Introduction
Insular ecosystems are very susceptible to environmental changes as indicated by the high extinction rates present in oceanic islands (Ricklefs and Schluter Reference Ricklefs and Schluter1993, Terborgh et al. Reference Terborgh, Lopez, Nuñez, Rao, Shahabuddin, Orihuela, Riveros, Ascanio, Adler, Lambert and Balbas2001). In fact, 93% of extinct bird species and 81% of extinct mammal species over the last five centuries were inhabitants of islands (see King Reference King and Moors1985 and Ceballos and Brown Reference Ceballos and Brown1995 respectively). Moreover, 70% of the endemic flora of a number of islands is listed as threatened, rare, or extinct (Davis et al. Reference Davis, Droop, Gregerson, Henson, Leon, Villa-Lobos, Synge and Zantovska1986). Islands show low species richness, limited available habitat, small population sizes and high susceptibility to species introduction. These factors do not facilitate the buffering of disturbances to these ecosystems (MacArthur and Wilson Reference MacArthur and Wilson1967, Abbott Reference Abbott1978, Elton Reference Elton2000). Nowadays disturbances caused by direct or indirect anthropogenic factors are usually more common and deleterious than those with a natural origin (Diamond Reference Diamond, Western and Pearl1989).
The Pacific Juan Fernández Archipelago (situated about 670 km off the coast of Chile) was declared a Biosphere Reserve in 1977 and represents a clear example of insular ecosystem degraded by human activity, being considered a mini-hotspot along with Galapagos Islands (Mittermeier et al. Reference Mittermeier, Myers, Robles Gil and Mittermeier1999). The Juan Fernández Archipelago presents the highest rate of both endemic plant species per area (Stuessy Reference Stuessy, Grau and Zizka1992, Stuessy et al. Reference Stuessy, Marticorena, Rodriguez, Crawford and Silva1992) and plant species richness per area (Arroyo Reference Arroyo, Mittermeier, Myers, Robles Gil and Mittermeier1999) on oceanic islands. However, much of the native forest has been destroyed and attempts at regeneration have been hampered by prior introductions of animals (e.g. goat Capra aegagrus hircus and rabbit Oryctolagus cuniculus) and plants (e.g. bramble Rubus ulmifolius and maqui Aristotelia chilensis) (Wester Reference Wester1991, Cuevas and Van Leersum Reference Cuevas and Van Leersum2001).
In contrast, the Juan Fernández Islands have a very limited native fauna, with no land mammals, reptiles, or amphibians. There are seventeen breeding land and sea bird species including five endemic species and three endemic subspecies, two of them inhabiting Robinson Crusoe Island’s forests (Juan Fernández Firecrown Sephanoides fernandensis and the Juan Fernández Tit-Tyrant Anairetes fernandezianus). Several non-endemic bird species have long-established populations in the archipelago (King Reference King1839, Reed Reference Reed1874): Green-Backed Firecrown Sephanoides sephaniodes, Austral Thrush Turdus falcklandii, and three birds of prey (Falco sparverius fernandensis, Buteo polyosoma exsul and Asio flammeus). Another two landbird species were directly introduced by humans (House Sparrow Passer domesticus and Rock Dove Columba livia) (Hahn et al. Reference Hahn, Römer, Vergara and Walter2009, Reference Hahn, Vergara and Romer2011a).
The Juan Fernández Firecrown has a small range and fragmented habitat. It is catalogued as ‘Critically Endangered’ (IUCN 2012) although historical records (684 individuals in 1988–1989) indicate smaller populations than new ones (1,100 individuals, Hahn et al. Reference Hahn, Römer and Schlatter2006, Hahn et al. Reference Hahn, Römer, Vergara and Walter2009; even 2,500–3,000 individuals were estimated by Hodum in litt. 2007; see BirdLife International 2013 and Oikonos 2012). The Juan Fernandez Tit-Tyrant also has a small range and is classified as ‘Near Threatened’. Its population is considered stable or slightly declining since 1994 (Hahn et al. Reference Hahn, Römer and Schlatter2006). Brooke (Reference Brooke1987) estimated around 5,000 individuals but more recent estimations show that the population size is about 2,500–3,000 individuals (Hahn et al. Reference Hahn, Römer and Schlatter2006).
Several ecological studies have predominantly focused on the Juan Fernandez Firecrown. The currently accepted drivers of the Juan Fernandez Firecrown’s decline are (i) the decline of certain endemic plants used by the Juan Fernández Firecrown as food source, (ii) the introduction of predators such as rats Rattus spp. and cats Felis catus and (iii) the unequal sex ratio (Cuevas and Van Leersum Reference Cuevas and Van Leersum2001, Bourne et al. Reference Bourne, Brooke, Clark and Stone1992, Jaksic Reference Jaksic1998, Roy et al. Reference Roy, Torres-Mura, Hertel, Lemus and Sponer1999, Hahn and Römer Reference Hahn and Römer2002, Ricci Reference Ricci2006). In addition, Austral Thrushes could have a potential dual detrimental effect on Juan Fernández Firecrowns. On the one hand they could act as nestling predators and on the other hand contribute to seed dispersal of introduced plants that compete with the endemic species used as a food source by firecrowns (Hahn et al. Reference Hahn, Vergara and Romer2011a, Hahn and Römer Reference Hahn and Römer2002).
Previous work on the proximate causes behind the decline in endemic forest bird species in the Juan Fernández Archipelago have not accounted for the possible impact of avian diseases. It is well known that introduction of vector-borne diseases by alien species has had deleterious consequences for endemic fauna in insular ecosystems, especially in birds (Lapointe et al. Reference Lapointe, Atkinson and Samuel2012, Atkinson and Samuel Reference Atkinson and Samuel2010). Island species evolve in environments virtually free of diseases, making them especially susceptible to pathogens (Murray Reference Murray2001). The role of introduced avian malaria in the decline and extinction of native Hawaiian birds is a paradigmatic example (van Riper III et al. Reference van Riper, van Riper, Goff and Laird1986, Jarvi et al. Reference Jarvi, Atkinson and Fleischer2001). The endemic bird’s susceptibility and the occurrence of suitable vectors and reservoirs determine the transmission of avian malaria caused by Plasmodium relictum in Hawaii (Lapointe et al. Reference Lapointe, Atkinson and Samuel2012). The virulence of this haemosporidian on Hawaiian avifauna is high, showing 50% mortality in hatch-year birds and 25% in adults (Atkinson and Samuel Reference Atkinson and Samuel2010). However, the Hawaiian thrushes appear to have a high tolerance to malaria, developing chronic disease with low parasitemia (Atkinson et al. Reference Atkinson, Lease, Drake and Shema2001), thus probably playing a role as reservoir of avian malaria for susceptible species. This role was also suggested for other thrush species in New Zealand (Tompkins and Gleeson Reference Tompkins and Gleeson2006) and Sao Miguel Island, Azores (Hellgren et al. Reference Hellgren, Krizanauskiene, Hasselquist and Bensch2011). In this sense, the identification of reservoir species (key-host species) to assess its potential impact on endemic ones is essential to determine if it is necessary to act by reducing or even removing their populations (Hellgren et al. Reference Hellgren, Krizanauskiene, Hasselquist and Bensch2011).
Disease caused by a novel parasite could be highly pernicious to the host, with susceptibility increased in the presence of other concurrent factors such as predation pressure (Kilpatrick Reference Kilpatrick2006). As commented above, the Juan Fernandez Firecrown is actually exposed to different stressors (habitat degradation and predators) which may reduce its resistance to parasites. In this sense, an additional threat to the Juan Fernandez Firecrown lies with the Austral Thrush, a recent coloniser of the Juan Fernandez Archipelago, which shares habitat with this endemic bird (Hahn et al. Reference Hahn, Vergara and Römer2011b). Previous studies have shown that Austral Trushes are commonly infected by different haemoparasites on the mainland (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). If this is also the situation on Juan Fernández Island then the occurrence of host-switching events between thrushes and other bird species is feasible. Another recent coloniser that could also operate as a reservoir is the Green-backed Firecrown, though this is perhaps unlikely due to the low prevalence of blood parasites exhibited by individuals of that species captured on the mainland (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). Although the Juan Fernandez Firecrown population appears subjected to several stressors and therefore might seem more vulnerable to the introduction of a novel parasite, other endemic bird species, like the Juan Fernandez Tit-Tyrant, may also be threatened by the arrival of pathogens.
The introduction of a novel parasite into a new ecosystem can produce unknown consequences for hosts (Martinez and Merino Reference Martínez and Merino2011) and study of the parasitic fauna infecting both endemics and recent colonisers is essential to determine the occurrence of novel parasites and their potential impact. In this sense, we have focused the present study on haemoparasites. Many of them (Plasmodium, Haemoproteus, Leucocytozoon and Trypanosoma) have previously been detected in the mainland populations of Austral Thrush and Green-backed Firecrown (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). Parasites within genera Isospora and Babesia are infrequently recorded in birds (Bennett et al. Reference Bennett, Whiteway and Woodworth-Lynas1982) but the lack of knowledge on the parasites present in the Juan Fernandez Archipelago prompt us to include them in the screening.
The objectives of the present study are: (i) determine the genera and diversity of haemoparasites infecting both endemic birds (Juan Fernández Firecrown and Juan Fernández Tit-Tyrant) and recent colonisers (Green-backed Firecrown and Austral Thrush) and (ii) assess the role of the latter (Green-backed Firecrown and Austral Thrush) as potential reservoirs of parasites.
Materials and methods
Capture and sampling of birds was conducted on the Robinson Crusoe Island for five days during January 2010 (14–18 January). The islands have a temperate oceanic climate. Data from the settlement's meteorological station indicate that average annual precipitation is 1,081 mm varying from 318 mm to 1,698 mm across the year. During late fall and the winter months (May to August) rainfall is higher than 100 mm (24 mm for January). We captured birds near to the CONAF (Corporacion Nacional Forestal) center located in San Juan Bautista village (S33°36.5', W78°50.5'). During the following four days the capture was performed in Plazoleta El Yunque (S33°38.975', W078°50.568'), a recreational area located inside the island's protected area as a National Park.
The birds were captured using mist nets. Once the nets were installed we checked them every five minutes to avoid excessive stress to the birds. All captures were performed between 08h00 and 13h00. The captured birds were weighed and measured (tarsus, wing, tail, and beak length). A slight puncture was performed on the tarsus or wing vein with a needle (0.5 mm). Approximately 20 µL of blood was taken to perform smears (see Merino et al. Reference Merino, Potti and Fargallo1997, Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008) and molecular analysis (stored on FTA cards, Whatman). Before releasing the birds, their claws were painted with white varnish to avoid resampling recaptured birds.
DNA analyses
Genomic DNA from samples stored in FTA cards was extracted according to Martínez et al. (Reference Martínez, Martínez-de la Puente, Herrero, del Cerro, Lobato, Rivero-de Aguilar, Vásquez and Merino2009). Partial amplification of the cytochrome b gene was accomplished by PCR using the non-specific primers PALU-F and PALU-R for detecting Haemoproteus/Plasmodium species (Martinez et al. Reference Martínez, Martínez-de la Puente, Herrero, del Cerro, Lobato, Rivero-de Aguilar, Vásquez and Merino2009) and primers Leunew1F (designed for this study, see Table 1) and LDRd for Leucocytozoon species (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). In addition, partial amplification of the 18S ribosomal RNA gene was performed using the primers Try-F and Try-R for detecting Trypanosoma species (designed for this study, see Table 1) and the generic primers hep900F and hep1615R for various hematic parasites as piroplasms, Hepatozoon, hemococcidians, and hematic stages of intestinal coccidians (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008; the primer hep800F mentioned by these authors is identical to primer hep900F). The sequences of the primers, size of the amplicons, and PCR conditions are shown in Table 1. All amplicons obtained after PCR assays were recovered from agarose gels and subjected to direct sequencing using an ABI 3730 XL automated sequencer (Applied Biosystems). To prevent contamination, we used different sets of pipettes and filter tips for extraction, PCR set up and downstream fragment analyses. DNA extraction and PCR set up were always performed in different laminar flow cabinets. We never amplified DNA from negative controls added in each PCR batch. A positive control for each pair of primers was routinely used.
Table 1. Pairs of primers used in the screening in the present study (PaluF/PaluR, Leunew1F/LDRd, TryF/TryR, and Hep900F/Hep1615R).

As the reference sequences deposited in GenBank are larger than the amplicons obtained with the primers used in the screening, we obtained a larger DNA fragment of the 18S ribosomal RNA gene by using the primers hep50F/EimRodR and EimRodF/hep1615R for Isospora and NBA1-bab/hep1615R for Babesia (Table 2). These longer fragments allow us to perform a more powerful phylogenetic analysis. Identity analysis was performed using BioEdit software (Hall Reference Hall1999).
Table 2. Pairs of primers used to obtain a larger 18S ribosomal RNA gene fragment for Isospora and Babesia (Hep50F/EimRodR, EimRodF/Hep1615R, and NBA1bab/Hep1615R).

Phylogenetic analysis
The sequences used in the phylogenetic analysis were obtained from the MalAvi database (2013) or GenBank. We selected Plasmodium and Leucocytozoon haplotypes isolated in continental Chile and lineages involved in the decline of some bird populations in other parts of the world (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008; Bensch et al. Reference Bensch, Stjernman, Hasselquist, Ostman, Hansson, Westerdahl and Pinheiro2000). In addition, we selected Babesia haplotypes isolated from birds and mammals, and Eimeria and Isospora haplotypes isolated from birds.
The alignments were performed using the MUSCLE algorithm implemented on the website of the European Bioinformatics Institute (European Bioinformatics Institute 2012). The Gblocks program (Castresana Reference Castresana2000, Talavera and Castresana Reference Talavera and Castresana2007) was used to eliminate poorly aligned positions and divergent regions of the 18S RNA alignments or to establish the tails of the cytochrome b alignment. Using the cytochrome b sequence AY099045 from Haemoproteus majoris (1123 bp) as a reference, primers Palu F/R amplify from position 403 to 793 and primers LeunewF1/LDRd from 404 to 743. Primers hep50F/hep1615R amplify from position 81 to 1634 (using as a reference 18S rRNA gene from Isospora gryphoni; AF080613; 1797 bp) and primers NBA1bab/hep1615R amplify from position 137 to 1559 (using as a reference 18S rRNA gene from Babesia sp. EU1; AY046575; 1727 bp). Plasmodium/Leucocytozoon, Babesia and Isospora/Eimeria alignments contained 421, 1396, and 1460 sites, respectively. The alignments were analysed using Bayesian inference implemented in the program MrBayes v3.2 (Ronquist and Huelsenbeck Reference Ronquist and Huelsenbeck2003). The analysis consisted of two runs of four chains each, with 2,000,000 generations per run and a burn-in of 500,000 generations (30,000 trees for consensus tree) for Plasmodium-Leucocytozoon species, 3,000,000 generations per run and a burn-in of 750,000 generations (45,000 trees for consensus tree) for Babesia species, and 200,000 generations per run and a burn-in of 50,000 generations (3,000 trees for consensus tree) for Isospora-Eimeria species. The substitution model (nst=2 and rates=invgamma) was selected using MEGA5 software (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). The final standard deviation of the split frequencies was < 0.01. Convergence was checked using the Tracer v1.5 software (Rambaut and Drummond Reference Rambaut and Drummond2007). All of the model parameters were higher than 100.
Results
Data on bird species captured in the present study are shown in Table 3. No individual was recaptured. Only six birds were found infected by microscopic examination of blood smears. Two tit-tyrant individuals were infected by trypanosomes and another two by both trypanosomes and Leucocytozoon, while one Austral Thrush appeared infected by Plasmodium and other by Leucocytozoon parasites. In all cases intensity of infection was low (less than 1 parasite per 10,000 erythrocytes). All infections detected by microscopy were also detected by molecular analyses.
Table 3. Bird species captured in the present study.

The molecular analysis showed that all sampled Austral Thrushes were parasitized by at least one haemoparasite species (see Table 4). Leucocytozoon was found in all individuals (100%), Plasmodium in seven (87.5%), Babesia in five (62.5%), Isospora in three (37.5%), and Trypanosoma in two (25%). The Juan Fernández Tit-Tyrant was the second most frequently parasitized species. Four of the seven captured individuals were infected by at least one haemoparasite species (see Table 5). Trypanosoma was detected in four individuals (57%), Leucocytozoon in two (28%), and Plasmodium in just one of them (14%). In contrast, only two Green-Backed Firecrown individuals were parasitized (4.5%; in all cases by Trypanosoma) and the Juan Fernández Firecrowns were not found infected by haemoparasites. We did not find any bird parasitized by Haemoproteus.
Table 4. Haplotypes of the parasites found in each Austral Thrush individual.
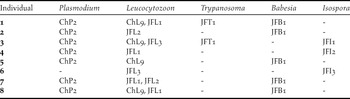
Table 5. Haplotypes of the parasites found in each Juan Fernández Tit-Tyrant individual.
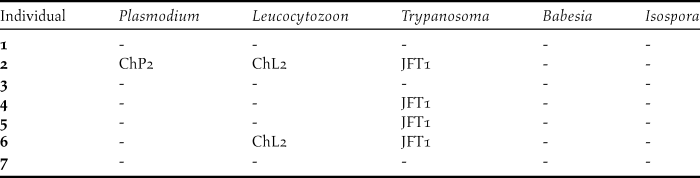
Only one haplotype was detected for Plasmodium (haplotype ChP2 with 331 bp), Trypanosoma (haplotype JFT1 with 100bp) and Babesia (haplotype JFB1 with 1350 bp). The Plasmodium haplotype was previously isolated from Austral Thrushes captured on the continent (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008) but also in other thrush species such as T. migratorius and Hylocichla mustelina from the USA (Ricklefs and Fallon Reference Ricklefs and Fallon2002, Martinsen et al. Reference Martinsen, Waite and Schall2007, Reference Martinsen, Perkins and Schall2008) and T. rufiventris from Uruguay (Durrant et al. Reference Durrant, Beadell, Ishtiaq, Graves, Olson, Gering, Peirce, Milensky, Schmidt, Gebhard and Fleischer2006). The Trypanosoma haplotype isolated in the present study had 100% identity with T. avium. However, the Babesia haplotype was detected for the first time, being Babesia kiwiensis the closest species (98% identity). On the other hand, we detected three and five haplotypes belonging to Isospora and Leucocytozoon genera, respectively. The Isospora haplotypes JFI1 and JFI2 (1522 and 1486 bp, respectively) differed in only one base but the haplotype JFI3 (725bp) showed a 98.8% identity with each JFI1 and JFI2. Three Leucocytozoon haplotypes (JFL1, JFL2 and JFL3) were detected for first time. The haplotypes JFL1 and JFL2 (301 bp) differed in just one nucleotide but their genetic identities with the haplotype JFL3 (283 bp) were close to 87%. However, the other two Leucocytozoon haplotypes (ChL9 and ChL2) were previously isolated from birds captured on the continent, the first from Austral Thrushes and the second from several bird species which do not inhabit the archipelago (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). The haplotypes isolated from each individual are shown in Tables 4 and 5. The geographical distribution of the haemosporidian haplotypes detected in the Juan Fernández birds is indicated in Figure 1.
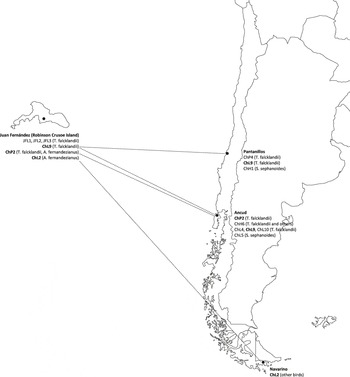
Figure 1. Geographical distribution of the haemosporidian haplotypes detected in Juan Fernández forest birds from both Robinson Crusoe Island and continental Chile. The dotted lines indicate the hypothetical origin of the haplotypes. The haplotypes JF were exclusively detected on the island. The haplotypes marked in bold were detected on both the island and continent. The rest of haplotypes were exclusively detected on the continent. The size of Robinson Crusoe Island is arbitrarily enlarged with respect to the American continent.
Phylogenetic analysis
The Plasmodium haplotype ChP2 isolated from Austral Thrushes was clustered together with the European haplotype Padom16, which corresponds to the morphospecies Plasmodium rouxi, and the American haplotype ChP4 (Figure 2).
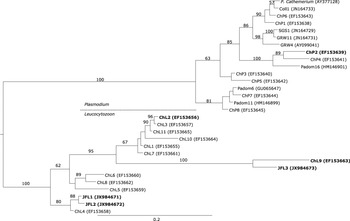
Figure 2. Phylogenetic analysis of the Plasmodium and Leucocytozoon haplotypes detected on the Juan Fernández Archipelago and continental Chile. Bayesian inference implemented in Mr Bayes program was used to build the tree. There were a total of 421 positions in the final dataset. The haplotypes detected in the present survey are in bold.
The novel Leucocytozoon haplotypes JF1 and JF2 were grouped together with haplotype ChL4 which was previously isolated from Austral Thrushes captured in continental Chile (Figure 2). In addition, the novel haplotype JFL3 was closely related to the haplotype ChL9 isolated from Austral Thrush and forming a sister group with six American haplotypes included in the tree (Figure 2). The haplotype ChL2 exclusively detected in Juan Fernández Tit-Tyrants was clustered together with other American Leucocytozoon haplotypes but it was not closely related to the haplotypes isolated from other birds in the archipelago (Figure 2).
The only haplotype belonging to Babesia genus formed a well-supported cluster together with Babesia kiwiensis (Figure 3). However, it was not related to other Babesia species isolated from seabirds (B. bennetti and B. poelea). The Isospora haplotypes JFI1 and JFI2 were clustered together with I. robini which was previously isolated from American Robin Turdus migratorius. However, the haplotype JFI3 was located as sister group of the latter clade with a low support (Figure 4). As we could only obtain a short DNA fragment for Trypanosoma (100 bp of the 18S ribosomal RNA gene) we did not build a phylogenetic tree. Nevertheless, as mentioned above this fragment showed 100% identity with T. avium.
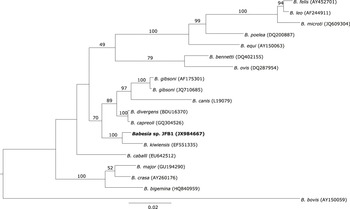
Figure 3. Phylogenetic analysis of the Babesia haplotype isolated from Austral Thrush. Bayesian inference implemented in Mr Bayes program was used to build the tree. There were a total of 1,396 positions in the final dataset. Haplotypes detected in the present survey are in bold.
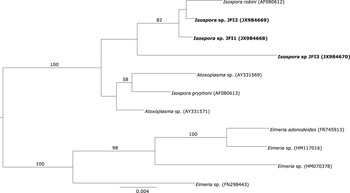
Figure 4. Phylogenetic analysis of the Isospora haplotypes isolated from Austral Thrush. Bayesian inference implemented in Mr Bayes program was used to build the tree. There were a total of 1,460 positions in the final dataset. Haplotypes detected in the present survey are in bold.
Discussion
Our results indicate that the Austral Thrush is a key-host species for the bird blood parasite community due to its high parasite prevalence and diversity. In fact, this species seems to be the potential source for two host-switching events involving Plasmodium and Trypanosoma parasites. Apparently, the most threatened endemic, Juan Fernandez Firecrown, is free of haemoparasites. However, we cannot rule out the possibility that infections were lethal for these birds, as we only captured healthy individuals. The endemic Juan Fernandez Tit-Tyrant appears affected by the hypothetical parasite switches. The Plasmodium haplotype ChP2 and Trypanosoma were detected in both host species, Austral Thrush and Juan Fernandez Tit-Tyrant. The haplotype (ChP2) was previously detected with a high prevalence (75%) in continental thrushes in the Chilean locality of Ancud (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). In addition, it seems to be host-specific because it was not detected in 14 other bird species sampled in the same locality, including tit-tyrants closely related to the endemic species on Robinson Crusoe Island (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). Thus, a recent host-switching event would be the most plausible explanation for the presence of this parasite in Juan Fernandez Tit-Tyrants. On the other hand, parasites belonging to the genus Trypanosoma were found in Juan Fernández Tit-Tyrants (prevalence 57%), Austral Thrushes (prevalence 25%), and Green-backed Firecrowns (prevalence 4.5%) inhabiting Robinson Crusoe Island. Trypanosomes also infect Austral Thrushes on the mainland but apparently no Green-backed Firecrowns or other species of tit-tyrants (authors’ unpubl. data, based on microscopic examination of blood smears). This fact points to an introduction of this parasite to the island by Austral Thrushes. At the moment, the impact of Plasmodium and Trypanosoma parasites on the stability of the Juan Fernández Tit-Tyrant population remains unknown. However, this bird species is presently suffering from a severe population decline (Hahn et al. Reference Hahn, Römer and Schlatter2006). As Trypanosoma infection is related with poor development and immune responses in nestlings of some passerine species (Merino et al. Reference Merino, Potti and Moreno1996, Martínez-de la Puente et al. Reference Martínez-de la Puente, Martínez, Rivero-de-Aguilar, del Cerro and Merino2013), it will be necessary to assess the virulence of these parasites by measuring the mortality caused on hatch-year and adult birds (see Atkinson and Samuel Reference Atkinson and Samuel2010).
Four Leucocytozoon haplotypes were detected in the Austral Thrushes. One of them (haplotype ChL9) was previously found in continental individuals (Pantanillos and Ancud localities; Fig. 1; see Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008) and three of them were detected for the first time in the archipelago (JFL1, JFL2 and JFL3). However, the haplotype ChL2 was only detected in Juan Fernández Tit-Tyrant. This haplotype was previously detected in some bird species from the mainland (Navarino locality; Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008) but not in Austral Thrushes or in the close relative Tufted Tit-Tyrant Anairetes parulus. Thus, its origin on the island is uncertain.
In a recent study performed on Sao Miguel Island (Azores) on the haemosporidians present in a bird community, the authors described some parasitological aspects similar to those found in the present survey (Hellgren et al. Reference Hellgren, Krizanauskiene, Hasselquist and Bensch2011). First, the Eurasian Blackbird Turdus merula was the main bird species harbouring haemoparasites, exhibiting the highest prevalence and parasite diversity. Second, most of the other sampled bird species were uninfected and only three of the 11 sampled bird species presented only one infected individual. However, these species showed higher prevalence when they were sampled in continental Europe. In our case, Green-Backed Firecrown and Austral Thrush are the only two species sampled that inhabit the archipelago and the continent, and their parasite diversity is higher on the mainland (see Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008 and Pérez-Rodríguez et al. Reference Pérez-Rodríguez, Ramirez, Richardson and Pérez-Tris2013 for a similar result on other islands). Third, Haemoproteus has not been found on the islands, possibly indicating a lack of suitable vectors.
The occurrence of the Austral Thrush as a parasite reservoir appears to be a threat to the two endemic Juan Fernández species, an actual threat to the Juan Fernández Tit-Tyrant and a potential threat in the case of the Juan Fernández Firecrown. Additionally, the well-known effects of habitat degradation and the pressure exerted by alien predators could play a role as immunosuppressive agents for the endemic birds (Kilpatrick Reference Kilpatrick2006). In fact, in a stress context some Plasmodium haplotypes could become pathogenic as previously reported in New Zealand for the system P. elongatum / Saddleback Philesturnus carunculatus (Alley et al. Reference Alley, Hale, Cash, Ha and Howe2010). Paradoxically, the same Austral Thrushes could play a role in the system as a stressor agent, promoting the displacement of native plants by dispersing foreign seeds and acting as predators at least to the Juan Fernández Firecrown (Smith-Ramírez et al. Reference Smith-Ramírez, Arellano, Mora, Hagen, Vargas and Miranda2013). Habitat loss and degradation on oceanic islands are not only leading to population decline of endemic birds but also facilitating the spread of invasive bird species (Catry et al. Reference Catry, Mellanby, Ali Suleiman, Haji Salim, Hughes, McKean, Anderson, Constant, Heany, Martin, Armitage and Wilson2000, Byers Reference Byers2002). Furthermore, an increase in Austral Thrush population density together with the low bird biodiversity present in the island (low dilution effect) would be key factors in enhancing parasite transmission (Martínez and Merino, Reference Martínez and Merino2011).
Some examples of introduction of avian blood parasites to islands with severe consequences for endemic avifauna exist. In these cases the bird host is as relevant as the parasitic specificity of the introduced lineages (Ewen et al. Reference Ewen, Bensch, Blackburn, Bonneaud, Brown, Cassey, Clarke and Pérez-Tris2012) and the vectors present in the colonised region. In this sense, the best example is the introduction of infected sparrows in Hawaii causing a population decline of native birds (van Riper III et al. Reference van Riper, van Riper, Goff and Laird1986). The low host specificity showed by the Plasmodium haplotype introduced by sparrows (GRW4) was an essential factor in facilitating the switch to native species. However, its transmission to native birds was impossible until the main competent vector for alien Plasmodium lineages, Culex quinquefasciatus, was introduced by humans at the beginning of 19th century (Hardy Reference Hardy1960, Warner Reference Warner1968). In New Zealand, this vector has spread over the past three decades and recent studies have showed a strong relationship between the avian malaria infections and the geographical distribution of the vector (Tompkins and Gleeson Reference Tompkins and Gleeson2006). In this case, one of the main reservoirs of infection to native species is also the non-native Eurasian Blackbird.
In conclusion, our results suggest that the Austral Thrush is a key-host species in the island as it showed both high haemoparasitic diversity and prevalence. The Juan Fernández Tit-Tyrants are infected with parasites possibly introduced by the thrushes. Although the endemic Juan Fernandez Firecrown is free of haemoparasites, the Austral Thrush is a potential threat due to its role as a reservoir. In the near future it would be very interesting (i) to check the virulence of the introduced parasites on the fitness of the Juan Fernández Tit-Tyrants, (ii) to perform a survey of the haemoparasites present in the recently introduced House Sparrow Passer domesticus, (iii) to identify potential vectors to assess a hypothetical parasite switch event to the endemic birds, and (iii) to establish a surveillance programme in order to control potential parasite switches and / or increases in parasite prevalence.
Acknowledgements
During the preparation of this work, J.M. and S.M. were supported by project CGL2009-09439 from the Spanish Ministry of Science and Technology, projects CGL2012-40026-C02-01 and 02 from the Spanish Ministry of Economy and Competiveness and the Fundación BBVA project BIOCON06/109. The joint project 2009CL0025 (CONICYT-CSIC 2009-137) between CSIC (Spain) and CONICYT (Chile) helped to cover travel and accommodation to Juan Fernández archipelago. Research has been supported by FONDECYT-Chile 1090794, FONDECYT 1140548 and the Institute of Ecology and Biodiversity (ICM-P05-002-Chile, and PFB-23-CONICYT-Chile), to R.A.V.











