No CrossRef data available.
Article contents
Feasibility and acceptability of intranasal povidone iodine decolonization among orthopedic trauma surgery patients
Published online by Cambridge University Press: 16 May 2022
Abstract
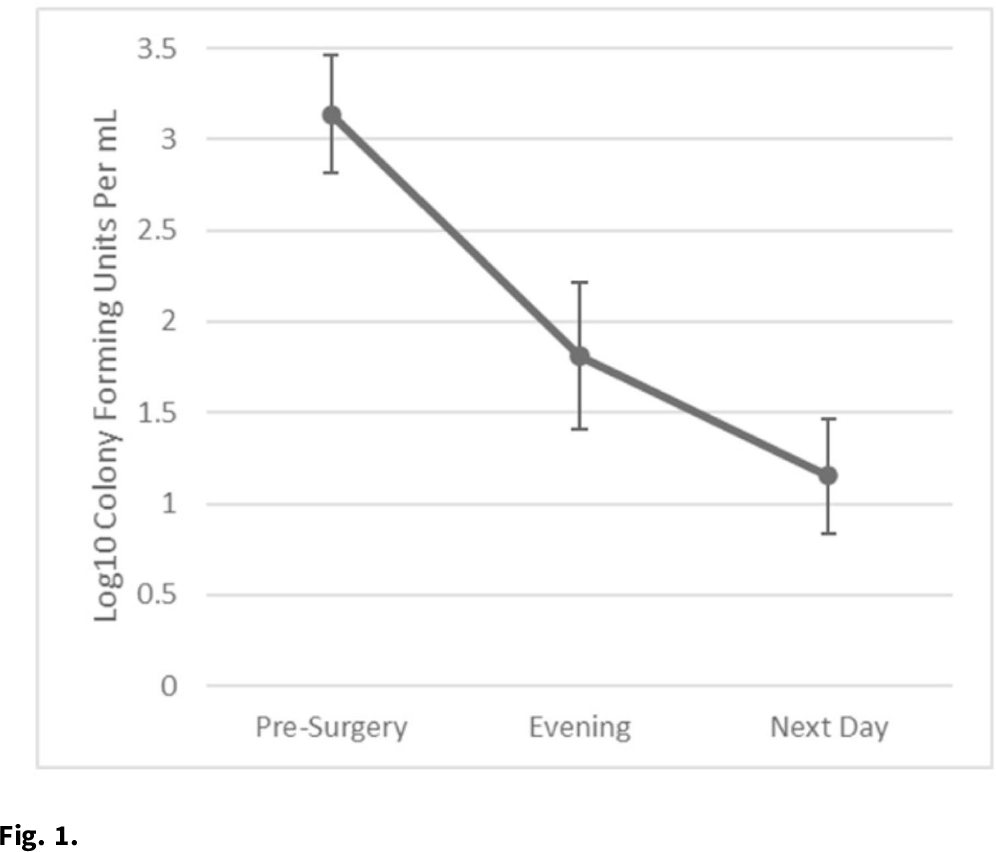
Background: Nasal decolonization significantly decreases the incidence of Staphylococcus aureus surgical-site infections (SSIs). Patient adherence with self-administration of a decolonization ointment (ie, mupirocin) is low, especially among patients having urgent surgery. Povidone-iodine decolonization may overcome patient adherence challenges because povidone-iodine needs to be applied only on the day of surgery. We assessed the effectiveness and acceptability of povidone-iodine decolonization given on the day of surgery among patients having orthopedic trauma surgery. Methods: Adult patients who underwent operative fixation of traumatic lower extremity fractures were consented to receive 10% intranasal povidone-iodine solution. Povidone-iodine was applied ~1 hour before surgical incision and was reapplied the evening after surgery. Patients were tested for S. aureus nasal colonization before surgery, the evening after surgery (before povidone-iodine reapplication), and the day after surgery. Swabs were inoculated into Dey-Engley neutralizer and processed in a vortexer. A series of dilutions were performed and plated on mannitol salt agar plates. S. aureus cultures were quantitatively assessed to determine the reduction in S. aureus after povidone-iodine use. Reductions in S. aureus nasal growth were evaluated using the Skillings-Mack test. SSIs manifesting within 30 and 90 days of surgery were identified using NHSN definitions. A survey was administered the morning after surgery to determine the acceptability of intranasal povidone-iodine. Results: In total, 51 patients participated in this pilot study between February 2020 and June 2021. Nasal samples from 12 participants (23.5%) grew S. aureus. The S. aureus concentration decreased significantly across the time points (P = .03) (Fig. 1). No SSIs were identified within 30 days of surgery. One SSI occurred within 90 days of surgery; this patient did not carry S. aureus, and cultures from the infected site were negative. Also, 31% of patients reported at least 1 mild side effect while using povidone-iodine: dripping (n = 7), itching (n = 6), dryness (n = 4), stinging (n = 4), staining (n = 3), unpleasant taste (n = 3), runny nose (n = 2), burning (n = 1), sneezing (n = 1), sore throat (n = 1), tickling (n = 1), and/or cough (n = 1). Also, 86% of patients stated that povidone-iodine felt neutral, pleasant, or very pleasant, and only 14% stated that it felt unpleasant or very unpleasant. Discussion: In this pilot study, 2 applications of nasal povidone-iodine on the day of surgery were acceptable for patients, and this protocol significantly reduced S. aureus concentration in nares of patients. Future large clinical trials should evaluate whether this 2-application regimen of povidone-iodine significantly decreases rates of SSI among orthopedic trauma surgery patients.
Funding: PDI Healthcare
Disclosures: None
- Type
- SSI
- Information
- Antimicrobial Stewardship & Healthcare Epidemiology , Volume 2 , Issue S1: SHEA Spring 2022 Abstracts , July 2022 , pp. s62 - s63
- Creative Commons
- This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Copyright
- © The Author(s), 2022. Published by Cambridge University Press on behalf of The Society for Healthcare Epidemiology of America




