Introduction
Nests can provide protection for adult birds, eggs and nestlings (Jiao et al. Reference Jiao, Guo, Huettmann and Lei2014). The selection of nest sites, whereby individuals choose nest sites based on environmental cues, often results in distinct patterns of habitat patch occupancy (Martin Reference Martin1998, Clark and Shutler Reference Clark and Shutler1999, Kolbe and Janzen Reference Kolbe and Janzen2002). Nest site selection may be influenced by factors at multiple hierarchical spatial scales. These include physical characteristics such as food resources (Kazantzidis et al. Reference Kazantzidis, Hafner and Gourner1996), vegetation cover (Eggers et al. Reference Eggers, Griesser and Nystrjan2006), human disturbance (Chen et al. Reference Chen, Liu, Yan and An2011, Mccarthy and Destefano Reference Mccarthy and Destefano2011) and interspecific social factors such as attraction, territoriality and competition (Reed and Dobson Reference Reed and Dobson1993, Quintana and Yorio Reference Quintana and Yorio1998). For large wading birds, such as Grey Heron Ardea cinerea in Zhanglong wetland, studies have found that the distance of nests from areas having infrequent human activities and vegetation height are two important factors affecting nest site selection (Wu et al. Reference Wu, Miao, Zou and Jiao2008). Nest site selection of Black-necked Crane Grus nigricollis at the northern limit of the Tibetan Plateau is determined by habitat type, human disturbance and water depth (Zhang et al. Reference Zhang, An, Shu and Yang2017). In addition, with the development of ecotourism, tourists’ approach or presence may prompt parent birds to leave their nests, rendering eggs open to attack (Edington and Edington Reference Edington and Edington1986, Bolduc and Guillemette Reference Bolduc and Guillemette2003), which may cause modified nesting behaviour and nest placement (Burger and Gochfeld Reference Burger and Gochfeld1993).
Habitat selection, particularly the choice of nest site, may be important for reproductive success (Martin Reference Martin1998, Chalfoun and Schmidt Reference Chalfoun and Schmidt2012) and is also closely associated to offspring production in birds (Clark and Shutler Reference Clark and Shutler1999). Birds are expected to select nest sites that maximise their potential reproductive success (Kim and Koo Reference Kim and Koo2009). Nesting habitat provides protection against predators (e.g. humans and birds of prey), adequate stability to support the nest as well as abundant food resources for breeding (Fasola and Alieri Reference Fasola and Alieri1992). Halfwerk et al. (Reference Halfwerk, Holleman, Lessells and Slabbekoorn2011) showed that nest sites near roads would have lower reproductive success because of the negative impact of traffic noise. Therefore, for endangered species, suitable nest site habitats may directly affect the success rate of reproduction, population size and dynamic change. Knowledge of nest site selection within breeding areas has become a key issue for endangered waterbird species conservation.
Oriental Stork Ciconia boyciana is large migratory wading waterbird species, which is listed as ‘Endangered’ on the IUCN Red List of Threatened Species (IUCN 2018) and in the first category of the nationally protected wildlife species in China (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013), with a global population of less than 2,500 mature individuals (BirdLife International 2018). By the end of the 20th century, the breeding range of Oriental Stork was limited to north-eastern China, in the Amur and Ussuri basins along the border of Russia (Liu et al. Reference Liu, Li and Li2007, Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). Its main wintering sites lie around lakes situated along the middle and lower Yangtze River floodplain and southern China, including Taiwan and Hong Kong, with small numbers wintering in the Korean peninsula, Japan, Philippines, north-eastern India, Myanmar and Bangladesh (Wang and Yang Reference Wang and Yang1995, BirdLife International 2018). According to the results of monitoring in 2008 (Zhu et al. Reference Zhu, Li, Wang and Chang2000), 157 storks inhabited Sanjiang Plain, Heilongjiang, in the summer, when 56 young storks were reared. In 2010, 38 storks were found in Zhalong National Nature Reserve (Gao et al. Reference Gao, Wang, Pang, Pan and Du2011). In China, there has been limited systematic and comprehensive investigation of the breeding populations of Oriental Storks, and only a few studies have reported short-term observations in local areas, therefore it was difficult to estimate the size of the breeding population (Tian et al. Reference Tian, Wang and Ma2011).
Studies on nest site selection of Oriental Storks have been reported in the breeding grounds. For example, tree height and distance from highways were two limitations on tree nesting among Oriental Storks in Honghe National Nature Reserve (Wang and Li Reference Wang and Li2006). Furthermore, a nest site selection model indicated that Oriental Storks prefer to make nests in areas with abundant food resources and lower levels of human disturbance in Zhalong, Honghe and Xingkaihu National Nature Reserves (Duan et al. Reference Duan, Tian, Zhu, Ding, Shan and Lu2011). Widespread deforestation and conversion of wetlands to farmlands in Sanjiang Plain reduced the areas of wetlands by 31% from 1995 to 2005 (Song et al. Reference Song, Wang, Du, Liu, Zeng and Ren2014). Transition to agricultural cultivation accounted for 91% of wetland losses, whereas transition to grassland and forest accounted for 7% (Song et al. Reference Song, Wang, Du, Liu, Zeng and Ren2014). This led to a subsequent decrease in population size and nesting habitat of Oriental Storks in traditional breeding regions. Since 1999, a small Oriental Stork subpopulation began to breed in the wintering and stopover sites, such as Anhui Wangjiang in 2001, Jiangsu Gaoyou in 2002, Dafeng in 2009 and Jiangxi Poyang in 2004 (Tian et al. Reference Tian, Wang and Ma2011). Yellow River Delta National Nature Reserve (YRD NNR) has been an important stopover site on the Oriental Stork flyway (Shimazaki et al. Reference Shimazaki, Tamura, Darman, Andronov, Parilov, Nagendran and Higuchi2004). Since 2003, the stork began breeding in this wetland. In order to satisfy the ecological conditions of Oriental Stork nest site selection, YRD NNR established a wetland recovery project. With the implementation of this project, open water in the recovered areas accounted for about 60% of the total areas (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). In 2009, to attract more storks breeding there, YRD NNR set up 21 artificial nests for the first time in the reed wetland restored areas at Dawenliu station (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). From 2003 to 2010, the cumulative number of breeding Oriental Storks was 91 pairs, with 213 stork chicks raised (data provided by YRD NNR).
In this study, we investigated the nesting factors that influence nest site selection of the breeding Oriental Storks in YRD NNR in 2017, to better understand the strategies of nest site selection. The objectives of our study were to: 1) document the basic characteristics of three different types of nest site habitat of Oriental Storks; 2) analyse differences in selections of three types of nest site and examine the main habitat variables influencing nest site selection. Based on our results, we provide suggestions for future conservation and habitat management of Oriental Stork.
Methods
Study area
The YRD NNR (37°35′ - 38°12′N, 118°33′ - 119°20′E) is located in the estuary of Yellow River. It is one of the main stopover sites for waterbirds on the East Asian-Australasian Flyway in China (Figure 1). This region is under the influence of the semi-humid continental monsoon climate, with an average annual temperature of 11.9°C, a frost-free period of 210 days and annual precipitation of 592.2 mm (Duan et al. Reference Duan, Tian, Zhu, Ding, Shan and Lu2011).

Figure 1. The study area in Yellow River Delta National Nature Reserve, Shandong, China.
The reserve holds the best preserved, most extensive and youngest wetland ecosystem in the warm temperate zone of China (Liu Reference Liu2013). Owing to deposition of large amount of sand and mud carried by Yellow River, about 1,333 ha of new land is formed there every year. The reserve includes areas of Yellow River Estuary (Dawenliu station and Yellow River Estuary station), and along the old course of Yellow River prior to 1976 (Yiqian’er station) (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). It covers a total area of 153,000 ha, including 59,400 ha of core areas, 11,200 ha of buffer zones and 82,400 ha of experimental areas.
Our study area was located at Dawenliu station, where most of the main nesting and foraging areas of Oriental Stork in YRD NNR have been found. Due to salinization of the soil in the reserve, the vegetation types are mainly halophytes and herbaceous plants. Therefore, the vegetation structure is simple and lacks large trees (Xue Reference Xue2010). As a result, only alternative elevated structures are suitable for construction of nests. In 2002, YRD NNR put a wetland recovery programme into practice at Dawenliu station. On implementation, the open water areas in the recovered regions accounted for about 60% of the total area (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). The predominant plants in this region are common reed Phragmites australis, cattail Typha orientalis, water pepper Polygonum hydropiper, black locust Robinia pseudoacacia, Chinese tamarisk Tamarix chinensis and winged salsa Suaeda heteroptera. Besides, the habitats are rich in fish, with species such as Carassius auratus, Aristichthys nobilis, Silurus asotus and other aquatic life (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013). The dominant habitat types in the recovered areas, reed wetland and shallow water wetland, provide a suitable environment for the storks to inhabit and forage. Consequentially, an increasing number of storks visit the reserve and the number of nests has gradually increased. Of note, a 15 km long highway, tourist attractions, village areas and a large number of oil exploitation machines can also be found in this region.
Data collection at nest sites
Nesting sites of storks in the study area were investigated from early February to mid-June 2017. A total of 62 nest sites variable data were collected after the end of the nesting period. A handheld GPS (eTrex 30, Garmin, China) was used for geo-referencing recording of latitude, longitude and altitude.
We set up quadrats and surveyed various nest variables. A large quadrat of 10 m × 10 m centered on each nest, and five 1 m × 1 m small quadrats were set up at four of the vertices and the centre point of the large one. Data were collected from all five small quadrats. The following variables were determined: (1) nest height; (2) vegetation height (mean height of the vegetation per small quadrat); (3) vegetation density (mean density of the vegetation per small quadrat); (4) vegetation coverage (ratio of the vertical projection areas of the vegetation to the sample quadrat areas); (5) water area percentage in a quadrat; (6) human disturbance (reed harvesting, agricultural production and photography); (7) distance from highways; (8) distance from byways (dirt roads or paths used for reed harvest and daily patrol); (9) distance from open water areas; (10) distance from the Yellow River; (11) distance from woods; (12) distance from the nearest conspecific nest; (13) distance from sightseeing sites; (14) distance from villages; (15) distance from oil wells; (16) distance from suitable foraging habitats (Zhu et al. Reference Zhu, Cao, Wang, Xu, Lei, Wu and Zhao2015: Figure 2); (17) usage times (the times nests were used by Oriental Storks in the last 6 years). Breeding pairs were considered as ‘successful’ if one or more chicks were confirmed to have been produced. We also recorded the clutch size and the number of nests with breeding success over the parental care period in order to calculate breeding success (number of nests with breeding success divided by total number of nests).
For our analysis, we intended to pursue a robust but basic comparison of used vs. available sites (Manly et al. Reference Manly, Mcdonald and Thomas2014). Therefore, we randomly selected 32 control plots within the study area. The control plots were also set up with a large 10 m × 10 m quadrat at the center of the plot, and the same variables as those for the nest sites were measured.
Statistical analyses
The factors from a total of 62 nest sites were used for the nest selection analyses. We used the one-sample Kolmogorov-Smirnov Test to examine whether the variables followed a normal distribution with statistical software SPSS20.0. For data that followed a normal distribution, we used the parametric Student’s t-test or one-way ANOVA to analyse the significance of the comparison between the nest site variables, otherwise the non-parametric Mean-Whitney U test was used.
Before analysis, we tested significance between all collected variables and dependent variables using single factor analysis and retained variables with P< 0.05. Then we performed conditional logistic regression on retained variables to model microhabitat characteristics affecting nest site selection (Hartman et al. Reference Hartman, Ackerman, Takekawa and Herzog2016).
To determine what influenced the nest selection of storks, an information theoretical approach was adopted. We examined logistic regression models with nests chosen or nests abandoned by storks as the dependent variables, then used second order Akaike’s Information Criterion (AICc) to calculate the value of each model before ranking them. We considered the model with the lowest AICc score to be the most parsimonious, and we used the difference in AICc values (△AICc) between the best model and each of the other models in the candidate set to assign model rank (Hartman et al. Reference Hartman, Ackerman, Takekawa and Herzog2016). We estimated the weight of variables of the overall model using Akaike model weights (wi) in order to show that the likelihood of a model on a given date, relative to those of other candidate models. We calculated evidence ratios to compare the relative weight of support between models.
No single model was clearly superior compared with others (△AICc > 2) (Anderson et al. Reference Anderson, Link, Johnson and Burnham2001, Burnham and Anderson Reference Burnham and Anderson2002). This conclusion was based on relevant literature, which indicated △AICc > 2 should be ignored and that all candidate models must be model-averaged including models of low Akaike weight (Anderson et al. Reference Anderson, Link, Johnson and Burnham2001). We sum the Akaike weights of variables that appeared in each model (Symonds and Moussalli Reference Symonds and Moussalli2011). For parameter bj, the model averaged estimate was calculated as:
 $$\overline {{b_j}} = \sum\nolimits_{i = 1}^R {{w_i}\widehat {b_{j,i}^ + }}$$
$$\overline {{b_j}} = \sum\nolimits_{i = 1}^R {{w_i}\widehat {b_{j,i}^ + }}$$
In which, wi is the Akaike weight of model i, and  $\widehat {b_{j,i}^ + }$
is the estimate of bj if predictor j is included in model i, otherwise it is equal to zero (Freckleton Reference Freckleton2011).
$\widehat {b_{j,i}^ + }$
is the estimate of bj if predictor j is included in model i, otherwise it is equal to zero (Freckleton Reference Freckleton2011).
The sum of Akaike weights over the subset of models that include a specific predictor was used to assess the relative importance of that predictor variable in explaining the variation of the overall model, which provides a more robust estimate assessment of variable importance relative to the single-model approaches (Burnham and Anderson Reference Burnham and Anderson2002). All the modelling analyses were performed using the R statistical software (R Version 3.3.3), and the multi-model inference package ‘MuMIn’ for model selection and averaging. A significance level of 0.05 (P) was used for all statistical tests, with means stated as Mean ± SE.
Results
Reproductive success and performance
After establishing nests in late February, eggs were usually laid during the last two weeks of March, and by the end of April chicks hatched. Nests were classified into three types (power pole nest, artificial nest and pylon nest) depending on the nest location construction.
Within the study area, a total of 68 power poles, 21 artificial poles and 37 pylon poles (provided by YRD NNR). Among these, 40 power pole nests, 14 artificial nests and eight pylon nests were used by storks in YRD NNR in 2017. A total of 57 nests showed breeding success (36 power pole nests, 14 artificial nests and seven pylon nests) (Table 1). Breeding success was highest in artificial nests (100%) and chick productivity in power pole nests (128 chicks) during the monitoring period (Table 1). The average clutch size in each nest was 3.56 ± 0.13 in power pole nests, 3.14 ± 0.23 in artificial nests and 3.14 ± 0.34 in pylon nests (Table 1).
Table 1. Nest number and breeding success of three nest types of the Oriental Stork in Yellow River Delta National Nature Reserve in 2017.

Characteristics of nest site habitat
Open reed wetland was an important habitat for the nest site selection of Oriental Storks. Power pole nests and artificial nests were all located in reed habitats (n = 50), and pylon pole nests located in farmland habitats (n = 7). They were all surrounded by open water, consisting of small pools and seasonal streams. Except distance from Yellow River (x 2 = 5.57, P = 0.062), all other habitat characteristics of the nest sites were significantly different among the three types (Table 2). Furthermore, usage times in power pole nests were the highest (4.00 ± 0.31 times), and there were significant differences among all three types of nest (x 2 = 14.66, P = 0.001) (Table 2). Except for distance from villages and suitable foraging habitats, all other habitat characteristics of nest sites were significantly different (Table 2). Distance from disturbance of successful nests was significantly further than for the 25 control plots (Table 2), such as sightseeing sites (2517.10 ± 214.09 m), oil wells (3243.39 ± 173.27 m) and highways (321.84 ± 23.54 m) (Table 2). The level of disturbance for the control plots was strong (3.52 ± 0.19 degree), significantly different from disturbance level for the successful nests (2.45 ± 0.07 degree; x 2 = 24.32, P = 0.000) (Table 2). Distances from villages and the suitable foraging habitats were no significant difference between the control plots and successful nests (x 2 = 1.69, P = 0.194; x 2 = 2.07, P = 0.150) (Table 2). In addition, usage times of the successful nests were significantly higher than that for control plots (x 2 = 40.26, P = 0.000) (Table 2).
Table 2. Nest site characteristics of three types of nest and comparison between successful and unselected nests of Oriental Stork in Yellow River Delta National Nature Reserve.
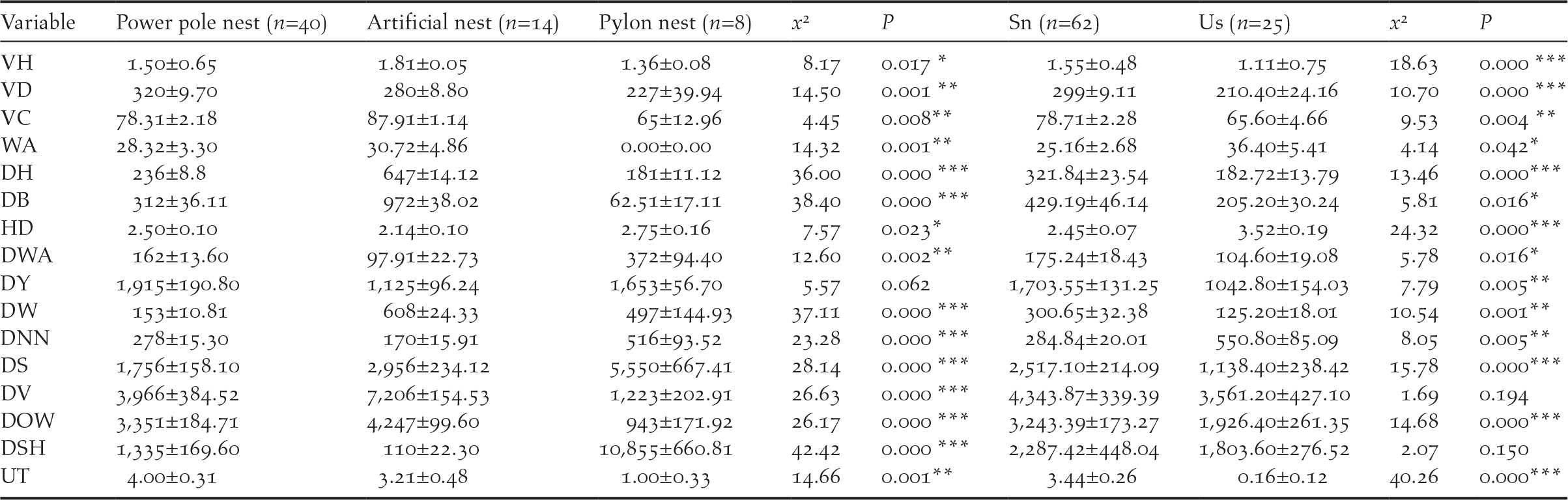
VH, vegetation height (m); VD, vegetation density (ind./m2); VC, vegetation coverage (percent); WA, water area percentage in the quadrat (percent); DH, distance from highways (m); DB, distance from byways (m); HD, human disturbance (degree); DWA, distance from open water areas (m); DY, distance from the Yellow River (m); DW, distance from woods (m); DNN, distance from the nearest conspecific nest (m); DS, distance from sightseeing sites (m); DV, distance from villages (m); DOW, distance from oil wells (m); DSH, distance from suitable foraging habitats (m); UT, Usage times (time).
***:P<0.001; **:P<0.01; *:0.01<P<0.05 Pp- Power pole nest; An- Artificial nest; Py- Pylon nest; Sn- Successful nest; Us- Unselected potential nests (control plots)
Factors influencing nest site selection
The results of logistic regression and model-averaging analysis showed that human disturbance was the most important factor affecting nest site selection of Oriental Storks in power pole nests (relative importance, RI = 0.98), and the coefficient was negative, which showed that storks preferred lower level of human disturbance (Table 3). For artificial nests, model-averaging analysis indicated that distance from highways had the highest importance among all variables (RI = 0.95). Coefficients being positive for ‘distance from highways’ meant that storks preferred artificial poles far from highways (Table 3). The analysis about microhabitats of pylon nests indicated that distance from sightseeing sites was an important factor affecting nest site selection (RI = 0.99). Similarly, the coefficient of ‘distance from the sightseeing sites’ was also positive, which showed storks preferred the pylon poles far from sightseeing sites (Table 3).
Table 3. Model-averaged coefficient estimates, standard errors (SE), lower (LCL) and upper (UCL) 95% confidence limits, and relative importance (RI) for variables examined for all models.

VH, vegetation height (m); VD, vegetation density (ind./m2); VC, vegetation coverage (percent); WA, water area percentage in the quadrat (percent); DH, distance from highways (m); HD, human disturbance (degree); DS, distance from sightseeing sites (m); DV, distance from villages (m); DOW, distance from oil wells (m).
Discussion
Reproductive characteristics and performance
Nest numbers and successfully breeding pairs of Oriental Storks were 62 and 57 respectively, during the monitoring period. The Oriental Storks in YRD NNR begin to reproduce in early February, sooner than in March in northern breeding areas (Xu et al. Reference Xu, Fei, Xu, Yang and Song1993). This difference could be caused by latitude. Compared with the traditional breeding grounds in north-east China, the YRD NNR is closer to the wintering grounds of the middle and lower Yangtze River, and a shorter migration distance could reduce the energy loss of long flights and probability of danger along the migration route. This may be a reason why more and more Oriental Storks breed in YRD NNR. In addition, previous studies have also shown that wintering and stopover areas with higher human population reduced White Stork Ciconia ciconia overall energy expenditure because of shorter daily foraging trips, shortened migration distances, or completely suppressed migration (Flack et al. Reference Flack, Fiedler, Blas, Pokrovsky, Kaatz, Mitropolsky, Aghababyan, Fakriadis, Makrigianni, Jerzak, Azafzaf, Feltrup-Azafzaf, Rotics, Mokotjomela, Nathan and Wikelski2016, Arizaga et al. Reference Arizaga, Resano-Mayor, Villanúa, Alonso, Barbarin, Herrero, Lekuona and Rodriguez2018). We will try our best to clarify the relationship between nest site distribution, migration and energy cost of Oriental Stork in future study. Breeding success of artificial nests was highest among the three types of nests monitored. This might be explained by the fact that the artificial nests were close to food resources and had a lower level of human disturbance, so parent birds could put more energy into chick-rearing, concurring with earlier result for White Storks (Tryjanowski et al. Reference Tryjanowski, Sparks, Jakubiec, Jerzak, Kosicki and Kuźniak2005). The average clutch size of power pole nests was larger than for other types of nests (3.56 ± 0.13 chicks/nest), more than the average clutch size (2.40 ± 0.27 chicks/nest) in the Zeya River of Russia (Collar et al. Reference Collar, Andreev, Chan, Crosby, Subramannya and Tobias2001), but less than in the middle and lower Yangtze River floodplain (4.13 ± 0.23 chicks/nest) (Yang et al. Reference Yang, Zhou, Zhu and Hou2007). Therefore, the clutch size not only depended on geographical latitude, but also on weather conditions and climate variability (Kulesza Reference Kulesza1990). Nest structure and size were also related to clutch size (Snow Reference Snow1976, Reference Snow1978). In addition, Dunn (Reference Dunn, Thusius, Kimber and Winkler2010) found that levels of resource abundance determine the variation in clutch size.
Effect of habitat characteristics on nest site selection
To improve breeding success, parent birds selected the most optimal nest site that could provide sufficient food and energy for their reproduction (Welcker et al. Reference Welcker, Speakman and Elliott2015). We expected to reveal factors that affected the utilisation of different types of nest sites. The power pole nests in the reed wetland represented more than 70% of storks nesting in the study areas, so the reed wetlands were considered as the main breeding habitat. Our results showed that the main factors affecting nest site selection of Oriental Storks were human disturbances. During the early nesting phase, the noise of harvesters prevented storks from nesting in this area. Furthermore, photographers tried to approach the nesting areas on foot, making some storks temporarily flee or even abandon their nests. The results were similar to those of previous studies in this area (Duan et al. Reference Duan, Tian, Zhu, Ding, Shan and Lu2011).
In terms of artificial nests, the most important factor affecting nest site selection was distance from highways. Due to the development of ecotourism, the reserve authority has built viewing platforms, parking lots, boardwalks and visitor centers. This has resulted in higher transport levels, and vehicle horns and noises reduce the use of nests closer to highways (Shu et al. Reference Shu, Hu, Leng, Zhu and Shan2006). Furthermore, traffic noise might interrupt stork communications, such as the sound of striking beaks with each other (Parris and Schneider Reference Parris and Schneider2009).
Distance from sightseeing sites had the greatest impact among other variables on pylon nests, exhibiting a negative effect on nest site selection. This was consistent with reports from other study areas, such as Honghe National Nature Reserve (Wang and Li Reference Wang and Li2006). During the breeding season, birds were more sensitive to disturbance. The adverse effects of tourism activities included changing the behaviour of the parent birds, causing abandonment of nests and affecting hatching (Bolduc and Guillemette Reference Bolduc and Guillemette2003). In addition, the frequent occurrence of tourists in sightseeing sites could prevent parent birds from feeding their chicks, causing chicks to lose weight and grow slowly (Zande and Vos Reference Zande and Vos1984), potentially resulting in nest failure and reduced reproductive success, which can affect population growth or species stability (Haysmith and Hunt Reference Haysmith and Hunt1995).
Furthermore, our study showed that usage times of successful nests were significantly higher than for unselected potential nests. Oriental Stork preferred old nests that had been used many times. Previous studies have shown that nest site characteristics, habitat quality and breeding density were related to the usage times of the White Stork nest in Europe (Switzer Reference Switzer1993, Beheler et al. Reference Beheler, Rhodes and Weeks2003). Suitable nest site habitat can provide enough food for storks the entire breeding season (Vergara et al. Reference Vergara, Aguirre, Fargallo and Davila2010). Another possible reason was that the older nests were generally larger and more solid, saving more energy and improving reproduction efficiency, similar to a report for other study (Zhou et al. Reference Zhou, Song and Ma1998). Vergara et al. (Reference Vergara, Aguirre, Fargallo and Davila2010) indicated that the age was also a major factor related to nest usage times and individual’s age determines its experience and therefore its use of resources in White Stork. Changing nest sites involves the cost of reproductive, so preferring old nests can be considered an adaptive strategy to improve fitness. We will continue to focus on this aspect in future work.
Implications and suggestions
Based on our results, we give the following suggestions for conservation of Oriental Stork. Firstly, conduct a systematic and comprehensive field survey to clarify the number of Oriental Stork in China, which would be helpful to better understand the current status of the species. Secondly, in order to protect breeding populations, it is necessary to reduce traffic and tourism development in breeding areas, such as enforcing lower speed for cars, forbidding use of car horns and keeping photographers a safe distance from nesting areas. Thirdly, as birds are more sensitive to disturbance in the early phase of reproduction (Ruhlen et al. Reference Ruhlen, Abbott, Stenzel and Page2003), lowering the noise and intensity of oil exploitation and reed harvesting activities during the early nesting phase could promote improved nesting success. Fourthly, due to the decrease in spaces for potential nests within the core areas of YRD NNR, it is necessary to erect additional artificial nests near suitable foraging habitats, away from highways and other disturbance factors, which could act as suitable nest sites for storks in the early nesting period. Finally, soft barriers should be established between tourists and nesting areas, such as fences and ditches, which could effectively reduce the human disturbance to storks. This should be managed sensitively, because ecotourism is an important benefit for nature reserves, and providing visitors with memorable experiences should be encouraged whilst ensuring that breeding storks are not disturbed.
Acknowledgements
We appreciate the staff of Yellow River Delta National Nature Reserve Management Center for their help in the field work. We are grateful to Mr. Ziqiang Huang for his assistance. The manuscript benefited from helpful suggestions by Dr. Gang Liu, Dr. Xingjia Xiang, Dr. Xi Wang, Dr. Jinming Zhao, Miss Mehtab Nazia, Mr. Chao Yu and Mr. Yiwei Bao. We also thank Tim Dodman and an anonymous reviewer for their helpful comments. This study was supported by the National Natural Science Foundation of China (L.Z., grant numbers 31472020 and 30870317).






