Article contents
The study of ultrasound-assisted extraction of flavonoids from Polygonum cuspidatum Sieb. et Zucc.
Published online by Cambridge University Press: 29 April 2019
Abstract
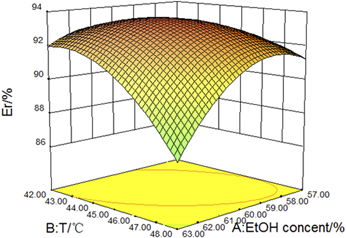
In this work, flavonoids in Polygonum cuspidatum Sieb. et Zucc. were extracted by ultrasound-assisted methodology and determined by ultraviolet–visible spectrophotometry. After that, extraction conditions were optimized by the single fact investigation, the central composite design, and response surface methodology (RSM) in turn. The results showed the optimal values of ethanol concentration, solid–liquid ratio, extraction temperature, extraction time, ultrasonic power, and number of extraction times were 60%, 1:20 (g/mL), 45 °C, 34 min; 80 W, and 5, respectively. The extraction ratio of flavonoids could be as high as 94.50%. The influence order of each factor was ultrasonic power > extraction time > extraction temperature > ethanol concentration. The results also showed that the experimental value was close to the predicted value (94.49%) of the established model by RSM, which proved that the established model was reasonable. The thermodynamic results showed that the extraction process was endothermic and could proceed spontaneously.
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 19: Focus Issue: Thermodynamics of Complex Solids , 14 October 2019 , pp. 3254 - 3262
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by




