Introduction
Cognitive deficits are a clinically significant feature among psychiatric disorders and often, in some combination, persist into remission (Millan et al. Reference Millan, Agid, Brüne, Bullmore, Carter, Clayton, Connor, Davis, Deakin, DeRubeis, Dubois, Geyer, Goodwin, Gorwood, Jay, Joëls, Mansuy, Meyer-Lindenberg, Murphy, Rolls, Saletu, Spedding, Sweeney, Whittington and Young2012). In major depressive disorder (MDD; Jaeger et al. Reference Jaeger, Berns, Uzelac and Davis-Conway2006;Withall et al. Reference Withall, Harris and Cumming2009) and schizophrenia (Green et al. Reference Green, Kern and Heaton2004), impairments are longitudinally predictive of poor functional outcome. Furthermore, cognitive improvements following pharmacological (Keefe et al. Reference Keefe, Bilder, Davis, Harvey, Palmer, Gold, Meltzer, Green, Capuano, Stroup, McEvoy, Swartz, Rosenheck, Perkins, Davis, Hsiao and Lieberman2007), neuropsychological (Fiszdon et al. Reference Fiszdon, Choi, Goulet and Bell2008) and psychosocial (Brekke et al. Reference Brekke, Hoe and Green2009) interventions in schizophrenia are associated with functional improvements, highlighting the potential that targeting neuropsychological deficits has in reducing disability.
At present, psychiatric pharmacotherapies are largely ineffective in ameliorating persisting cognitive deficits (Goldberg et al. Reference Goldberg, Goldman, Burdick, Malhotra, Lencz, Patel, Woerner, Schooler, Kane and Robinson2007). By contrast, cognitive rehabilitation seems to be more promising. Initial studies used operant conditioning procedures in improving behavioural performance (Cohen, Reference Cohen1956; Wagner, Reference Wagner1968). A second wave of studies in the early 1990s brought together a range of techniques under the collective term ‘cognitive remediation’ (CR). CR programmes are highly heterogeneous, differing on whether they consist of ‘drill-and-practice’ or compensatory strategy learning, therapist facilitation, computer training, or the length and intensity of treatment. Various structured programmes have codified combinations of these approaches, including the Neuropsychological and Educational Approach to Remediation (NEAR; Medalia et al. Reference Medalia, Revheim and Herlands2002).
The efficacy of CR has been well established in chronic schizophrenia (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). Neuropsychological functioning seems to benefit most from CR with mean effect sizes typically in the medium range (Medalia & Choi, Reference Medalia and Choi2009). Smaller effects are observed for functional improvements, which are reported to be mediated by gains in memory and executive functioning (Wykes et al. Reference Wykes, Newton, Landau, Rice, Thompson and Frangou2007; Fiszdon et al. Reference Fiszdon, Choi, Goulet and Bell2008). Recently, CR has been successfully applied to a range of other psychiatric disorders, including MDD (Elgamal et al. Reference Elgamal, Mckinnon, Ramakrishnan, Joffe and MacQueen2007; Naismith et al. Reference Naismith, Redoblado-Hodge, Lewis, Scott and Hickie2010b, Reference Naismith, Diamond, Carter, Norrie, Redoblado-Hodge, Lewis and Hickie2011), bipolar disorder (Deckersbach et al. Reference Deckersbach, Nierenberg, Kessler, Lund, Ametrano, Sachs, Rauch and Dougherty2010), anorexia nervosa (Wood et al. Reference Wood, Al-Khairulla and Lask2011), obsessive–compulsive disorder (Buhlmann et al. Reference Buhlmann, Deckersbach, Engelhard, Cook, Rauch, Kathmann, Wilhelm and Savage2006) and substance use disorders (Bickel et al. Reference Bickel, Yi, Landes, Hill and Baxter2011). Perhaps the basis for a potential ‘cross-diagnostic’ utility of CR is its focus on cognitive deficits readily found in psychiatric illnesses. Indeed, a recent study found that CR effectiveness was unrelated to diagnosis in schizophrenia-spectrum disorders (Lewandowski et al. Reference Lewandowski, Eack, Hogarty, Greenwald and Keshavan2011). The enthusiasm for cross-diagnostic implementation is reflected in studies using mixed psychiatric samples (Choi & Medalia, Reference Choi and Medalia2005; Naismith et al. Reference Naismith, Redoblado-Hodge, Hermens, Carter, Scott and Hickie2010a). Pragmatically, a cross-diagnostic implementation may prove to be more cost-effective in mental health clinics that are not constrained by diagnostic groupings. Indeed, this approach is particularly appropriate for younger patients in the early stages of psychiatric disease, where the longitudinal trajectory is not yet evident (McGorry et al. Reference McGorry, Hickie, Yung, Pantelis and Jackson2006; Hickie et al. Reference Hickie, Scott, Hermens, Naismith, Guastella, Kaur, Sidis, Whitwell, Glozier, Davenport, Pantelis, Wood and McGorry2012).
CR studies have generally focused on later stages of psychiatric illness (cf. Wykes et al. Reference Wykes, Newton, Landau, Rice, Thompson and Frangou2007). By contrast, identification and treatment at earlier stages are crucial, given the cumulative effects of mental disorders (McGorry et al. Reference McGorry, Hickie, Yung, Pantelis and Jackson2006; Hetrick et al. Reference Hetrick, Parker, Hickie, Purcell, Yung and McGorry2008), which are underscored by findings that longer durations of untreated illness are associated with structural brain abnormalities in MDD (Sheline et al. Reference Sheline, Gado and Kraemer2003) and schizophrenia (Lappin et al. Reference Lappin, Morgan, Morgan, Hutchison, Chitnis, Suckling, Fearon, McGuire, Jones, Leff, Murray and Dazzan2006; Malla et al. Reference Malla, Bodnar, Joober and Lepage2011), and neuropsychological deterioration (Amminger et al. Reference Amminger, Edwards, Brewer, Harrigan and McGorry2002; Lappin et al. Reference Lappin, Morgan, Morgan, Dazzan, Reichenberg, Zanelli, Fearon, Jones, Lloyd, Tarrant, Farrant, Leff and Murray2007; Gaynor et al. Reference Gaynor, Dooley, Lawlor, Lawoyin and O'Callaghan2009). Early intervention studies have largely focused on pharmacological (Kahn et al. Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe, Keet, Gheorghe, Rybakowski, Galderisi, Libiger, Hummer, Dollfus, Lopez-Ibor, Hranov, Gaebel, Peuskens, Lindefors, Riecher-Rossler and Grobbee2008) and cognitive behavioural (Lynch et al. Reference Lynch, Laws and McKenna2010) therapies, which typically affect state-dependent cognitive function but have little effect on pre-existing or persisting cognitive impairments. This is despite evidence demonstrating prominent memory deficits in emerging MDD (Naismith et al., unpublished observations) and prodromal psychosis (Fusar-Poli et al. Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes, Stieglitz, Vita, McGuire and Borgwardt2012), and broader neuropsychological dysfunction in first-episode MDD and schizophrenia across multiple cognitive domains (Mesholam-Gately et al. Reference Mesholam-Gately, Giuliano, Goff, Faraone and Seidman2009; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012). Taken together with findings that impairments seem to be related to higher rates of relapse and recurrence (Majer et al. Reference Majer, Ising, Kunzel, Binder, Holsboer, Modell and Zihl2004), the early treatment of persisting cognitive impairment may prove to be an important step in reducing the everyday impact and chronicity of mental disorders. To this end, several studies have demonstrated the effectiveness of CR in improving neurocognition in early-onset adolescents with psychosis (Ueland & Rund, Reference Ueland and Rund2004, Reference Ueland and Rund2005; Wykes et al. Reference Wykes, Newton, Landau, Rice, Thompson and Frangou2007), and also in combination with functional gains and neuroprotection in ‘early-course’ schizophrenia (Eack et al. Reference Eack, Greenwald, Hogarty, Cooley, DiBarry, Montrose and Keshavan2009, Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010). There are also two CR trials currently being conducted in first-episode psychosis (Breitborde et al. Reference Breitborde, Moreno, Mai-Dixon, Peterson, Durst, Berstein, Byreddy and McFarlane2011; Vesterager et al. Reference Vesterager, Christensen, Olsen, Krarup, Forchhammer, Melau, Gluud and Nordentoft2011). To the best of our knowledge, there have been no published studies demonstrating the effectiveness of CR in solely first-episode psychiatric patients.
The paucity of early intervention studies is surprising given evidence suggesting that CR programmes at earlier stages of schizophrenia (Gupta et al. Reference Gupta, Holshausen and Bowie2011) and at younger ages (Wykes et al. Reference Wykes, Reeder, Landau, Matthiasson, Haworth and Hutchinson2009) are associated with greater cognitive and functional gains. Conversely, CR at more chronic stages and older ages may yield poorer outcomes (Dickinson et al. Reference Dickinson, Tenhula, Morris, Brown, Peer, Spencer, Li, Gold and Bellack2010). Clearly, there is sufficient theoretical impetus behind investigating CR in the early stages of major psychiatric illnesses. To this end, the NEAR programme may be particularly well suited. NEAR uses both drill-and-practice and compensatory approaches, and is based on theories of how people learn best (Medalia & Freilich, Reference Medalia and Freilich2008). Principles such as errorless learning, positive reinforcement and shaping help to guide therapists in promoting behavioural change. Of particular relevance to early intervention, NEAR has been shown to be effective in a mixed sample including first-episode schizophrenia patients (Redoblado-Hodge et al. Reference Redoblado-Hodge, Siciliano, Withey, Moss, Moore, Judd, Shores and Harris2010), and in a relatively young sample of MDD patients (Naismith et al. Reference Naismith, Redoblado-Hodge, Lewis, Scott and Hickie2010b). Additionally, a structured psycho-education programme may be an important adjunct, and has recently been demonstrated to be effective in late-life depression (Naismith et al. Reference Naismith, Diamond, Carter, Norrie, Redoblado-Hodge, Lewis and Hickie2011). Psycho-education is primarily aimed at improving awareness of cognitive deficits, and is reported to improve insight in early schizophrenia (Ruzanna et al. Reference Ruzanna, Marhani, Parveen and Cheah2010). Facilitating awareness is pivotal, given that insight is commonly limited in both MDD (Yen et al. Reference Yen, Chen, Lee, Tang, Ko and Yen2005) and schizophrenia (Medalia & Thysen, Reference Medalia and Thysen2008), with downstream effects on motivation (Markland et al. Reference Markland, Ryan, Tobin and Rollnick2005), a crucial factor in CR success (Medalia & Saperstein, Reference Medalia and Saperstein2011). Indeed, it is also difficult to see how patients would benefit from CR if they do not understand the reasons for treatment and the precise cognitive functions being targeted.
Overall, the aim of the current study was to examine the clinical effectiveness of NEAR in out-patients diagnosed with first-episode MDD or psychosis, two of the most common presenting disorders in our youth mental health clinics (Scott et al. Reference Scott, Naismith, Whitwell, Hamilton, Chudleigh and Hickie2009, Reference Scott, Hermens, Glozier, Naismith, Guastella and Hickie2012). These disorders were chosen on the basis that cognitive impairments are common in both, and have been shown to respond to CR. Two primary hypotheses were postulated. First, CR patients were expected to demonstrate greater gains in cognitive and psychosocial functioning than a treatment-as-usual (TAU) cohort, which was included to control for practice effects (Goldberg et al. Reference Goldberg, Keefe, Goldman, Robinson and Harvey2010) and the effects of ongoing psychosocial and pharmacological treatment (Keefe et al. Reference Keefe, Bilder, Davis, Harvey, Palmer, Gold, Meltzer, Green, Capuano, Stroup, McEvoy, Swartz, Rosenheck, Perkins, Davis, Hsiao and Lieberman2007; Brekke et al. Reference Brekke, Hoe and Green2009). Second, greater cognitive and functional gains were expected in the CR group even while controlling for diagnosis to demonstrate the cross-diagnostic utility of CR. A further aim of the current study was to determine whether functional improvements would be mediated by improvement in memory and executive functioning, consistent with the literature.
Method
Subjects
The study was conducted at the Brain and Mind Research Institute (BMRI), Sydney, Australia. Consecutive referrals were obtained from clinicians in affiliated out-patient clinics (Headspace Central Sydney; The Clinical Centre, BMRI; Community Mental Health Services, NSW Health). Patients were included if they met the following inclusion criteria: (1) a lifetime history of a single episode of MDD or a psychotic disorder according to DSM-IV-TR (APA, 2000); (2) clinically stable as determined by a treating psychiatrist; and (3) fluent in English.
Participants were excluded if they: (i) were currently diagnosed with substance dependence, a developmental disorder or a neurological condition; (ii) had a history of traumatic brain injury; (iii) were currently prescribed a first-generation antipsychotic medication; (iv) had an estimated pre-morbid intelligence quotient (IQ) of <80; or (v) did not demonstrate significant cognitive dysfunction as defined by a neuropsychological test score of at least 1.5 standard deviations (s.d.) below their predicted pre-morbid IQ (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012). This criterion was chosen to prevent ceiling effects in the primary outcome measures, and from clinical experience, which has demonstrated that most patients with severe mental illnesses meet this criteria. Indeed, in a large sample of healthy adults, Schretlen et al. (Reference Schretlen, Munro, Anthony and Pearlson2003) found that the average person had at least one neuropsychological test score falling 1.9 s.d. below their predicted pre-morbid IQ, across a comprehensive neuropsychological battery not dissimilar from the current study. Thus, including those with milder deficits may erroneously include patients not cognitively impaired as a result of psychiatric morbidity, but normal intra-individual variability.
Written informed consent was obtained from all participants. The current study was approved by the Human Research Ethics Committees of the University of Sydney and Macquarie University.
Design
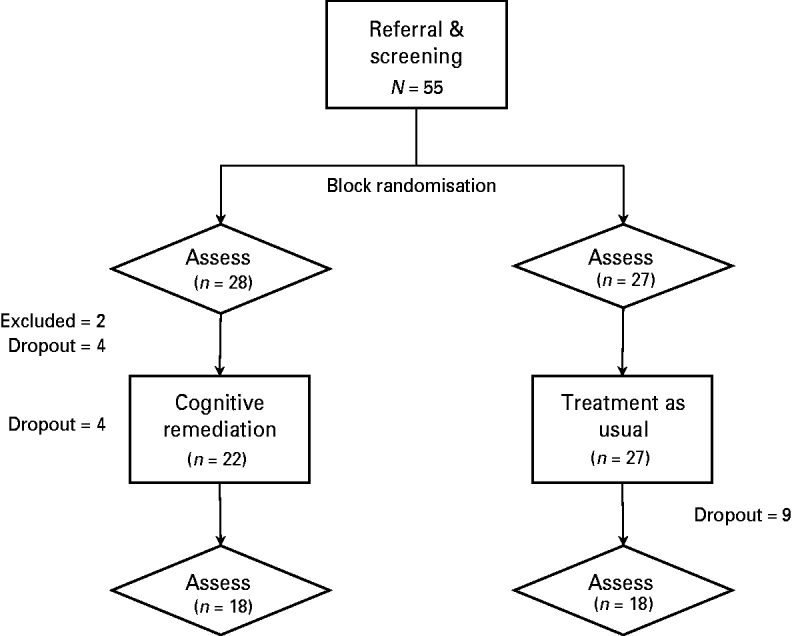
Following referral and screening, participants were block randomized to either CR or TAU (see Fig. 1). Neither clinicians (raters and therapists) nor participants were blinded to allocation. Participants were initially assessed on a range of measures, and subsequently commenced either a 10-week period of CR while still receiving TAU or continued to receive TAU (only) for the same period. Patients were reassessed using the same measures at follow-up.

Fig. 1. Flowchart of study design.
Measures
All participants were assessed by the first author (R.S.C.L.). At baseline, clinical and demographic information was ascertained through an interview. Two measures were also administered to estimate pre-morbid ability: the Wechsler Test of Adult Reading (WTAR; Wechsler, Reference Wechsler2001) and Information (Wechsler, Reference Wechsler1997a). Baseline and follow-up assessments included clinical, neuropsychological and functional measures. Clinical measures included the total score on the 17-item Hamilton Depression Rating Scale (HAMD-17; Hamilton, Reference Hamilton1960) and on the Brief Psychiatric Rating Scale – Expanded (BPRS-E; Ventura et al. Reference Ventura, Green, Shaner and Liberman1993). Primary outcomes included several cognitive and functional measures. Neuropsychological tests were organized into five theoretically derived cognitive domains (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012). ‘Processing Speed’ was measured using the Trail Making Test – Part A (TMT-A; Franzen et al. Reference Franzen, Paul and Price1990) and Category Fluency (Spreen & Benton, Reference Spreen and Benton1977). ‘Attention and Working Memory’ was assessed using Longest Digit Span Forward and Longest Digit Span Backward (LDSF and LDSB; Wechsler, Reference Wechsler1997a), Spatial Span length (Cambridge Neuropsychological Test Automated Battery, CANTAB; www.camcog.com), Rapid Visual Processing Hits score (RVP Hits; CANTAB), and Mental Control (Wechsler, Reference Wechsler1997b). ‘Immediate Learning and Memory’ was measured using Logical Memory I (Wechsler, Reference Wechsler1997b), Rey Auditory Verbal Learning Test total score (RAVLT Total; Taylor, Reference Taylor1959) and Paired Associates Learning (PAL; CANTAB). ‘Delayed Learning and Memory’ was assessed using Logical Memory II Percentage Retention (LM Retention; Wechsler, Reference Wechsler1997b), RAVLT 20-Minute Percentage Retention (RAVLT Retention; Taylor, Reference Taylor1959) and Rey–Osterrieth Complex Figure 3-Minute Recall (CFT 3min; Corwin & Bylsma, Reference Corwin and Bylsma1993). ‘Executive Functioning’ was indexed by TMT – Part B (TMT-B; Franzen et al. Reference Franzen, Paul and Price1990), Intra/Extradimensional Set Shift Errors (IED Errors; CANTAB) and Letter Fluency (FAS; Spreen & Benton, Reference Spreen and Benton1977). Alternate forms were used at follow-up where available (Reitan & Wolfson, Reference Reitan and Wolfson1985; Crawford et al. Reference Crawford, Stewart and Moore1989; Hubley & Tremblay, Reference Hubley and Tremblay2002). Scores were standardized into z scores based on demographically adjusted norms from internal (Wechsler, Reference Wechsler1997a, Reference Wechslerb, CANTAB) and external samples (Meyers & Meyers, Reference Meyers and Meyers1995, Reference Meyers and Meyers1996; Tombaugh et al. Reference Tombaugh, Rees, McIntyre, Spreen and Strauss1996, Reference Tombaugh, Kozak and Rees1999; Rickert & Senior, Reference Rickert and Senior1998). Functional outcome was assessed using the standardized total score of the Social Functioning Scale (SFS; mean = 100, s.d. = 15; Birchwood et al. Reference Birchwood, Smith, Cochrane, Wetton and Copestake1990). A higher score on neuropsychological and functional measures indicated more superior functioning.
Intervention
The NEAR CR programme involved once-weekly 2-h sessions for a total of 10 weeks, and was adapted to include a psycho-education component (Medalia & Freilich, Reference Medalia and Freilich2008). Each session started with psycho-education regarding cognitive deficits and relevant compensatory strategies adapted from a previously implemented programme in the elderly (Naismith et al. Reference Naismith, Diamond, Carter, Norrie, Redoblado-Hodge, Lewis and Hickie2011). Patients also received a bound workbook of PowerPoint slides. The talks were given by either the therapist facilitating the groups (R.S.C.L.) or invited guest speakers with expertise in the area of interest. Following psycho-education, patients engaged in therapist-led drill-and-practice group activities, and computer-assisted cognitive training using a range of software programs that were educational, designed to target cognitive deficits in the laboratory and/or available online. The cognitive training component was specifically tailored to each individual's neuropsychological profile. Patients were encouraged throughout the program to experiment with compensatory strategies during warm-up activities, computer training, and in their own time outside the clinic.
All patients also received TAU care, involving ongoing monitoring by a psychiatrist of symptoms and medication use, in addition to case management by mental health clinicians. Participants were not concurrently involved in structured cognitive (e.g. social cognitive remediation) or psychosocial (e.g. social skills training) rehabilitative interventions at any time during the study.
Data analysis
IBM SPSS version 20.0 (SPSS Inc., USA) was used for all statistical analyses. Neuropsychological standardized scores were reduced to five composite scores by averaging z scores from each cognitive domain (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012). Composite scores were used instead of individual test scores because the latter are less reliable (Salthouse, Reference Salthouse2012) and unnecessarily inflate the type I error rate. Analyses were conducted using all available data excluding those from participants who did not meet entry criteria. Thus, an intent-to-treat (ITT) approach was adopted, assuming data were missing at random (MAR), including any significant predictors of drop-out as covariates, consistent with previous research (Wykes et al. Reference Wykes, Reeder, Huddy, Taylor, Wood, Ghirasim, Kontis and Landau2012). One-way ANOVAs were conducted to determine: (1) whether those who withdrew from the study were different from those who completed the study; and (2) whether any baseline variable differed between treatment conditions despite randomization. Any differences at baseline were also included as covariates in subsequent and respective primary outcome analyses. Repeated-measures ANOVAs were conducted on symptomatology (HAMD-17 and BPRS-E) and medication-use variables to ensure primary outcome analyses were not confounded by symptom or medication change. Primary outcomes were analysed using ANOVAs for each cognitive composite and psychosocial functioning, with treatment condition as the between-subjects factor. Subsequently, ANCOVAs were conducted to include any predictors of drop-out, baseline differences and diagnosis as covariates. If functional change was significantly improved in CR compared to TAU, it was regressed on treatment condition and each separate cognitive change score to determine whether cognitive improvement predicted functional improvement over and above the direct effects of treatment. For the primary cognitive outcomes based on Bonferroni adjustments for multiple comparisons, α was set at 0.01. For all other statistical inferences, α was set at 0.05.
Results
In total, 55 participants were recruited and assessed at baseline. Two participants were excluded from further participation because of inadequate English or borderline impaired pre-morbid IQ (<80). In addition, 17 participants dropped out prior to completing the study because of hospitalization for relapse (n = 3), work commitments (n = 3) or disinterest (n = 11). In total, 36 participants completed the whole study, with seven and 11 first-episode depression and psychosis patients in each condition respectively. There was no differential drop-out rate between conditions (p > 0.05), and those who dropped out did not differ from those who completed the study on any demographic or clinical variables, medication use, neuropsychological or psychosocial functioning (p > 0.05). The test–retest interval averaged 21 and 19 weeks for CR and TAU participants respectively (p > 0.05).
Baseline differences
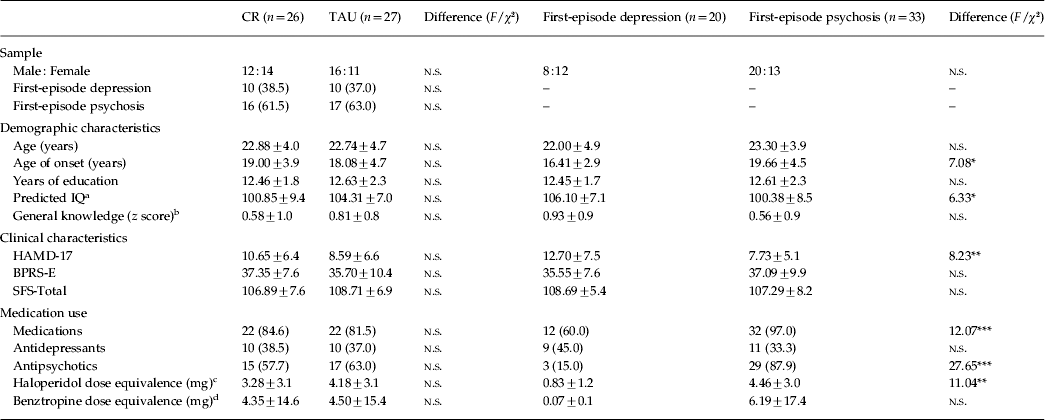
Baseline sample characteristics are displayed in Table 1, and stratified according to treatment condition and diagnosis. Participants were relatively young (mean age = 22.8 years, s.d. = 4.3, range 16–34) and well educated (mean = 12.6 years, s.d. = 2.1, range 9–18). There were no significant differences between treatment conditions on any baseline sample characteristics, including the primary functional outcome (p > 0.05). However, in terms of diagnosis, first-episode psychosis patients had a significantly later age of onset (F 1,47 = 7.08, p < 0.05) and lower predicted IQ (F 1,50 = 6.33, p < 0.05), were less depressed (F 1,51 = 8.23, p < 0.01), had a greater proportion using medication (χ21,53 = 12.07, p < 0.001) and antipsychotics (χ21,53 = 27.65, p < 0.001), and also a higher mean haloperidol dose equivalency (F 1,37 = 11.04, p < 0.01).
Table 1. Sample characteristics of intent-to-treat (ITT) participants at baseline

CR, Cognitive remediation; TAU, treatment as usual; HAMD-17, 17-item Hamilton Depression Rating Scale; BPRS-E, Brief Psychiatric Rating Scale – Expanded; SFS, Social Functioning Scale; n.s., not significant.
Values given as n (%) or mean ± s.d.
a Wechsler (Reference Wechsler2001) .
b Information subtest (Wechsler, Reference Wechsler1997a).
c Based on conversion table in Kane et al. (Reference Kane, Leucht, Carpenter and Docherty2003) .
d Based on conversion table in Minzenberg et al. (Reference Minzenberg, Poole, Benton and Vinogradov2004) .
*p ⩽ 0.05, **p ⩽ 0.01, ***p ⩽ 0.001.
CR participants also performed significantly worse than TAU participants in attention and working memory at baseline (F 1,47 = 4.06, p = 0.05). No other cognitive domains were significantly different between conditions (p > 0.05). Diagnostically, first-episode psychosis patients performed significantly more poorly than depressed patients on every cognitive composite score (p < 0.005; see Fig. 2).

Fig. 2. Neuropsychological profile (z scores and standard errors) of cognitive composite scores according to diagnosis.
Primary outcomes
There were no differential changes to psychiatric symptoms or medication dosage between groups (p > 0.05), or changes to the type of medication(s) prescribed between assessments.
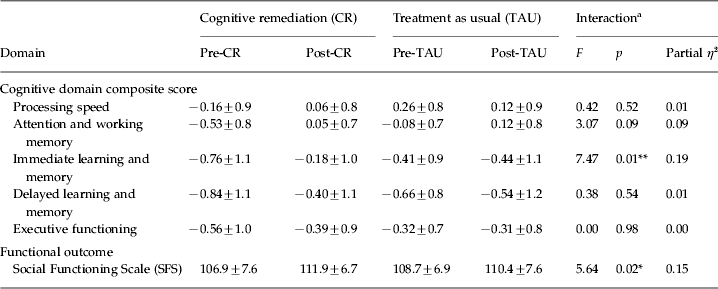
Repeated-measures ANOVA for the primary cognitive composite scores indicated that CR patients improved significantly more than TAU patients on attention and working memory (F 1,34 = 8.10, p < 0.01; see Table 2) and immediate learning and memory (F 1,34 = 15.51, p < 0.001). Controlling for diagnosis and baseline attention and working memory using repeated-measures ANCOVA, only immediate learning and memory remained significant (F 1,32 = 7.47, p < 0.01). Specifically, CR independently contributed to 18.9% of the variance in immediate learning and memory improvement, which is considered a relatively large effect according to Cohen's criteria (Reference Cohen1988) . No other repeated-measures ANCOVA interactions by group were significant (p > 0.05).
Table 2. Mean scores (± standard deviation) for each composite neuropsychological measure and functional outcome stratified by treatment condition and time

a ANCOVA with diagnosis and baseline attention and working memory composite score as covariates, or for functional outcome, ANCOVA with diagnosis as a covariate.
*p ⩽ 0.05, **p ⩽ 0.01.
With regard to psychosocial functioning, controlling for diagnosis, CR patients demonstrated significantly greater improvement than TAU patients on the SFS (F 1,32 = 5.64, p = < 0.05). The effect size of relative functional gain in CR patients was large, with treatment accounting for 14.6% of the variability in improvement.
Mediation models
Controlling for the effects of treatment (β = 0.30, t 33 = 1.91, p = 0.07), improvement in delayed learning and memory independently predicted psychosocial gains at the trend level (β = 0.29, t 33 = 1.86, p = 0.07). The overall model was significant (R 2 = 0.21, F 2,33 = 4.38, p < 0.05). No other changes to cognition were predictive of psychosocial improvement over and above treatment.
Discussion
Our results suggest that the neuropsychological profiles of patients recruited into the current study were largely consistent with those seen in larger samples with similarly young (Hermens et al., unpublished observations) and first-episode (Mesholam-Gately et al. Reference Mesholam-Gately, Giuliano, Goff, Faraone and Seidman2009; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012) cohorts. Pre-morbid intellectual functioning and educational attainment in our sample were generally fairly high. Neuropsychological functioning was most reduced in the learning and memory domains, consistent with studies demonstrating verbal learning and memory as most impaired in average to high IQ first-episode psychosis patients (Leeson et al. Reference Leeson, Sharma, Harrison, Ron, Barnes and Joyce2011), and highly educated early-episode youth with severe mental illnesses (Hermens et al., unpublished observations). In addition to memory, all other cognitive domains were significantly more reduced in patients with psychosis compared to those with only depression, in keeping with the literature (Schatzberg et al. Reference Schatzberg, Posener, DeBattista, Kalehzan, Rothschild and Shear2000). Overall, the current sample is representative of those experiencing a first episode of MDD and psychosis.
The primary aim of the current study was to determine the effectiveness of CR as an early intervention in first-episode depressive and psychotic disorders. A modified NEAR programme was implemented in young out-patients, involving once-weekly cognitive training and psycho-education groups for a total of 10 weeks. The results suggest that CR is effective in improving immediate learning and memory. The effect size was large, and not inconsistent with older MDD patients (Naismith et al. Reference Naismith, Redoblado-Hodge, Lewis, Scott and Hickie2010b, Reference Naismith, Diamond, Carter, Norrie, Redoblado-Hodge, Lewis and Hickie2011), and greater than those found previously in chronic schizophrenia cohorts (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). The larger effects currently observed may be suggestive of more favourable responses to neuropsychological intervention when implemented in the early stages of mental illness. The results are also consistent with findings that verbal learning and memory benefit most from CR in a young mixed psychiatric sample (Naismith et al. Reference Naismith, Redoblado-Hodge, Hermens, Carter, Scott and Hickie2010a).
Importantly, CR as an early intervention also seems to be effective in improving psychosocial functioning. Effect size was again in the large range, and generally greater than identified in previous studies in chronic schizophrenia (Medalia & Choi, Reference Medalia and Choi2009), and contrary to findings in more chronic MDD cohorts, which have largely failed to detect functional improvements (Naismith et al. Reference Naismith, Redoblado-Hodge, Lewis, Scott and Hickie2010b, Reference Naismith, Diamond, Carter, Norrie, Redoblado-Hodge, Lewis and Hickie2011). This discrepancy may be again indicative of a better treatment response when CR is implemented at earlier stages of mental illness.
Secondary to our primary outcome analyses, regression modelling suggested that improved delayed learning and memory mediated the effect of CR on psychosocial improvements. The overall model was significant and explained 21% of the variance in functional change. This was found despite a non-significant primary outcome analysis for this domain, which may suggest that the latter was underpowered, particularly given the inclusion of diagnosis and baseline attention and working memory as covariates with the currently modest sample size. The regression model is in keeping with previous findings demonstrating that functional improvements are mediated by memory improvements (Wykes et al. Reference Wykes, Newton, Landau, Rice, Thompson and Frangou2007; Fiszdon et al. Reference Fiszdon, Choi, Goulet and Bell2008), although executive functioning improvements have also been shown to predict functional gains following CR (Reeder et al. Reference Reeder, Newtown, Frangou and Wykes2004, Reference Reeder, Smedley, Butt, Bogner and Wykes2006; Wykes et al. Reference Wykes, Reeder, Huddy, Taylor, Wood, Ghirasim, Kontis and Landau2012). The failure to find a relationship between executive functioning change and functional improvement was not entirely surprising given that executive functioning did not respond to CR in the current study. Nonetheless, the current results suggest that delayed learning and memory potentially mediates psychosocial gains, although this requires further validation as our results were only significant at a trend to marginal level, and cognitive functioning improvements may require more time to generalize to real-world functioning.
In addition to neuropsychological functioning, several other mechanisms may explain social and role functioning improvements attributable to CR. Social functioning improvements may be mediated by aspects of social cognition, either wholly (Addington et al. Reference Addington, Girard, Christensen and Addington2010; Schmidt et al. Reference Schmidt, Mueller and Roder2011) or partly (Hoe et al. Reference Hoe, Nakagami, Green and Brekke2012). Thus, cognitive functioning may be a necessary, but insufficient, condition for functional improvement. With regard to role functioning, self-efficacy, job opportunities and social networks, to name a few, may moderate functional change over and above cognition. For instance, as a result of the current CR treatment, participants' self-efficacy beliefs regarding their own abilities may be strengthened in response to observed improvements on training tasks and, as a result, stimulate their attempts to seek out employment or return to study. That is not to say that cognitive functioning plays no part in role functioning. Indeed, it is difficult to imagine how someone can hold down a moderately demanding job or area of study with clinically significant cognitive impairments. Instead, cognitive improvements may influence role functioning once a patient has returned to a higher level of functioning and is expected to maintain those roles, consistent with evidence that neurocognition is associated with job tenure, but not job attainment (Gold et al. Reference Gold, Goldberg, McNary, Dixon and Lehman2002). Thus, targeting self-efficacy may prove crucial in improving occupational or academic functioning in the initial stages of illness (Caprara et al. Reference Caprara, Barbaranelli, Pastorelli and Cervone2004). Taken together, factors other than neuropsychological functioning, such as social cognition and self-efficacy, may be crucial rate-limiting factors in the translation of cognitive gains into the real world. Furthermore, these factors could potentially account for a significant portion of the 79% of functional improvement currently left unexplained.
The current study also demonstrated that cognitive and functional improvements were detected even while controlling for the effects of diagnosis. This is consistent with previous findings of CR's cross-diagnostic utility (Lewandowski et al. Reference Lewandowski, Eack, Hogarty, Greenwald and Keshavan2011), and provides additional support for the need to expand the application of CR into other psychiatric populations with demonstrable neuropsychological dysfunction.
Limitations and future directions
There were several limitations to the current study. The lack of blinded assessments may have biased the clinical and neuropsychological results, although previous findings have found negligible effects of methodological rigour on treatment effect (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). Similarly, current practice in conducting clinical trials favours the use of ‘active’ controls over the use of ‘passive’ controls. The rationale is that the use of active controls can account for non-specific therapist-related effects, such as demand characteristics. However, as with blindedness, previous meta-analytic research has found minimal effect for control type on the effect size of improvements (McGurk et al. Reference McGurk, Twamley, Sitzer, McHugo and Mueser2007;Lewandowski et al. Reference Lewandowski, Eack, Hogarty, Greenwald and Keshavan2011). Lastly, in terms of sample size, the current study was also relatively modest and may have been underpowered to detect small to moderate effects typical of CR studies. The sample size, however, was not incidental, and is reflective of the difficulty in implementing a neuropsychological intervention in youth cohorts, where engagement may be particularly difficult and drop-outs not uncommon. To this end, future studies may benefit from incorporating motivational interviewing techniques into CR programmes to help facilitate engagement and, as a result, adherence (Markland et al. Reference Markland, Ryan, Tobin and Rollnick2005). Future studies should also examine the factors that predict treatment response, which was not currently conducted because of the restrictive sample size. The modest sample size also meant that more sophisticated modelling of the relationships between neuropsychological variables and functional improvements was not feasible (see Wykes et al. Reference Wykes, Reeder, Huddy, Taylor, Wood, Ghirasim, Kontis and Landau2012 for an example of this type of analysis).
Summary
The current study is the first to demonstrate the effectiveness of CR in clinically stable out-patients diagnosed with a solely first episode of major depression or psychosis. Improvements were detected in both immediate learning and memory and psychosocial functioning. Improvements in delayed learning and memory contributed to psychosocial gains at a trend level, over and above the functional benefits of treatment. We propose that other factors, such as social cognition and self-efficacy, may also mediate the effects between cognitive and functional gains, and should be further explored. Future studies should also follow up patients longitudinally to determine the baseline predictors of functional improvement, to allow enough time for cognitive gains to translate into the real world, and to determine the durability of improvements. Despite several limitations in the current study design, it provides evidence of the potential that CR has as a potent early and targeted intervention strategy for those presenting with severe mental illness. Future studies need to examine this cohort further under more rigorously controlled conditions to provide more cogent evidence that CR should be incorporated into the standard treatment of psychiatric illnesses.
Acknowledgements
We thank C. Chudleigh for help with facilitating treatment, K. Diamond for providing resources and clinical supervision, and M. Kaur for her administrative assistance. We also thank all the clinicians who made referrals to the current research programme without whom the current study would not have been possible.
This research was supported by a National Health and Medical Research Council (NHMRC) Program Grant (No. 350241) and an NHMRC Australia Fellowship awarded (No. 511921) to I.B.H. S.LN. was funded by an NHMRC Clinical Research Fellowship (No. 402864).
Declaration of Interest
I.B.H. has led projects for health professionals and the community supported by governmental, community agency, and drug industry partners (Wyeth, Eli Lilly, Servier, Pfizer, AstraZeneca) for the identification and management of depression and anxiety. He has served on advisory boards convened by the drug industry in relation to specific antidepressants, including nefazodone, duloxetine, and desvenlafaxine, and has participated in a multicenter clinical trial of agomelatine effects on sleep architecture in depression. He has participated in Servier-sponsored educational programs related to circadian-based therapies. D.F.H. has previously received honoraria for educational seminars from Janssen-Cilag. M.A.R.H. has received financial support from Eli Lilly for a multisite trial of cognitive remediation in psychosis, and is a co-investigator in an Australian Rotary-funded project investigating the synergistic effects of cognitive remediation and vocational training in psychosis. She was also a co-investigator in a drug trial with Novartis Pharmaceutical who sponsored overseas travel in 2011, and was also involved in NHMRC-funded trials in psychosis.