Introduction
Eleonora’s Falcon Falco eleonorae is a long-distance migratory raptor that overwinters in Madagascar and breeds on steep sea cliffs and rocky islets across the Mediterranean basin, with a few scattered colonies along the Atlantic coast of Morocco and the Canary Islands (Walter Reference Walter1979, Cramp and Simmons Reference Cramp and Simmons1980, Del Hoyo et al. Reference Del Hoyo, Elliot and Sargatal1992). The chicks hatch in late summer, coinciding with the influx of Palearctic migratory birds crossing the Mediterranean Sea and heading south to their African winter quarters (Moreau Reference Moreau1972, Berthold Reference Berthold2001, Hahn et al. Reference Hahn, Bauer and Liechti2009).
The entire breeding population is estimated at 14,300–14,500 pairs (BirdLife International 2015) with up to 80% breeding in Greece (Dimalexis et al. Reference Dimalexis, Xirouchakis, Portolou, Latsoudis, Karris, Fric, Georgiakis, Barboutis, Bourdakis, Ivovic, Kominos and Kakalis2008). Eleonora’s Falcon is not globally threatened, but evaluated as Least Concern within Europe (BirdLife International 2015) and listed as a protected species in Algeria (Samraoui and Samraoui Reference Samraoui and Samraoui2008). Although the global population trend appears to be positive (BirdLife International 2015), at smaller scales, studies reveal contrasting patterns and substantial population fluctuations, suggesting the unequal influence of local factors acting across the species’ range. Beyond the natural causes influencing mortality rates and breeding success, such as food availability and intra-specific predation (Gangoso et al. Reference Gangoso, Afán, Grande and Figuerola2015a), the species has historically suffered from persecution and plundering of nests in many islands (Clark Reference Clark1974). In addition, human disturbance, often associated with tourism development, has also been shown to negatively influence breeding success and the degree of colony occupancy (Martínez-Abraín et al. Reference Martínez-Abrain, Oro, Ferris and Belenguer2002, Orta and Kirwan Reference Orta, Kirwan, del Hoyo, Elliott, Sargatal, Christie and de Juana2014). Predation of eggs and small chicks by introduced mammals is also an important threat at breeding grounds (Walter Reference Walter1979, Bonnin Reference Bonnin2004, Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2006). For instance, rats Rattus sp. have been shown to cause 20–29% of eggs loss on some Greek islands (Ristow and Wink Reference Ristow, Wink, Newton and Chancellor1985). Therefore, precise information at a local scale is necessary to evaluate current status and identify potential threats that may affect the long-term persistence of isolated populations.
The status of some Algerian colonies has only been evaluated intermittently (Laferrère Reference Laferrère1960, Mayaud Reference Mayaud1960, Ledant et al. Reference Ledant, Jacobs, Jacobs, Malher, Ochando and Roché1981, Isenmann and Moali Reference Isenmann and Moali2000, Samraoui et al. Reference Samraoui, Alfarhan, Al-Rasheid and Samraoui2011) and the reproductive success of these colonies remains poorly known (Télailia et al. Reference Télailia, Saheb, Boutabia, Bensouilah and Houhamdi2013). Nonetheless, available information suggests that the Algerian population as a whole may be declining in recent times, but precise long-term data are lacking. Hence, identifying key environmental determinants affecting the dynamics of existing colonies is essential. Here, we determined variation in population size over time and investigated the influence of habitat characteristics on breeding performance of this singular species.
Methods
Our study was conducted between July and October in 2010 and 2012 on Kef Amor (37°5.070’N, 7°19.870’E, 1.6 ha, 30 m asl), a small rocky island off the coast of Chetaïbi, west of the town of Annaba. In addition, irregular surveys were carried out at Srigina (36°56.254’N, 6°53.184’E, 1.0 ha, 20 m asl), a rocky islet located 5 km offshore of the town of Skikda, between 2006 and 2012 (Figure 1). These islands have a continental origin and the climate is typically Mediterranean, characterised by a dry and hot period extending from May to October. Average monthly temperatures in the summer fluctuate between 18°C (June and September) and 31°C (August) with an average daily high temperature always exceeding 28°C. During the same season, wind predominantly blows from north and north-east and the infrequent precipitation occurs most often as thunderstorms. Vegetation of Srigina is relatively poor and dominated by Mediterranean dwarf palm Chamaerops humilis, the naturalized succulent Carpobrotus edulis, Barbary Fig Opuntia ficus-indica, Anthemis maritima, and Malva arborea. The flora of Kef Amor is even more depauperate and consists mainly of Malva arborea. In contrast to Srigina, where a lighthouse is permanently inhabited and where rodents and, as recently as 2006 and 2009, a dozen cats were introduced (Baaloudj et al. Reference Baaloudj, Samraoui, Alfarhan and Samraoui2014), Kef Amor is devoid of mammals. Both islands are regularly and increasingly visited by fishermen and tourists during the summer months (authors’ pers. obs.).
Figure 1. Map of study site showing the location of the surveyed Eleonora’s Falcon colonies.
Weekly surveys in Kef Amor were undertaken throughout the breeding period, whereas surveys in Srigina were carried out sporadically. To reduce disturbance, visits started at dawn and the time spent at each nest was minimised. Nests were accessed on three occasions; first to quantify clutch size and record their geographic position using a hand-held GPS (Garmin); second, to verify hatching success; and third, to quantify the final number of fledglings. Additional data on nestling colour polymorphism was also collected at Kef Amor in 2011. In nestlings, the colour morph was determined from the colour pattern of their undertail coverts. The reliability of this method was confirmed by molecular analyses (Gangoso et al. Reference Gangoso, Grande, Ducrest, Figuerola, Bortolotti, Andrés and Roulin2011). Nests were classified in four categories based on nest site characteristics and degree of protection, from less to more protected: open eyrie, ledge (nest leaning against a large rock), rock (nests between two or three rocks) and crevice (hole or fissure). At the end of the breeding season, sun exposure was visually estimated as the percentage of nesting ground exposed to the sun at 12h00 (local time).
Six breeding parameters were measured: clutch size, hatching success, breeding outcome (a binary variable accounting for nest failure or success, the latter defined as nests having fledged at least one chick), fledging success (percentage of hatched chicks that fledged), nesting success (percentage of successful nests), and productivity (number of young fledged per successful pair). Egg length (mm) and width (mm) were measured to the nearest 0.1 mm with Vernier callipers. Egg volume (Vol) was calculated as follows: Vol (cm3) = 0.000509*length*width2 (Hoyt Reference Hoyt1979). The date of the first hatched chick was considered as the hatching date of a nest. When hatching was not witnessed, age of chicks was visually estimated based on experience.
Statistical analyses
The population trend during the two study years was assessed by calculating the annual growth rate, using the formula ((f/s)^(1/t)-1)*100, where f and s represent the final and initial values, respectively, and t indicates the time elapsed between the two.
In order to assess the influence of environmental factors (sun exposure, and type of nests), hatching date, year and potential trade-offs (egg-volume) on clutch size and productivity, we used a generalized linear model (GLM) with a logit link function and Poisson error distribution. Possible yearly variation of the influence of hatching date was assessed by including the interaction between hatching date and year. To identify the effect of environmental determinants (same as above) on breeding outcome, we used logistic regression. All statistical analyses were carried out using R (R Development Core Team 2015). All means reported below are given ± SD.
Results
In 2010 and 2012, 117 and 80 nests were recorded on Kef Amor, while the number recorded on Srigina dwindled from 18 (2006) to seven (2009) and three (2010). Not all nests could be accessed at Kef Amor and so these numbers may represent an underestimate of the total number of breeding pairs, which is estimated at 130 and 100 in 2010 and 2012, respectively. This represents a negative population trend characterised by an annual decrease of 12.29%.
Nests were predominantly located on the southern slope, where vegetation is concentrated. They were also located well above the shoreline, generally on the central part of the islet at the highest elevation (25–40 m). With regard to the microhabitat characteristics associated with nest-site location and exposure, most nests were located in crevices (34.3%), amidst rocks (32.1%), or in ledges with a tall rock (26.4%) while a smaller proportion were located on bare ground (7.1%). Sun exposure and nest type were significantly correlated (Kruskal-Wallis rank sum test: χ2= 53.2, df = 3, P < 0.0001; Figure 2a). Pairwise comparisons using Mann-Whitney tests with Bonferroni p-values adjustments indicated that crevices were significantly less exposed than any other nest type (P < 0.001 in all three comparisons) while “rock” differed from “open” nests (P = 0.02) and was marginally different from “ledge” (P = 0.05).
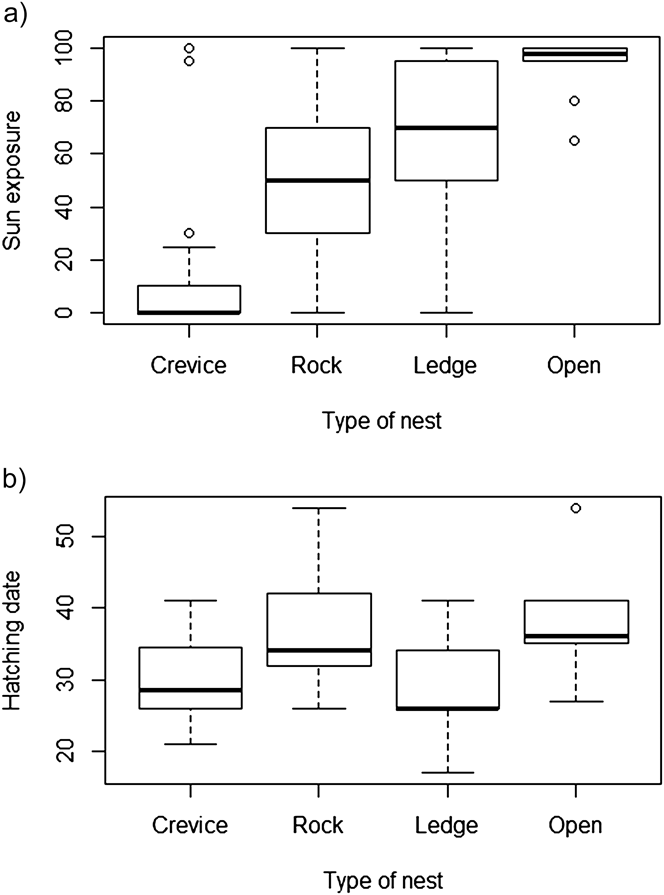
Figure 2. Relationship between: (a) nest type and exposure to the sun (2010 and 2012); (b) nest type and hatching date (1 = 1 August) (2010 and 2012).
In 2010 and 2012 at Kef Amor, egg-laying started on 22 July and 20 July, respectively, with mean hatching dates occurring on 28.1 ± 4.3 and 32.5 ± 8.1 (1 = 1 August) and differing significantly (Wilcoxon rank sum test: P = 0.01). Hatching date varied according to nest type (Kruskall-Wallis rank sum test: χ2= 11.0, df = 3, P = 0.012). Pairwise comparisons using Mann-Whitney tests with Bonferroni p-values adjustments indicated that hatching in “crevices” occurred significantly earlier than in “open” (P = 0.03) or “rock” (P = 0.02) nests (Figure 2b). Egg volume was not associated with hatching date (t value = 0.23, P = 0.82) nor was there a significant change between years (t value = -1.15, P = 0.25).
The clutch size ranged from one to three eggs. The mean clutch size was significantly different between years (2010: 2.6 ± 0.5, n = 90 and 2012: 2.9 ± 0.4, n = 34; Mann-Whitney U test: W = 1066.5, P < 0.002). Results of GLMs, however, indicated that there was no relationship with hatching date (z value = -0.22, P = 0.83), egg volume (z value = 0.05, P = 0.96), sun exposure (z value = -0.23, P = 0.82) or year (z value = 0.81, P = 0.42).
Hatching success was 56% and 41% in 2010 and 2012, respectively. Fledging success was 60% in both study years, whereas nesting success was significantly higher in 2010 (33%) than in 2012 (21%) (Wilcoxon rank sum test: P = 0.002). Productivity was also higher in 2010 (0.8 chick/nest) than in 2012 (0.4 chick/nest) (Wilcoxon rank sum test: P < 0.0002).
Breeding outcome and productivity differed according to nest type (χ2 = 18.5, df = 3, P = 0.003) and (χ2 = 15.7, df = 3, P = 0.001), respectively (Figure 3). Shaded nests always performed best whereas exposed nests fared worst (0% success rate). Results of the logistic regression indicated that nest exposure, and to a weaker extent, an interaction of year and hatching date, were the only factors that significantly influenced breeding outcome (Table 1, Figure 4). Likewise, productivity was significantly influenced by nest exposure and, to a lesser extent, by an interaction between hatching date and year (Table 2, Figure 5). The frequency of pale morph nestlings was higher than that of dark ones (79 and 21%, respectively, n = 43).
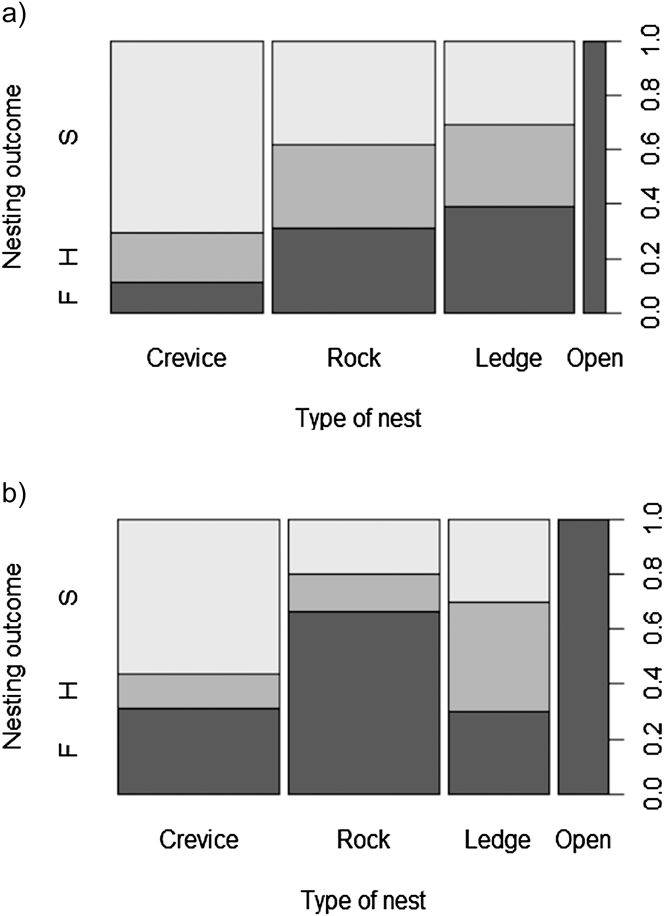
Figure 3. Relationship between breeding outcome and nest type (a) 2010, (b) 2012. S = successful nests, H = hatched nests and F = failed nests.
Table 1. Parameter estimates for the logistic regression analysis of breeding outcome of Eleonora’s Falcon at Kef Amor in 2010 and 2012.
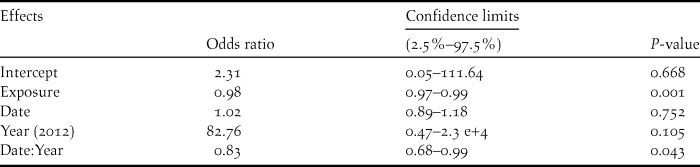
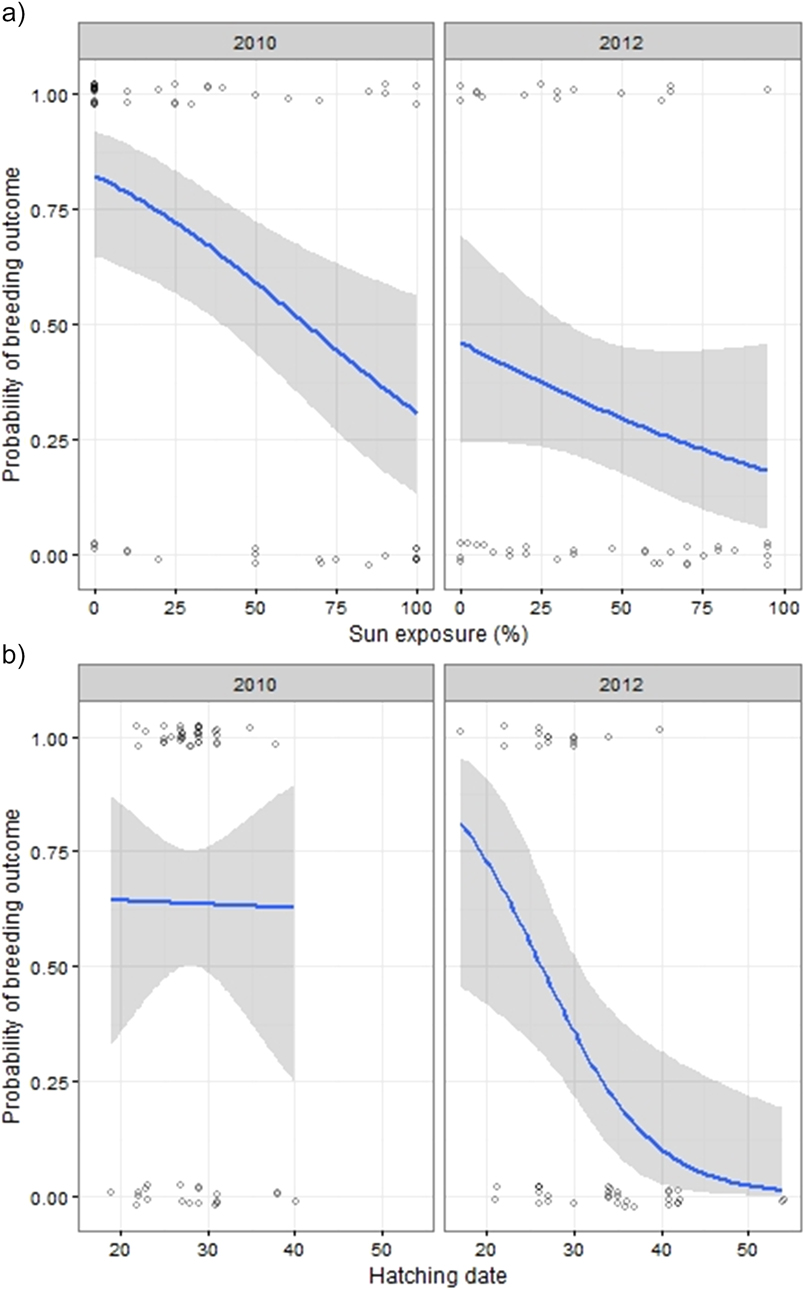
Figure 4. Estimated probability of breeding outcome plotted against exposure (a), year and hatching date (1 = 1 August) (b). Data points are scattered around their true value (0/1) to enhance their visibility.
Table 2. Parameter estimates for the GLM analysis of productivity of Eleonora’s Falcon at Kef Amor in 2010 and 2012.

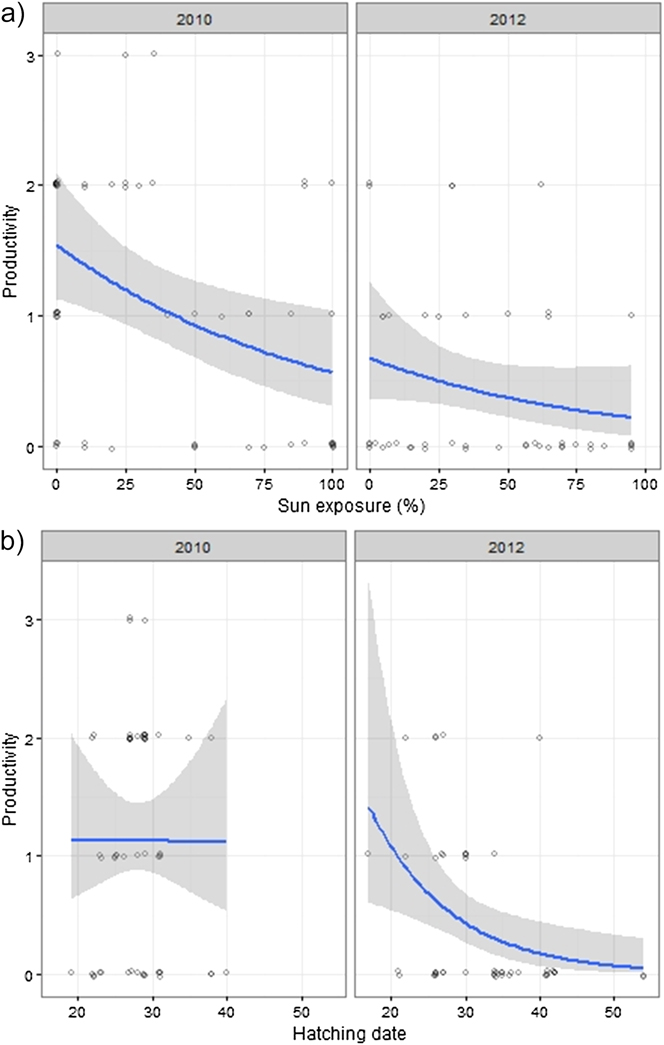
Figure 5. Fitted values of productivity plotted against exposure (a), year and hatching date (1 = 1 August) (b). Data points are scattered around their true value (0/1) to enhance their visibility.
Discussion
The total number of Eleonora’s Falcon pairs breeding in Algeria is estimated to be around 300 (authors’ unpubl. data), with Kef Amor representing the largest colony in the country. Our results, however, indicate that this population has undergone a sharp decline in recent years. At Kef Amor, in the two study years, the population decreased at an annual rate of 12.29%, from 130 to 100 nests. Moreover, the colony on Srigina underwent a clear and dramatic decline from 18 nests in 2006 to seven nests in 2009. Only three nests were recorded a year later, representing a steep annual drop rate of 36.11%. In general, population size limitation in birds of prey is largely driven by the availability of resources as well as the presence of environmental and/or intrinsic limiting factors (Newton Reference Newton1989). It is important to note that Eleonora’s Falcon is a long-distance migrant and hence, population changes may be influenced by factors operating in widely separated geographically breeding and non-breeding areas and during migration (Newton Reference Newton2004, López-López et al. Reference López- López, Liminana, Mellone and Urios2010, Mellone et al. Reference Mellone, López-López, Limiñana, Piasevoli and Urios2013). Here, we studied only the factors occurring during the breeding season at the Algerian breeding grounds, but given that the Eleonora’s Falcon population is increasing in other areas of their range (BirdLife International 2015) and that the winter distribution of different breeding populations is similar (Kassara et al. Reference Kassara, Dimalexis, Fric, Karris, Barboutis and Sfenthourakis2012, Mellone et al. Reference Mellone, López-López, Limiñana and Urios2012) it is unlikely that the reduction in breeding population size found in Algeria was related to factors operating during the winter. Therefore, the lower number of breeding pairs recorded is likely to reflect a population decline, yet other factors, such as food availability or unfavourable weather conditions during courtship or incubation periods, may also cause early breeding failure and thus, influence the number of breeding pairs recorded.
The breeding period of the Eleonora’s Falcon at Kef Amor (20–22 July) was similar to that recorded in other breeding areas, such as Sardinia where 73% of the clutches were initiated between 16th and 31st July (Badami Reference Badami, Chancellor, Meyburg and Ferrero1998) or the Canary Islands, where mean laying date occurs on July 21st (Gangoso et al. Reference Gangoso, Grande, Ducrest, Figuerola, Bortolotti, Andrés and Roulin2011). However, the initiation of egg-laying in the smaller colony of Srigina in 2006 was much later (end of July) (Télailia et al. Reference Télailia, Saheb, Boutabia, Bensouilah and Houhamdi2013, authors’ unpubl. data). It has widely been reported that older individuals usually nest earlier and attain higher reproductive success than do younger, inexperienced, or low quality conspecifics (Newton Reference Newton1979, Ferrer and Bisson Reference Ferrer and Bisson2003, Ristow and Wink Reference Ristow and Wink2004, Kassara et al. Reference Kassara, Dimalexis, Fric, Karris, Barboutis and Sfenthourakis2012, but see Zabala and Zuberogoitia Reference Zabala and Zuberogoitia2015). Therefore, the apparent delayed laying date in Srigina, despite corresponding to a different year, might suggest that this colony represents a suboptimal habitat, which is mainly occupied by young or low-quality birds. Nonetheless, and because the age of these breeding birds was unknown, we cannot rule out that other individual or environment-related factors or even a combination of these are responsible for the differences found between breeding colonies (Margalida et al. Reference Margalida, González, Sánchez, Oria, Prada, Caldera, Aranda and Molina2007). In 2012, mean clutch size (2.3 eggs) and hatching success (41%) recorded at Kef Amor were low compared with studies conducted in Greece (clutch size: 2.4, hatching: 64%; Xirouchakis et al. Reference Xirouchakis, Fric, Kassara, Portolou, Dimalexis, Karris, Barboutis, Latsoudis, Bourdakis, Kakalis and Sfenthourakis2012). However, the same authors recorded low success rates in some colonies and attributed the poor performance to adverse weather conditions (such as low winds strength in August) and habitat degradation. Badami (Reference Badami, Chancellor, Meyburg and Ferrero1998) reported a clutch size of 2.56 ± 0.65 (n = 156) with 13–67% of the eggs failing to hatch. The same author indicated that predation risk in this colony was low. Ristow and Wink (Reference Ristow, Wink, Newton and Chancellor1985) reported a clutch size of 2.3 eggs laid per pair with 1.3 young hatching on average and 1.2 young fledging. This led to a stable population for more than 10 years. In the Canary Islands, mean clutch size was 2.7 ± 0.50 and mean number of hatchlings was 2.26 ± 0.69 for the period 2007–2009 (n = 200 nests) (Gangoso et al. Reference Gangoso, Grande, Ducrest, Figuerola, Bortolotti, Andrés and Roulin2011). Nonetheless, it is important to recall that clutch size, and especially productivity, may vary widely among years; a common fact in species that depend on external food sources that are unpredictable in space and time.
During the breeding season, Eleonora’s Falcon specialises in the hunting of small migratory birds, whose availability largely depends on the population dynamics of their main prey in Europe and on local environmental conditions, mainly wind patterns, ultimately affecting breeding success (Ristow et al. Reference Ristow, Wink and Wink1983, Gangoso et al. Reference Gangoso, López-López, Grande, Mellone, Limiñana, Urios and Ferrer2013). Despite the clear importance of prey availability, the significant contribution of nest type to breeding success suggests that the degree of protection of the nest-site during incubation and chick-rearing period may be a key factor in this breeding population. Seasonal winds at the Eleonora’s Falcon’s breeding grounds can be occasionally very strong, and protection against exposure to the wind has been suggested as a factor influencing nest-site location, type, and orientation. Nonetheless, contrasting results have been reported (Walter Reference Walter1979, Wink et al. Reference Wink, Wink and Ristow1982, Urios and Martínez-Abraín Reference Urios and Martinez-Abraín2006), suggesting that wind exposure is probably not so decisive as to drive a preference for sheltered nests in this species. In contrast, predation pressure from mammal or avian predators, including conspecifics, could be of higher importance, because these can reach unprotected nests much more easily than protected ones (Ristow and Wink Reference Ristow, Wink, Newton and Chancellor1985, Xirouchakis et al. Reference Xirouchakis, Fric, Kassara, Portolou, Dimalexis, Karris, Barboutis, Latsoudis, Bourdakis, Kakalis and Sfenthourakis2012). Likewise, adults breeding in exposed nest-sites are more prone to momentarily abandon the nest due to disturbance, which also entails a high risk of clutch overheating. This may be especially important in a breeding colony as Kef Amor with increasing human frequentation. For Eleonora’s Falcons, an estimated 8% of egg loss due to solar radiation is considered normal (Ristow Reference Ristow1999, Gschweng et al. Reference Gschweng, Tataruch, Fröhlich and Kalko2011). However, the effect of exposure to sun may become critical in warm and deserted Algerian grounds, where excessive diurnal heat gain from solar radiation can quickly cause nest failure (Salzman Reference Salzman1982, Amat and Masero Reference Amat and Masero2004, Clark et al. Reference Clark, Mathieu and Seddon2015). In a breeding colony from the Aegean Sea, Wink et al. (Reference Wink, Wink and Ristow1982) found that breeding success in nests exposed to the sun was significantly lower than in sheltered nests. Indeed, we found that all those nests at Kef Amor that were exposed to the sun failed. Our results indicating that most breeding adults occupy nest sites sheltered from sun exposure support this explanation and are in close agreement with previous studies reporting that Eleonora’s Falcons preferred areas with lower average insolation and radiation (Badami Reference Badami, Chancellor, Meyburg and Ferrero1998, Urios and Martinez-Abraín Reference Urios and Martinez-Abraín2006, Xirouchakis et al. Reference Xirouchakis, Fric, Kassara, Portolou, Dimalexis, Karris, Barboutis, Latsoudis, Bourdakis, Kakalis and Sfenthourakis2012). In addition, on two separate cases, the contents of exposed nests located in a small ravine were washed away during storms, which agrees with the finding that nests above an elevation threshold are aimed at avoiding wave action during storms (Urios and Martínez-Abraín Reference Urios and Martinez-Abraín2006). But the relationship between nest type and breeding success can be also explained by the fact that young and inexperienced individuals often occupy more exposed sites and usually attain lower reproductive outcomes than older birds. Thus, the lower breeding success found in more exposed nest-sites can mirror an age-related effect. However, precise data on ringed individuals of known age would be needed to clarify the extent to which this is the case in our study population, where high quality and sheltered sites are increasingly available in parallel to the decline in the number of breeding pairs.
The influence of hatching date on breeding outcome was of lesser importance and varied between the two study years. This latter influence seems to be closely linked to the variation in environmental conditions driving prey availability, yet we cannot discard the effect of other factors not assessed in this study, such as the age or body condition of individual breeders. Since food provisioning is essentially carried out by males in Eleonora’s Falcon, the age effect of males on productivity may be predominant as was found for other raptors (Margalida et al. Reference Margalida, Mañosa, González, Ortega, Sánchez and Oria2008).
Eleonora’s Falcons have a delayed breeding season, which allows them to avoid interspecific competition for resources during summer, exception for some seabirds, such as the Scopoli’s Shearwater Calonectris diomedea. Although both species do not share their diet, they can compete for nest-sites. When a cavity is relatively large (over 2 m wide), Eleonora’s Falcon and Scopoli’s Shearwater may share it and successfully raise their chicks. However, we noted fives cases of suspected competition for crevices, where eggs of Scopoli’s Shearwater or chicks of Eleonora’s Falcon were destroyed. Falcons are at a disadvantage as they start egg laying when shearwater eggs have already hatched or are in the latest stages of the incubation period (Walter Reference Walter1979). This interspecific competition was not noted on Srigina, possibly because shearwaters are able to dig burrows in the soft ground of this islet.
Alternative breeding strategies based on differential habitat selection and asymmetric competitive behaviour has been associated with melanin-based coloration in Eleonora’s Falcon (Gangoso et al. Reference Gangoso, Afán, Grande and Figuerola2015a). Melanin-based colour morphs also differ in physiological characteristics, such as their antioxidant machinery (Galván et al. Reference Galván, Gangoso, Grande, Negro, Rodríguez, Figuerola and Alonso-Alvarez2010) and immune capacity (Gangoso et al. Reference Gangoso, Grande, Ducrest, Figuerola, Bortolotti, Andrés and Roulin2011, Reference Gangoso, Roulin, Ducrest, Grande and Figuerola2015b). The percentage of dark-phase falcons at Kef Amor (21%) is similar to that found in other breeding colonies and years: Morocco (18–28%), the Aegean Sea (21–39), and the Canary Islands (18.5–28.6%) (Walter Reference Walter1979, Ristow et al. Reference Ristow, Scharlau, Wink, Meyburg and Chancellor1989, Gangoso et al. Reference Gangoso, Grande, Ducrest, Figuerola, Bortolotti, Andrés and Roulin2011). In contrast, Badami (Reference Badami, Chancellor, Meyburg and Ferrero1998) found a lower percentage (8–17%) of dark-phase birds in Sardinia.
Overall, with the near extinction of the Srigina colony, the recorded poor breeding performance and sharp decline of the colony on Kef Amor raise serious concern about the conservation of Eleonora’s Falcon in Algeria and North Africa (Samraoui et al. Reference Samraoui, Alfarhan, Al-Rasheid and Samraoui2011). We found that breeding success was low and partially but substantially influenced by local environmental factors. Nonetheless, the effect of human activities may also contribute to the current decline in different ways. Although not quantified, there was a marked increase in the number of summer visitors to Kef Amor over the last five years (2007–2012), many of whom camped overnight to carry out recreational fishing. During the same period, the number of motorboats crisscrossing the area around the island also increased substantially. Recreational activities are known to impact negatively population dynamics of this species (Clark Reference Clark1974, Martínez-Abraín et al. Reference Martínez-Abrain, Oro, Ferris and Belenguer2002). Human disturbance can also cause nest abandonment, increased predation and exposure of clutches and chicks to lethal heat, thus decreasing breeding performance (Hunt Reference Hunt1972, Tremblay and Ellison Reference Tremblay and Ellison1979, Anderson and Keith Reference Anderson and Keith1980, Burger Reference Burger1981, Vos et al. Reference Vos, Ryder and Graul1985). In addition, on Srigina the sharp decline in Eleonora’s Falcon coincided with the introduction of cats by lighthouse keepers to control the already present rats. Although population trends in the western part of Algeria are unknown, the presence of rats on the Habibas islands (located off shore of the town of Oran, in the western part of Algeria) may equally be detrimental to the breeding performance of the Eleonora’s Falcon on that island (Samraoui and Samraoui Reference Samraoui and Samraoui2008). Invasive mammals represent a major threat not only to falcons, but also to all bird species breeding on islands worldwide (Blackburn et al. Reference Blackburn, Cassey, Duncan, Evans and Gaston2004, Hilton and Cuthbert Reference Hilton and Cuthbert2010). Moreover, humans and introduced species may carry the added risk of introducing pathogens and vectors to insular habitats devoid or with low occurrence of these (Gutiérrez-López et al. Reference Gutiérrez-López, Gangoso, Martínez de la Puente, Fric, López-López, Mailleux, Muñoz, Touati, Samraoui and Figuerola2015).
The recognition that human activities are putting insular species at risk has yet to make its way among local stakeholders as formal protection, which is, when present, insufficient to provide reliable refuges to breeding birds. With the expansion of tourism and recreational activities across the Algerian coast, the fate of breeding birds seems sealed if urgent action is not taken to mitigate and control these burgeoning activities (Burger Reference Burger2003, Burger et al. Reference Burger, Gochfeld, Jenkins and Lesser2010).
Acknowledgements
We thank two anonymous reviewers for their helpful comments and suggestions. We are also most grateful to all volunteers who helped during fieldtrips with a special mention to Abdennour Boucheker. The work was supported by the Algerian Ministère de l’Enseignement Supérieur et de la Recherche Scientifique (DGRSTD/M.E.S.R.S.) and Distinguished Scientist Fellowship Program (DSFP), King Saud University, Saudi Arabia. LG was supported by a contract under the Excelence Projects from Junta de Andalucía, Spain (RNM-7038).











