Introduction
The Baltic Sea ice is one of the key components in the North European climate system and in the physical and ecological environments in the area. The Baltic Sea is connected with the North Sea by a narrow and shallow strait and is therefore characterized as a semi-enclosed brackish water basin where sea ice occurs annually. The salinity of the Baltic is controlled by the balance between the inflow of saline water from the North Sea and of fresh water from riverine input and precipitation. The greater the distance from the North Sea, the lower the salinity. As a brackish water basin, the Baltic Sea provides excellent conditions to study specific physical phenomena, especially the role of salts in freezing processes.
Studies on ice structure have been conducted in both fast-ice and pack-ice areas in the Baltic Sea. Reference PalosuoPalosuo (1963) observed the snow depth and the thickness of different ice layers and took ice samples to determine the structure and salinity of ice at many stations on the Baltic Sea coast during three winters. Reference Gow, Weeks, Kosloff and CarseyGow and others (1992) studied the planar/ non-planar structure of the ice/water interface for ice samples in the northernmost Baltic basin, the Bay of Bothnia. Ice crystallography structures were observed along the Hanko Peninsula in the Gulf of Finland where the surface water salinity is 0.5−6.1 psu (practical salinity units) (E. Palosuo and others, unpublished data). Studies in pack-ice areas have focused mainly on the deformation, dynamics and remote-sensing analyses, including ice thickness and structure in the Bay of Bothnia (e.g. Reference LeppärantaLeppäranta, 1981,Reference Leppäranta1987; Reference Askne, Leppäranta and ThompsonAskne and others, 1992; Reference Gow, Weeks, Kosloff and CarseyGow and others, 1992).
A Finnish-Japanese cooperative research programme entitled "Ice Climatology of the Okhotsk and Baltic Seas" gave us an opportunity to study time-series observations of ice growth and structure at the Hanko Peninsula in the Gulf of Finland. The first series was collected in winter 1999. The aim was to obtain time-series data of snow and ice structure and to understand the development of snow and ice in the brackish water. One particular question was the formation of snow ice. This paper reports the results from this investigation of ice structure and properties, and discusses different growth processes.
Observation Area and Methods
Ice observations were conducted once a week from 20 January at the onset of freeze-up until 12 April 1999. A total of 13 ice samples were collected during the period with an auger drill, a chain-saw and a handsaw on the fixed fast-ice area at Santala Bay, near the mouth of the Gulf of Finland (Fig. 1). These samples were analyzed for crystal structure, salinity and oxygen isotopes. An approximately 50 g snow sample from the near-surface layer and 50−100 mL water samples from the surface and ice/water interface were taken for salinity and oxygen isotope measurements.

Fig. 1. Location of the santala bay sampling site.
The ice samples were put into plastic bags and kept at −20°C in a storage chamber at Tvarminne Zoological Station, University of Helsinki, and analyzed in March and August in Tvarminne and at the State Technical Research Centre (VTT), Espoo, respectively. In the cold room, each sample was split lengthwise to obtain 5 mm thick vertical sections along the entire length. Horizontal thick sections were also produced at selected depths. These thick sections allowed us to examine bubble and brine layer distributions under scattered light. Then the sections were smoothed by planing to a thickness of < 1 mm and illuminated between crossed polarizers to identify individual grains and their structures. Analysis for c-axis orientation was conducted on grains on the horizontal thin sections with a universal stage. The entire length of the sample was cut at about 5 cm intervals as near as possible to structural boundaries. Then the pieces were melted in plastic bags for determination of the salinity with a conductivity meter (Radiometer, CDM 83 at Tvarminne, and Schott handylab LF1 at Espoo). The standard sea-water formula was used to determine the salinity from the conductivity (Reference Fofonoff and MillardFofonoff and Millard, 1983; Reference FofonoffFofonoff, 1985) to an accuracy of 0.1 psu. The oxygen isotope composition (δ 18O) of the ice and snow samples was determined with a mass spectrometer (Finnigan MAT Delta E, accuracy of 0.1‰) at the Institute of Geology, Tallinn Technical University, Estonia.
Results and Discussion
Temporal changes in the thickness of the snow, granular ice and columnar ice are illustrated in Figure 2. The water-level elevation was not measured, but was estimated to be near the snow/ice interface by assuming snow and ice densities of 300 and 900 kg m−3, respectively. The ice thickness increased at a constant rate from 18 cm on 20 January to 41 cm on 10 February, and thereafter remained almost constant until 24 February. Then the thickness gradually increased again to a maximum of 53 cm on 24 March. Thereafter, the ice decayed abruptly to 12 cm on 12 April.
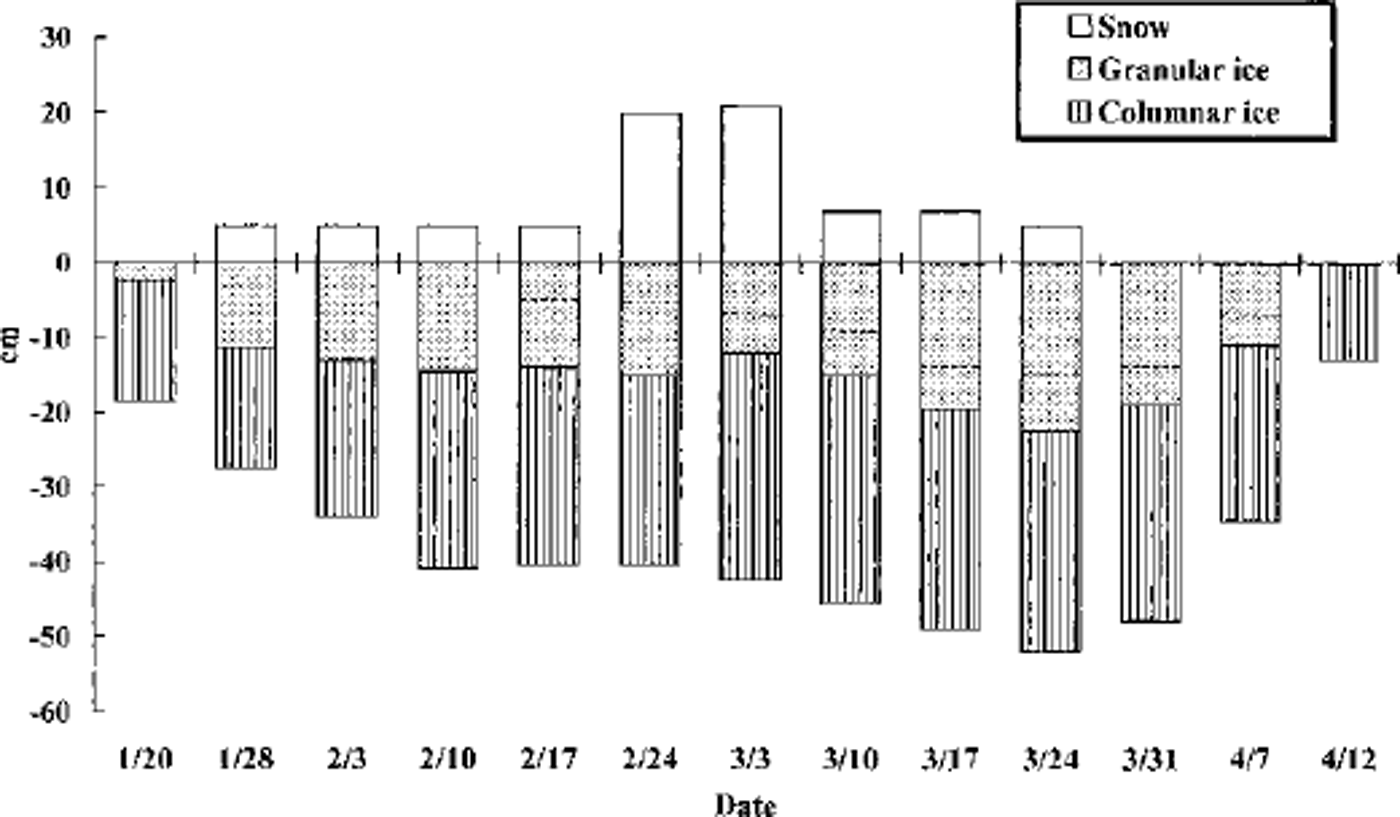
Fig. 2. Temporal changes of snow depth, and granular-ice and columnar-ice thicknesses during the sampling period. the snow/granular-ice interface is shown as the reference level. the dashed line in the granular ice indicates the boundary between superimposed ice and snow ice. bates are month/day.
Photographs of vertical thin sections are shown in Figure 3. All samples contained ice of both granular and columnar structure. The former occupied the upper layer and accounted for approximately 30−40% of the entire ice thickness, while the latter involved the lower layer. The columnar ice had a characteristic sea-ice structure, i.e. a preferred horizontal c axis, jagged grain boundaries and a substructure within the grains associated with brine layers.

Fig. 3 Photographs of vertical thin sections of the ice samples at santala bay on (a) 28 january, (b) 17 february, (c) 3 march, (d) 17 march and (e) 31 march.
Figure 4 shows profiles of salinity and oxygen isotopic composition of the snow, ice and water samples. The δ18O values of the snow cover were lower than −10%, and, in the minimum, exceeded −20‰, being similar to those in Arctic regions (Reference DansgaardDansgaard, 1964). The water salinity was 2−4 psu.

Fig. 4. Profiles of salinity and oxygen isotoPic composition of snow, ice and water samples at santala bay on (a) 28january, (b) 17 february, (c) 3 march, (d) 17 march and (e) 31 march.
The above-mentioned crystal structure reflected a clear difference in the salinity and in the isotopic composition (Fig. 4). The salinity of the granular ice was higher than that of the columnar ice, implying that the mechanisms of entrapment of brine in the two ice types are quite different. In columnar ice, brine is mostly rejected during the ice growth, whereas in granular ice brackish water is incorporated into the pore spaces between the snow grains. The filling of pores with brine in permeable snow is likely to lead to higher salinities than brine incorporation in columnar ice. Granular ice had higher negative δ18O values than did the columnar ice, possibly because of the effect of snow cover which had extremely large negative values.
The δ18O data are used to determine the fraction of snow contributing to the granular layers and to the entire ice thickness (Reference Jeffries, Shaw, Morris, Veazey and KrouseJeffries and others, 1994). Their simplified version of the original model by Reference Lange, Schlosser, Ackley, Wadhams and DieckmannLange and others (1990) has the form
where fs is the snow fraction, fsw is the sea-water fraction of the granular ice, and 6 is the mean δ 18O value of the granular-ice layer for which the snow fraction is being determined.
For δs, we use measured δ18O values of the snow on each sampling day. The δ18o values of bottom columnar ice were about 2‰ higher than those of surface sea water of about −9‰, which might be related to the isotopic fractionation that occurs during freezing. The fractionation factor is comparable to the values obtained previously (e.g. Reference Craig and HornCraig and Horn, 1968; Reference O’NeilO’Neil, 1968). Therefore, taking into account the fractionation effect, we adopt the measured δ 18O values of bottom ice at each sampling as δsw.
We tried to apply the equations to the early five samples with 518O data. The fs values calculated according to the model were 14−74% of the granular-ice thickness. The ratio of the granular-ice thickness to the entire thickness was estimated at 14−42%. Multiplying fs by this ratio, the snow fraction of the total ice thickness, Fm, is calculated at 2−26%. These Fm values are comparable to or larger than the values estimated for Antarctic sea ice (Reference Lange, Schlosser, Ackley, Wadhams and DieckmannLange and others, 1990; Reference Eicken, Lange, Hubberten and WadhamsEicken and others, 1994; Reference Jeffries, Shaw, Morris, Veazey and KrouseJeffries and others, 1994, Reference Jeffries, Worby, Morris and Weeks1997), where maximum snow contribution was 14−16% in summer ice in the Bellingshausen and Amundsen Seas Reference Jeffries, Worby, Morris and WeeksJeffries and others, 1997). Therefore, in spite of the limited data, they indicate that a significant amount of snow contributes to the development of the sea ice in years such as 1999 when large snow accumulations occurred in this region.
The whole upper layer of granular ice was composed of very uniform and small grains in the early samples obtained during the period 20 January−3 February (Fig. 3a). However, after 17 February there was a structural boundary within the upper layer (Fig. 3b-e), with the ice layer above the boundary consisting of slightly larger and vertically elongated grains, while the underlying layer had smaller grains. The δ18O values for the granular ice with the smaller grains were between those for the water and the snow cover, whereas the granular ice containing the elongated grains had values similar to those in the snow cover (Fig. 4). This result suggests that the origins of the two granular-ice layers were different; the former was originated from a mixture of snow and sea water (i.e. snow ice), while the latter was formed from snowmelt water alone (i.e. superimposed ice) (Reference Jeffries, Shaw, Morris, Veazey and KrouseJeffries and others, 1994; Reference Kawamura, Ohshima, Takizawa and UshioKawamura and others, 1997). The growth of superimposed ice became significant when the ice developed. The maximum superimposed-ice thickness of 28% of the total ice thickness occurred on 17 March.
The ice thickness increased steadily during the period 3−24 March (Fig. 2). Intensive investigation of the ice-structure components shows that sea-ice growth during this period is not caused by columnar ice but by the upward growth of granular ice. This result also shows that the snow cover made a large contribution to sea-ice growth in this area. Moreover, comparing thin-section photographs (Fig. 3), we can conclude the upward ice growth is due primarily to superimposed-ice formation and secondarily to snow-ice formation.
Because superimposed ice is a result of melting and refreezing of snow cover, snowmelting is essential for its formation. Snowmelting could have been brought about by strong solar radiation, although the air temperature during this growth period was as low as 0°C at Tvärminne, about 10 km from Santala Bay. On the other hand, snow ice is formed when a snow layer soaked with sea water is refrozen. Therefore, a prerequisite for snow-ice formation is the depression of the snow/ice interface below the water level, i.e. a negative freeboard. We have observed negative freeboards on sampling days with thick snow cover (e.g. 24 February, 3 March), and would expect such conditions after heavy snowfalls.
A steady decrease in the total ice thickness was clearly observed after 24 March (Fig. 2). This occurs in both granular- and columnar-ice layers. The most plausible explanation in the case of granular ice is surface melting of the ice together with the snow cover as a result of a high air temperature above 0°C after 17 March, and, in the case of columnar ice, bottom melting due to an increasing water temperature after 26 March.
A closer inspection of the salinity and δ 18O profiles in Figure 4 shows that there is a sharp change in salinity at the boundary between the granular- and columnar-ice layers. The salinity of granular ice at the boundary decreased sharply both from 17 February to 3 March and from 17 to 31 March, while underlying columnar ice kept salinity almost constant or gradually decreasing (Fig. 5). This result suggests that brine drainage might occur mainly in the granular-ice but not in the columnar-ice layer. On the other hand, the δ 18O values of both granular and columnar ice did not change (Fig. 5), implying that the δ 18O values might be affected only by the solid part of the ice but not by brine.
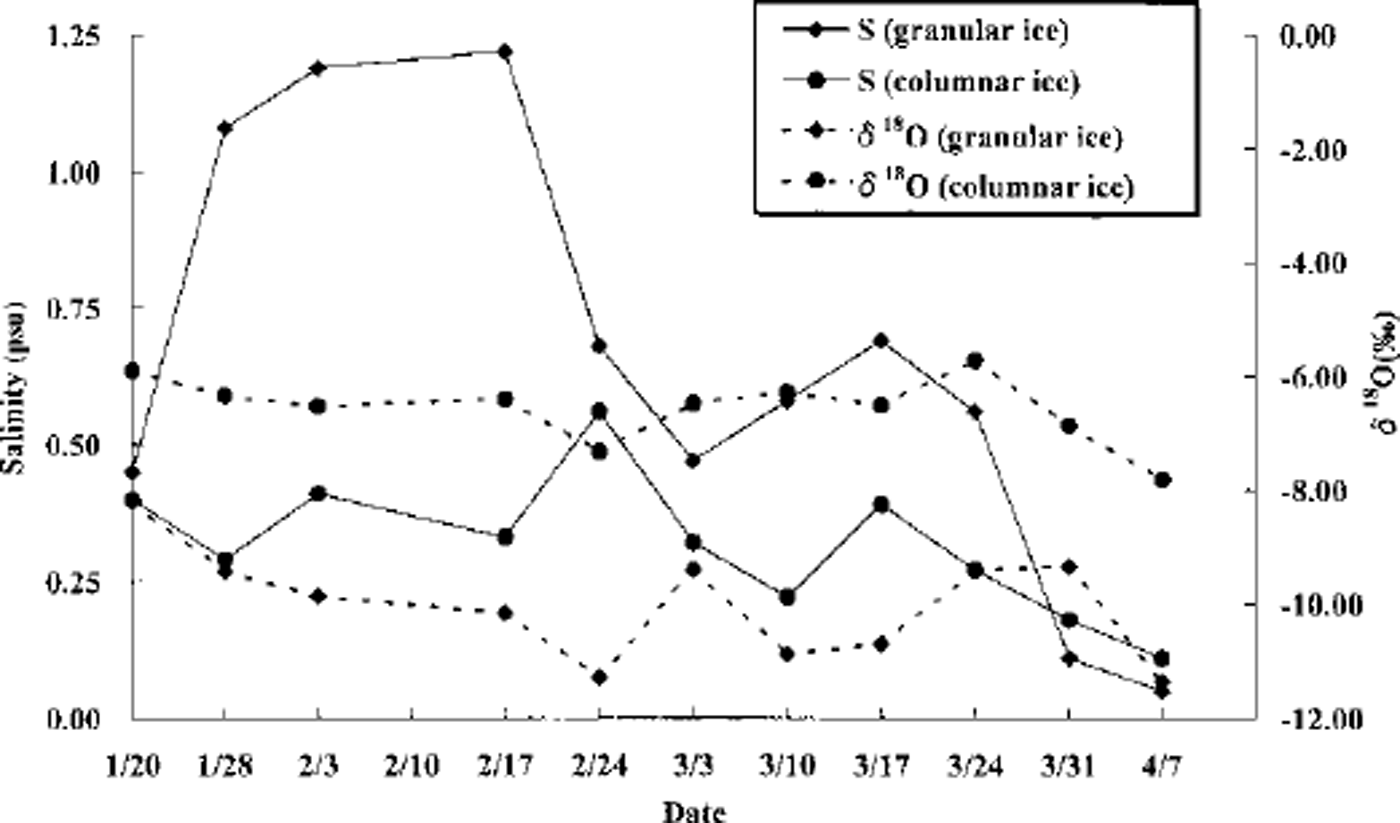
Fig. 5. Temporal changes of the salinity and oxygen isotopic composition in the granular- and columnar-ice layers at their boundary. dates are month/day.
Conclusions
Ice samples were collected once a week during the period January-April 1999 at Santala Bay near the mouth of the Gulf of Finland. Temporal variability in the ice structure as well as in the salinity and oxygen isotopic composition was examined.
The ice-structural analysis reveals that granular ice composed approximately one-third of the whole length and that it was made up of both snow ice and superimposed ice which grew upward. This result clearly shows a significant contribution from snow cover to sea-ice growth in this area.
The salinity of the granular ice was higher than that of the columnar ice. The salinity of granular ice at the granular-/columnar-ice boundary decreased sharply. These results imply that the mechanism of entrapment and retention of brine pockets may differ between the two ice types.
We propose further studies for the ice structure and properties in the brackish water as follows: Santala Bay provides excellent conditions to study temporal changes of ice properties in brackish water. Observations of ice should be carried out together with meteorological and oceanographic observations, preferably from the beginning of the ice season. Snow cover should be sampled more systematically.
Acknowledgements
We wish to thank the personnel of the Tvärminne Zoological Station for their kind permission to use their facilities. Thanks are extended to E. Uematsu for drawing. This work is a part of the project "Ice Climatology of the Okhotsk and Baltic Seas", financed by the Japanese-Finnish Bilateral Programs with the Japan Society for the Promotion of Science and the Academy of Finland, and also the Finnish Ministry of Trade and Industry and the Japanese Ministry of Education, Science, Sports and Culture (Monbusho) through grant-in-aid for scientific research.









