No CrossRef data available.
Article contents
Bounahasite, Cu+Cu2+2(OH)3Cl2, a new mineral from the Bou Nahas Mine, Morocco
Published online by Cambridge University Press: 09 December 2022
Abstract
The new mineral bounahasite, Cu+Cu2+2(OH)3Cl2, was found in the oxidation zone of the Bou Nahas Mine, Morocco. It forms pseudo-hexagonal plates up to 3 × 30 × 40 μm in size combined in loose clusters with native copper and paratacamite. The mineral is green with vitreous lustre. The cleavage is parallel to {110}, perfect. Dcalc is 3.90 g/cm3. The infrared spectrum is reported. The composition (wt.%) is Cu2O 23.26, CuO 51.72, Cl 23.36, H2O 8.71, O = Cl2 –5.27, total 101.78. The empirical formula calculated on the basis of 3 Cu atoms per formula unit is: Cu+Cu2+2(OH)2.97Cl2.03. The mineral is monoclinic, P21/n, a = 8.5925(1), b = 6.4189(1), c = 10.4118(2) Å, β = 111.804(2)°, V = 533.17(2) Å3 and Z = 4. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 7.71(70)($\bar{1}$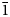 01), 5.34(22)(011), 3.856(100)(012, $\bar{2}$
01), 5.34(22)(011), 3.856(100)(012, $\bar{2}$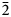 02), 2.673(36)(022), 2.665 (30)(103) and 2.350 (71)($\bar{1}$
02), 2.673(36)(022), 2.665 (30)(103) and 2.350 (71)($\bar{1}$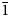 23, 301, $\bar{2}$
23, 301, $\bar{2}$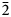 14). The crystal structure, refined from single-crystal X-ray diffraction data (R1 = 0.028), is based on two alternating sheets coplanar to (110): one consists of alternating edge-sharing Cu2+(OH)6 octahedra and two Cu2+(OH)4Cl2 octahedra, whereas the other one is based on Cu+Cl4 tetrahedra forming edge-sharing Cu+2Cl6 dimers.
14). The crystal structure, refined from single-crystal X-ray diffraction data (R1 = 0.028), is based on two alternating sheets coplanar to (110): one consists of alternating edge-sharing Cu2+(OH)6 octahedra and two Cu2+(OH)4Cl2 octahedra, whereas the other one is based on Cu+Cl4 tetrahedra forming edge-sharing Cu+2Cl6 dimers.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Owen Missen




