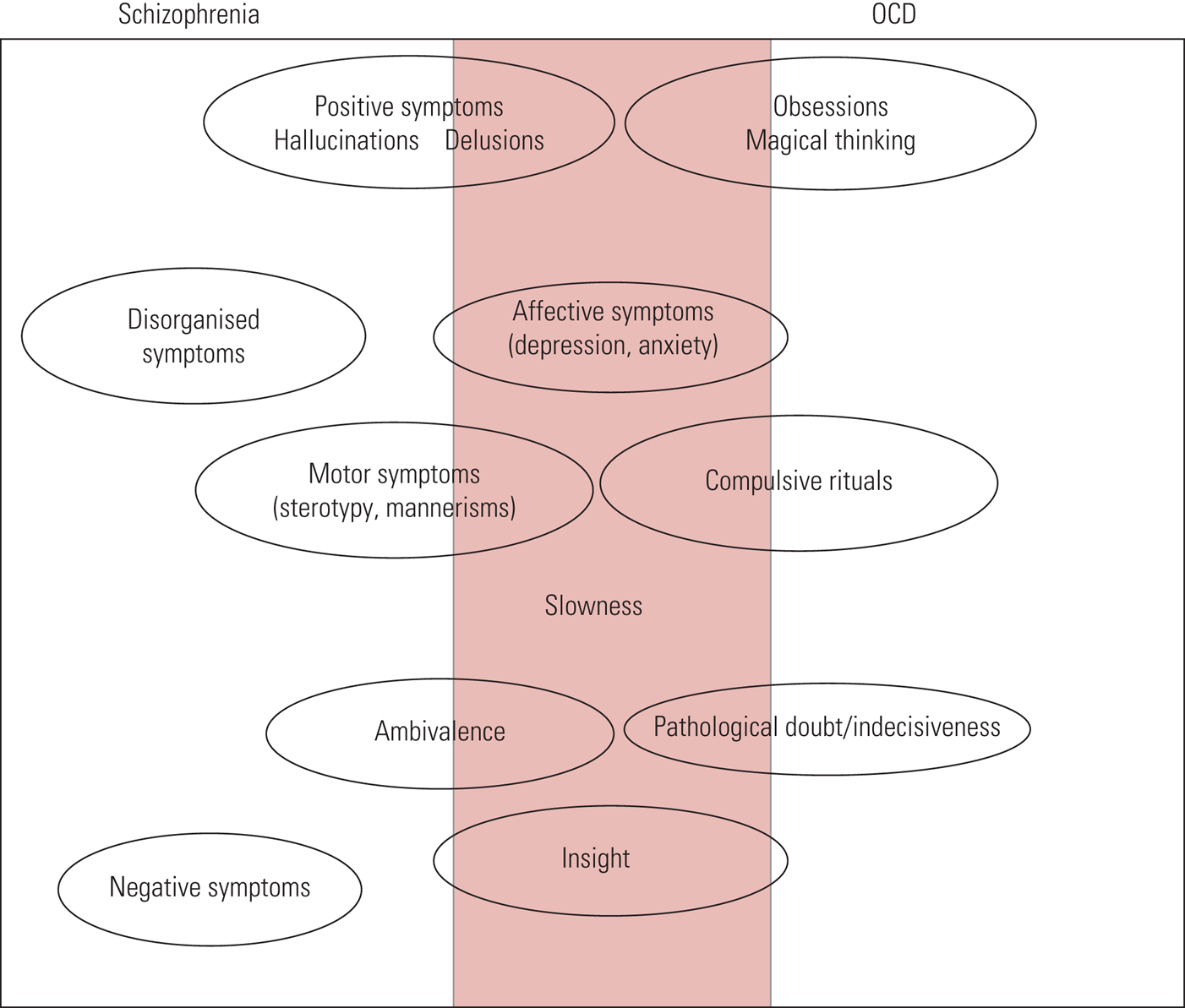
In terms of symptoms, as shown in Fig. 1, there is significant overlap between the features of schizophrenia and OCD. As a result, one might be misdiagnosed for the other.

FIG 1 Schizophrenia versus obsessive–compulsive disorder (OCD): psychopathological features (Poyurovsky Reference Poyurovsky, Faragian and Kleinman-Balush2013, reproduced with permission of Cambridge University Press).
Persons with comorbid schizophrenia and OCS tend to have an earlier onset of schizophrenia, more severe psychiatric symptoms, more negative symptoms, a greater genetic predisposition to psychosis and lower socioeconomic status than those without OCS (Owashi Reference Owashi, Ota and Otsubo2010). Schizophrenia with either OCS or OCD is associated with greater impairment in abstract thinking than non-comorbid schizophrenia (Cunill Reference Demjaha, Egerton and Murray2013). In a comparison of people with schizophrenia with and without comorbid OCS/OCD, it was found that those with OCS/OCD had fewer positive symptoms and greater comorbidity with personality disorders (Devi Reference Doyle, Chorcorain and Griffith2015). In another study, people with first-episode psychosis (FEP) and comorbid OCD tended to be younger, with more depressive symptoms and a greater likelihood of having suicidal plans or attempts than those with FEP and no OCD (Hagen Reference Hagen, Solem and Hansen2013). The obsessions most often reported in this comorbid presentation related to aggression, contamination and symmetry, or were somatic in nature. Checking, cleaning, arranging and counting were the most common compulsions (Rajkumar Reference Rabe-Jablonska2008).
Most persons with comorbid psychosis and OCD tend to have insight into the OCS. Those with impaired insight into OCS tend also to have poor insight into schizophrenia, and vice versa (Poyurovsky Reference Poyurovsky, Faragian and Kleinman-Balush2007). One study found that there were more neurocognitive deficits associated with comorbidity than with schizophrenia alone (Sahoo Reference Sa, Hounie and Sampaio2018). As shown in Fig. 1, the features that are most commonly seen in comorbid schizophrenia and OCD are affective symptoms, slowness and insight.
Putting it all together, the obsessions most commonly observed in the comorbid disorders were of contamination or thoughts that were aggressive or somatic in nature (El Dawla Reference El-Shiekh, Michail and Al-Said2015; Sahoo Reference Sa, Hounie and Sampaio2018). Other commonly described obsessions are pathological doubt (Doyle Reference Dykema2014; Grover Reference Grover, Sahoo and Surendran2015) and sexual (Grover Reference Grover, Sahoo and Surendran2015) and religious obsessions (Seedat Reference Scotti-Muzzi and Saide2007). The most frequent compulsions were washing, cleaning, checking and repeating (Krüger Reference Krüger, Bräunig and Höffler2000; Seedat Reference Scotti-Muzzi and Saide2007; Doyle Reference Doyle, Chorcorain and Griffith2014; El Dawla Reference El-Shiekh, Michail and Al-Said2015; Sahoo Reference Sa, Hounie and Sampaio2018). However, Kim et al (Reference Kim, Ryu and Nam2012) described similarity in phenomenology of forbidden thoughts, hoarding, cleaning, symmetry and counting, such that the quality of these experiences may not be used as distinguishing features.
Schizophrenia with comorbid OCD is more likely to be attended by motor symptoms (including akathisia and catatonia) than non-OCD schizophrenia (Krüger Reference Krüger, Bräunig and Höffler2000). This would suggest that there is basal ganglia–frontal lobe involvement linking the two disorders and a possible link between these disorders and movement disorders (Krüger Reference Krüger, Bräunig and Höffler2000).
Investigations
Most psychiatric assessments are done as unstructured interviews. However, there are standardised interviews that have greater reliability. The Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS) are internationally recognised instruments to measure the presence and severity of psychotic features (Fulford Reference Galletly, Castle and Dark2014), and the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) is considered the gold standard for rating the severity of obsessions and compulsions (Bejerot Reference Bejerot, Edman and Anckarsäter2014).
Neurocognitive differences found in people with schizophrenia and comorbid OCD include deficits in processing speed, verbal fluency and cognitive flexibility (Sahoo Reference Sa, Hounie and Sampaio2018). Other difficulties described include deficits in visuospatial skills, delayed non-verbal memory and cognitive shifting (Berman Reference Berman, Merson and Viegner1998).
Management
Management is usually aimed at reducing the distress associated with OCS/OCD and, where possible, reducing the severity of these symptoms while eliminating psychotic symptoms. Where the latter is not possible, the goal would be to keep psychotic symptoms low or tolerable. It is not always feasible to aim for elimination of OCS/OCD. Often, a fine balance has to be struck with the reduction of psychotic symptoms and a corresponding increase in OCS, especially where antipsychotic use is thought to be an inducing factor for OCS.
Current treatment guidelines
Treatment recommendations from current guidelines are limited in this area. Table 1 summarises recommendations pertaining to this comorbidity in schizophrenia: what may be observed is that it is often not addressed. The American Psychiatric Association's practice guidelines (Working Group on Schizophrenia Reference Villari, Frieri and Fagiolini2004) acknowledge that its advice is not up to date and the guideline as a whole needs to be revised and updated. There is at present no Cochrane review in this area.
TABLE 1 Position of treatment guidelines on obsessive–compulsive disorder or obsessive–compulsive symptoms comorbid with psychosis

Management strategies derived from appraisal of the evidence are summarised in Table 2 and further discussed below.
TABLE 2 Evidence-based psychopharmacological treatments

a Evidence levels are from Shekelle's method of classification, with level Ib being the strongest evidence currently available in this subject area (Shekelle Reference Seedat, Roos and Pretorius1999).
Addition of fluvoxamine
Fluvoxamine is an SSRI with a UK licence for depression and OCD (Joint Formulary Committee Reference Bottas, Cooke and Richter2019). There is limited evidence to support its efficacy and safety in the treatment of comorbid schizophrenia and OCD. One of the few studies was by Reznik & Sirota (Reference Remington, Honer and Raedler2000), who conducted a randomised controlled trial (RCT) that compared the use of an antipsychotic (perphenazine or haloperidol) plus fluvoxamine with the use of an antipsychotic alone. Participants were 30 adults with schizophrenia who had been stabilised on their antipsychotic regimen. The study showed that the addition of fluvoxamine resulted in a significant reduction of compulsions but not obsessions. There was also a significant reduction in pathological slowness and doubt. There was a general improvement in the severity of psychotic illness and insight, although these were not at statistically significant levels. No one dropped out of the study. However, the study sample was small and the process of randomisation is not clearly explained. The estimates of treatment effect would have benefited from the inclusion of confidence limits. It is important to note that, in this potentially useful study, fluvoxamine was administered for 8 weeks, up to a maximum dose of 200 mg daily. The British National Formulary recommended maximum dose for OCD is 300 mg daily (Joint Formulary Committee Reference Bottas, Cooke and Richter2019).
Poyurovsky et al (Reference Poyurovsky, Isakov and Hromnikov1999) presented an open study in which they also studied the efficacy and safety of the addition of fluvoxamine to an existing variety of antipsychotic medications in participants with stabilised psychosis. These antipsychotics included haloperidol, levomepromazine, perphenazine, clozapine, haloperidol decanoate and fluphenazine decanoate. In this study, fluvoxamine was used for a longer period (12 weeks) but up to the same maximum dose of 200 mg daily. Limited information is given about the demographic characteristics of participants. The study showed significant improvement in obsessions but not compulsions and significant improvement in both positive and negative symptoms of psychosis. However, there were only ten participants, three of whom dropped out before the end of the study. The reasons for drop-out were aggressive behaviour and exacerbation of psychosis. These reasons for drop-out are important in the clinical use of fluvoxamine. They underlie the importance of close monitoring of patients when commenced on new psychotropics, especially the SSRIs.
Pharmacokinetically, fluvoxamine inhibits many cytochrome P450 (CYP) enzymes to varying extents. It inhibits CYP1A2 (affecting clozapine, haloperidol and olanzapine), CYP3A4 (affecting aripiprazole, clozapine, haloperidol, quetiapine and ziprasidone), CYP2D6 (aripiprazole, chlorpromazine, clozapine, haloperidol, olanzapine), CYP2C9, CYP2C19 and CYP2B6 (Stahl Reference Shekelle, Woolf and Eccles2013). Hence, it can affect the plasma levels of antipsychotics and the dosage may need to be adjusted when these are combined.
In summary, fluvoxamine is evidenced to be effective in reducing OCS in comorbid schizophrenia and OCD, but it appears that caution is needed in its use, owing to the potential for negative consequences of its addition to established antipsychotics and the potential for interaction with other psychotropics.
Addition or switch to aripiprazole
Several studies have supported the addition of the antipsychotic aripiprazole to existing treatments. These include an open-label study and a case series. Glick et al (Reference Gordon2008) presented an open label study of 15 people with schizophrenia who had comorbid OCS in which monotherapy with aripiprazole resulted in moderate but clinically significant symptom improvement.
Eryılmaz et al (Reference Fenton and McGlashan2013) presented a series of four cases where significant clinical improvement in OCS was achieved with the addition of aripiprazole to clozapine. The combination of aripiprazole and clozapine was considered well tolerated. Villari et al (Reference van Os and Kapur2011) also presented a case series in which two people with clozapine-associated OCS improved with aripiprazole augmentation. In each case, aripiprazole was taken at doses of up to 30 mg/day. In Englisch et al (Reference Eryılmaz, Sayar and Ozten2009), a case series of seven patients demonstrated improvement in what they describe as clozapine-induced obsessive–compulsive syndrome following augmentation with aripiprazole. The mean dose prescribed was 22.9 mg/day for an average of 9.7 weeks. Improvement was significant on the mean total Y-BOCS. Psychosis improved but to a less significant level than OCS.
It has not been useful to differentiate between the effects of the addition of aripiprazole or switching to aripiprazole because the number of studies was relatively low.
Aripiprazole is a second-generation antipsychotic medication that has partial dopamine D2 agonist activity, partial serotonin 5-HT1A agonist and 5-HT2A antagonist activity (Stahl Reference Shekelle, Woolf and Eccles2013). Considering the mechanisms of action of aripiprazole, it appears that the reduced blockade of D2 receptors and/or serotonin blockade bring about improvements. Study data suggest that aripiprazole has to be administered for a longer period than usually used for relief of psychotic symptoms and at the higher end of the dose range.
Switch to amisulpride
The use of amisulpride has not been widely studied. In an open-label study Kim et al (Reference Kim, Shin and Kim2008) demonstrated an improvement in OCS in people with schizophrenia taking atypical antipsychotics, following a change in antipsychotic to amisulpride. This is a second-generation antipsychotic that works via highly selective antagonism of D2/D3 receptors with minimal inhibition of 5-HT2A (Stahl Reference Shekelle, Woolf and Eccles2013).
Addition of cognitive–behavioural therapy
Cognitive–behavioural therapy (CBT) is licensed for the treatment of OCD (National Institute for Health and Care Excellence 2009), and there is also evidence that it may be helpful for OCD comorbid with psychosis.
Schirmbeck & Zink (Reference Schirmbeck, Esslinger and Rausch2013) present a narrative review of case reports and a case series in which CBT was used in the treatment of comorbid OCS. Out of 30 individuals with schizophrenia and OCS who received CBT including exposure and response prevention (ERP) or just ERP alone, 24 showed symptom improvement. An increase in psychotic symptoms was reported in two case reports. In one of these, psychotic symptoms worsened with the introduction of CBT, but became controlled with increased medication. In the case series (Tundo Reference Tonna, Ottoni and Affaticati2012), CBT was discontinued in one case owing to exacerbation of psychosis and subsequent hospital admission after more than 6 months of psychotherapy.
Tundo et al presented open naturalistic studies (Reference Tonna, Ottoni and Affaticati2012, Reference Tundo, Salvati and Di Spigno2015) that utilised the same data-set. The second was a reanalysis of data presented in the first, to clearly separate individuals who had OCS before psychosis from those who developed OCS during or after the onset of psychosis. Both groups were compared. All patients had been stabilised on psychotropics. The latter study included 21 participants. CBT sessions were scheduled ‘flexibly and jointly’ by both the therapist and the patient (Tundo Reference Tundo, Salvati and Di Spigno2015). Patients had an average of four sessions each month for the first 4 months and thereafter continued therapy with one to four sessions per month. CBT duration was not determined in advance. Therapy was delivered by CBT therapists with 5 or more years’ experience of treating OCD. CBT consisted of imaginary and in vivo exposure, ritual prevention and/or delay, cognitive therapy and other ad hoc interventions used to supplement exposure and ritual prevention strategies. These ad hoc interventions were not outlined.
Results showed a non-statistically significant improvement over time (at 6 months and 12 months) on the Y-BOCS and the Clinical Global Impression scale's Severity of illness component (CGI-S). At 12 months, drop-out rates were lower and improvement was found to be higher in individuals with OCD who developed this after the commencement of antipsychotic treatment, although not significantly (Tundo Reference Tundo, Salvati and Di Spigno2015).
A case study of an individual with schizophrenia and OCD who received CBT for 3 weeks reported that he ceased to fulfil the diagnostic criteria for OCD at the end of treatment (Hagen Reference Hanisch, Friedemann and Piro2014). His recovery was maintained at 3- and 6-month follow-up. With this method of study, it is impossible to generalise this finding to similar cases. Details of how CBT was applied were not given.
Available evidence for the use of CBT suggests that it may be helpful when added to existing medications and may not be suitable for every patient. However, more studies are needed to understand its usefulness.
Addition of escitalopram
Moving to lower-level evidence, Stryjer et al (Reference Stahl2013) used an open-label prospective study to examine the efficacy of escitalopram in the treatment of 15 individuals with OCD and schizophrenia. A significant improvement was observed in the total Y-BOCS scores and in the scores of both the Y-BOCS Obsession and the Y-BOCS Compulsion subscales at the end point. In addition, a significant improvement was observed in the total scores on the PANSS and particularly in the scores on the anxiety, tension, depression and preoccupation items. Escitalopram was prescribed at 20 mg daily and was well tolerated by the cohort.
Escitalopram is an SSRI, licensed in the UK and USA for the treatment of OCD, with evidence of use at doses exceeding 20 mg/daily for resistant cases (Joint Formulary Committee Reference Bottas, Cooke and Richter2019). However, there is no evidence in the current literature for the use of higher than recommended doses of escitalopram in OCS/OCD comorbid with psychosis.
Escitalopram is the S-enantiomer of racemic citalopram. Being highly selective for serotonin receptors, it has minimal effects on noradrenaline and dopamine reuptake. It has no or very low affinity for serotonergic or other receptors, including alpha- and beta-adrenergic, dopamine, histamine, muscarinic and benzodiazepine receptors (Glass Reference Glick, Poyurovsky and Ivanova2009).
Reduction in dosage of clozapine
Reduction in the dosage of antipsychotics seems to be the strategy more often employed by clinicians. While intuitive, it is not firmly supported by current evidence. Grover et al (Reference Grover, Sahoo and Surendran2015) used a retrospective cohort study design to demonstrate that clozapine can lead to aggravation or de novo presentation of OCS/OCD, but this can be managed with reduction in dose. This was a multispecialty, tertiary-centre study in which one-fifth (n = 42; 19.1%) of participants had OCS/OCD.
Clinical experience suggests that reduction of antipsychotic dosage may be associated with an exacerbation of psychotic symptoms and associated suffering, making this strategy less ethical to be explored in treatment studies. However, a balance of risks and benefits needs to be evaluated in each case and, where obsessive symptoms are very severe, a reduction in antipsychotic dosage could be an acceptable option. Dykema (Reference Egerton, Brugger and Raffin2018) described observable improvement and subsequent cessation of obsessive–compulsive symptoms in a man following the discontinuation of clozapine, initially prescribed to treat delusional beliefs. In this case, clozapine was discontinued over a 4-week period while the patient was under the care of an assertive community treatment team. Dykema emphasised that this decision was made following the weighing of risks versus benefits of discontinuing clozapine. The patient did not experience a return of psychotic symptoms.
Addition of sertraline
At evidence level IV (the weakest evidence), treatment with sertraline, an SSRI, is supported by a case report by Coskun & Zoroglu (Reference Cunill, Huerta-Ramos and Castells2009). This involved an adolescent boy with a diagnosis of schizophrenia who developed severe religious obsessions in a dose-dependent fashion during clozapine treatment. Addition of sertraline at 100 mg daily brought about improvement. Sertraline is recommended for clinical treatment of OCD (National Institute for Health and Care Excellence 2009), but this was the only evidence supporting this strategy for OCS in psychosis.
Addition of paroxetine
Zink et al's (Reference Zhou, Baytunca and Yu2007) case report describes various interventions trialled in a 30-year-old man with clozapine-induced obsessive–compulsive symptoms. Clomipramine was added, then clozapine dose reduced, then clomipramine changed to paroxetine. Remission was noted at a paroxetine dose of 60 mg/day. The limitation of this study method is that it is difficult to attribute the improvements wholly to the addition of paroxetine. Paroxetine is another SSRI licensed for the treatment of OCD (National Institute for Health and Care Excellence 2009). It would appear that, as in non-comorbid OCD, higher doses are needed for the treatment of comorbid OCD.
Addition of lamotrigine
Augmentation of clozapine treatment with lamotrigine, a glutamate modulator, appeared to reduce OCD symptoms in a 19-year-old male with both psychotic and obsessive–compulsive symptoms (Rodriguez Reference Reznik and Sirota2010). In this particular patient, the addition of fluvoxamine and clomipramine had been ineffective. Lamotrigine stabilises presynaptic neuronal membranes by blocking voltage-dependent sodium and calcium channels, and reduces the release of excitatory amino acids such as glutamate and aspartate (Stahl Reference Shekelle, Woolf and Eccles2013). It is unclear whether the reduction in OCS/OCD is from the known mechanisms of action of lamotrigine.
Addition of electroconvulsive therapy
From available evidence, electroconvulsive therapy (ECT) may be used as both an initial treatment and for maintenance treatment in OCS/OCD comorbid with psychosis. However, the studies are few and are not necessarily distinguished by the presence of severe or debilitating psychosis.
Lavin & Halligan (Reference Lavin and Halligan1996) reported a case of a 37-year-old woman with schizophrenia who experienced a marked reduction in obsessive–compulsive symptoms with ECT. Her medication regimen included sertraline (200 mg/day), haloperidol (10 mg at bedtime) and levothyroxine (45 μg/day). After her medications were tapered, she was started on a trial of bilateral ECT. Marked improvement was shown during a course of 12 treatments.
In a case report by Hanisch et al (Reference Hemrom, Pushpa and Prasad2009), a 48-year-old woman was treated for schizoaffective disorder and OCS (mainly repeating rituals, avoidance behaviour, collecting and hoarding). She did not respond to a combined treatment of antipsychotics and high-dose SSRIs. She improved during a course of ECT and was maintained on out-patient ECT fortnightly together with pharmacological agents (sertindole and mirtazapine) for 42 weeks.
A case report from India by Rao et al (Reference Rajkumar, Reddy and Kandavel2011) is thought to be the first report of the successful use of maintenance ECT in the treatment of clozapine-associated OCS in schizophrenia. They present a 40-year-old woman with treatment-resistant schizophrenia who developed OCS following the switch to clozapine. The symptoms did not abate with the addition of up to 80 mg daily of fluoxetine. The basis for the use of ECT is stated as associated suicidality and the lack of response to interventions. She received a total of 24 ECT sessions over an 8-week period. While the ECT was ongoing she had only occasional controllable and non-distressing obsessions and no suicidality. On stopping ECT, there was a relapse of OCS and suicidality, which disappeared when maintenance ECT was reintroduced for 3 months. This is an example where known strategies such as reduction and discontinuation of clozapine and addition of an SSRI did not help.
In summary, from the cases discussed, clinical care involving ECT was not implemented until pharmacological interventions had been trialled and shown to be ineffective. This practice is in line with the current use of ECT in clinical situations, although it is not a recommended treatment for refractory OCD (National Institute for Health and Care Excellence 2009).
Other interventions
Clomipramine
People with OCD may benefit from the use of clomipramine (National Institute for Health and Care Excellence 2009). However, this may not be ideal in combination with clozapine for comorbid OCD and psychosis, as together they increase seizure risk. Additionally, the efficacy of clomipramine has been questioned in some case studies (Zink Reference Zhou, Baytunca and Yu2007; Rodriguez Reference Reznik and Sirota2010). However, no high-level, robust evidence exists to discredit the role of clomipramine in the treatment of comorbidity.
Addition of repetitive transcranial magnetic stimulation
There is higher-level though limited evidence examining the use of repetitive transcranial magnetic stimulation (rTMS) in individuals with psychosis and comorbid OCS/OCD. Mendes-Filho et al (Reference Mendes-Filho, de Jesus and Belmonte-de-Abreu2016) studied the effect of rTMS in adults with schizophrenia or schizoaffective disorder in a double-blind RCT. Although their study was well designed, it had only 12 participants (6 in each arm). It did not show any significant reduction in OCS. The characteristics of the participants were similar to those commonly encountered in rehabilitation settings, with long duration of psychosis and all taking clozapine medication. The authors felt that an effect may have been found if the sample size had been larger.
Earlier, Mendes-Filho et al (Reference Mendes-Filho, Belmonte-de-Abreu and Pedrini2013) had described cases in which OCS in three adults with clozapine-treated schizophrenia or schizoaffective disorder were shown to improve after receiving rTMS. However, they relapsed within 4 weeks of completion of treatment. More studies are needed to position this treatment method in the hierarchy of treatments that may reduce OCS/OCD in people with schizophrenia or schizoaffective disorder.
Spontaneous resolution of symptoms
Grover et al (Reference Grover, Sahoo and Surendran2015) describe the spontaneous improvement of OCS in a subgroup of patients in their retrospective cohort study. However, there is no biomarker with which this subgroup may be identified. Waiting for spontaneous resolution to happen is not a reported strategy in studies and may not be an ethical and moral strategy in clinical settings.
Summary
The main evidence-based interventions for managing comorbid OCS/OCD in psychosis are:
• addition of or switching to aripiprazole or amisulpride
• addition of SSRIs, notably fluvoxamine, escitalopram, sertraline or paroxetine
• reduction of dosage of antipsychotic medication (clozapine)
• addition of lamotrigine
• addition of ECT
• addition of CBT.
Discussion
The population
Although significant advances have been made in various aspects of psychiatric care, the area of comorbid psychosis and OCS/OCD requires further research. A review of the literature on this comorbidity was carried out by Grover et al (Reference Hagen, Hansen and Joa2018). Their results were broadly similar to those stated here. These studies raise questions that are not easily answered from current knowledge. An obvious question is whether the population experiencing this comorbidity is homogeneous and whether a single aetiological pathway can be attributed to this illness. From the clinical experience and limited evidence presented above, it appears that some people have OCS/OCD before the onset of psychotic illness, whereas others develop OCS/OCD during the course of psychosis. This could be during the early, intermediate or later period of their illness. Within this subgroup, the development of OCS/OCD during psychosis may be associated with or even caused by psychotropic medications.
Treatment
This article shows that the available treatments are imprecise. As may be gathered from Table 2, the level and strength of evidence are mostly low, and therefore more research is needed to direct or develop treatments in this area. This should delineate who responds to what, when and why.
There are as yet no biomarkers from which one may be able to classify these patients. In practice, one often has to rely on recall of the history to apply what hopefully may be an appropriate management strategy.
What may be garnered from available evidence does support the use of SSRIs – fluvoxamine, escitalopram, sertraline and paroxetine – as would be expected from the treatment of stand-alone OCD. There is no indication in the literature as to why these SSRIs in particular are beneficial compared with other SSRIs, such as clomipramine, that are thought to be less effective for some of these patients. This does bolster the hypothesis that comorbid OCD and psychosis is an illness with heterogeneous mechanisms having similar phenotypic expressions.
The special case of clozapine
Clozapine deserves a more detailed examination because it has demonstrated higher efficacy compared with other antipsychotics and is the recommended treatment for treatment-resistant schizophrenia (Barnes Reference Barnes2011). It is a dopamine D1, dopamine D2, 5-HT2A, alpha1-adrenoceptor and muscarinic-receptor antagonist. Its propensity to cause or be associated with OCS/OCD may be related to its antagonist activity at 5-HT receptors. OCD, but not OCS, is listed in the British National Formulary as a ‘rare or very rare’ side-effect of clozapine (Joint Formulary Committee Reference Bottas, Cooke and Richter2019). However, lower-level evidence exists of the worsening of OCS with clozapine treatment (Leung Reference Leung and Palmer2016). It is our thinking that this association of clozapine with onset or worsening of OCS is underresearched and underrecognised in clinical practice. This would be the clinical circumstance in which the more appropriate action would be to reduce the dosage of clozapine. Addition of evidenced SSRIs (escitalopram, sertraline, paroxetine) might lead to increased serum levels of clozapine and associated side-effects due to cytochrome P450 (CYP) 2D6 enzyme inhibition. Adjustment of the dosage of clozapine is normally advised on addition of an SSRI. However, the addition of fluvoxamine to clozapine, in particular, is to be avoided owing to its propensity to markedly inhibit the metabolism of clozapine, through CYP1A2 inhibition, with a potential risk for clozapine toxicity.
Conclusions
Obsessive–compulsive symptoms and/or obsessive–compulsive disorder are common in people with schizophrenia. Where present, they are associated with poorer quality of life, lower level of functioning, more severe illness and suicidality. The risk of developing OCS or OCD increases with the use of antipsychotics, especially clozapine, which has a relatively high antiserotonergic activity. The occurrence of OCS or OCD has been observed with long-term use of clozapine.
Evidenced-based interventions, summarised in Table 2, offer a more tailored approach to management. At present, pharmacological interventions predominate. However, evidence is limited, and more research is needed to improve patient care in this area.
Acknowledgement
We thank the Mental Health Library at Parkwood Institute, St Joseph's Healthcare, London, Ontario, for the literature search that made this article possible. We also thank the Oxford Assertive Community Treatment Team, Woodstock, Ontario, for enthusing the lead author with this subject matter and creating the time and space that made this article possible.
Author contributions
All authors made substantial contributions to the conception or design of the work, including the acquisition, analysis and interpretation of data; all authors were involved in drafting the work and revising it; all authors gave final approval of the version to be published; and all authors agree to be accountable for all aspects of this work.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2020.57.
MCQs
Select the single best option for each question stem
1 According to El Dawla et al (Reference El-Shiekh, Michail and Al-Said2015), the prevalence of OCD in people with schizophrenia is:
a 1–5%
b 5–10%
c 10–20%
d 20–30%
e none of the above.
2 Which of the following can be associated with an increased risk of comorbid OCD in schizophrenia?
a clozapine
b short duration of treatment
c short duration of illness
d abnormalities in the occipital cortex
e none of the above.
3 Which of the following features is most likely to be present in comorbid schizophrenia and OCD?
a negative symptoms
b disorganised behaviour
c hallucinations
d presence of insight
e suicidality.
4 Persons with schizophrenia and comorbid OCS when compared with those with non-comorbid schizophrenia tend to have:
a an earlier onset of schizophrenia
b more severe psychiatric symptoms
c more negative symptoms
d lower socioeconomic status
e all of the above.
5 Evidence-based interventions to reduce OCS or OCD in people with severe psychosis include:
a reduction in dosage of antipsychotic
b addition of sertraline or escitalopram
c switching antipsychotic to amisulpride
d addition of aripiprazole
e all of the above.
MCQ answers
1 c 2 a 3 d 4 e 5 e








eLetters
No eLetters have been published for this article.