GWG has been extensively studied over the past two decades and an excessive GWG has been associated with adverse pregnancy-related and childhood outcomes. These include gestational diabetes and increased risk of high BW and overweight and obesity in the offspring (Diesel et al., Reference Diesel, Eckhardt, Day, Brooks, Arslanian and Bodnar2015; Guo et al., Reference Guo, Liu, Ye, Zhuang and Ren2015; Hedderson, et al., Reference Hedderson, Gunderson and Ferrara2010; Ludwig & Currie, Reference Ludwig and Currie2010; Oken et al., Reference Oken, Taveras, Kleinman, Rich-Edwards and Gillman2007; Wrotniak et al., Reference Wrotniak, Shults, Butts and Stettler2008). There is also evidence that GWG might have different effects on offspring BW and childhood weight/BMI, depending on the timing of the weight gain. The majority of the studies that have investigated trimester-specific effects of GWG on BW or adiposity at birth found effects for all trimesters (Abrams & Selvin, Reference Abrams and Selvin1995; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012; Starling et al., Reference Starling, Brinton, Glueck, Shapiro, Harrod, Lynch and Dabelea2015; Wander et al., Reference Wander, Sitlani, Badon, Siscovick, Williams and Enquobahrie2015) although some suggested stronger, or specific, effects for the second trimester (Abrams & Selvin, Reference Abrams and Selvin1995; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012; Sekiya et al., Reference Sekiya, Anai, Matsubara and Miyazaki2007; Wander et al., Reference Wander, Sitlani, Badon, Siscovick, Williams and Enquobahrie2015), while others found that first and second trimester gain were more strongly associated with BW or adiposity at birth (Brown et al., Reference Brown, Murtaugh, Jacobs and Margellos2002; Starling et al., Reference Starling, Brinton, Glueck, Shapiro, Harrod, Lynch and Dabelea2015). In terms of later childhood outcomes, stronger effects of GWG on childhood BMI have been detected for first and second trimester gain (Andersen et al., Reference Andersen, Gamborg, Sorensen and Nohr2011; Fraser et al., Reference Fraser, Tilling, Macdonald-Wallis, Sattar, Brion, Benfield and Lawlor2010; Gaillard et al., Reference Gaillard, Steegers, Franco, Hofman and Jaddoe2015; Karachaliou et al., Reference Karachaliou, Georgiou, Roumeliotaki, Chalkiadaki, Daraki, Koinaki and Chatzi2015; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012).
In order to understand whether these aforementioned associations are due to an in utero programming of the fetus (within-pair effects) or to shared mother–child obesity-promoting genes and/or environment (between-pair effects), epidemiological study designs (family-studies) comparing these within- and between-pair effects can be adopted. With regards to offspring BW, the studies that have applied this technique using sibling comparisons all found a significant within-pair association with total GWG, independent of shared genetic and/or environmental confounding (Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014; Lawlor et al., Reference Lawlor, Lichtenstein, Fraser and Langstrom2011; Ludwig & Currie, Reference Ludwig and Currie2010). To our knowledge, only one study was able to examine trimester-specific effects of GWG on BW using a similar methodology, and the authors found that GWG during the second trimester had a stronger influence on offspring weight at birth within sibling pairs, compared to the other trimesters (Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014). In terms of the effects of GWG on later infancy and childhood weight and BMI, few studies using a within-family (sibling-comparison) design have investigated these associations and their results are inconsistent (Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014; Branum et al., Reference Branum, Parker, Keim and Schempf2011; Ludwig et al., Reference Ludwig, Rouse and Currie2013). One study found a positive within-pair association of GWG and offspring childhood BMI (Ludwig et al., Reference Ludwig, Rouse and Currie2013), whereas the two other studies failed to find any significant associations when controlling for shared genes and environment (Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014; Branum et al., Reference Branum, Parker, Keim and Schempf2011). None of these latter studies have, however, looked into whether different trimesters during pregnancy could be especially important in terms of offspring childhood weight or BMI, nor have they utilized offspring of MZ twins to address these possible associations. Although the offspring of MZ twins are less genetically related compared to full siblings, the amount of unique environmental variation in GWG is likely to be larger in a twin pair (two separate mothers) than that of a sibling pair (same mother). Another advantage of the MZ twin design is that parity (which is a known confounder) can be kept the same within the twin pair. Our aim was therefore to examine the association of GWG (both total and trimester-specific) with offspring BW and infant/childhood weight and BMI at the ages of 1, 5, and 10 within MZ twin mother pairs and their children, while taking shared genetic and environmental confounding into account.
Materials and Methods
In order to create a study population of MZ twin mothers (born between 1962 and 1975) and their children (born between 1984 and 2009), several national registers were first linked using the personal identification number unique to each Swedish citizen. The study period was selected in order to include mothers old enough to have had children who were at the minimum age of 10 years at the time. A subsequent data collection was carried out, once the MZ twins were identified and recruited, where medical records were retrieved to obtain data from both mother and child (as explained in more detail below).
Register Linkage
The linkage was carried out between the register of the Total Population (to identify the female twins), the Swedish Multi-Generation register (in order to link the twin mothers to their children), the Medical Birth register, the Cancer register, and the Cause of Death register. The three last-named registers were used to exclude individuals due to the following reasons: diseased or severely ill (severe types of cancers) mothers and/or children, children with extremely low BW (<1000 g), mothers who had lost custody of their child, and mothers and/or children with protected identity. These individuals were not contacted for participation in the study.
Recruitment Process
The resulting database contained 5,836 female DZ and MZ twin individuals where both twins had given birth to at least one child. Information letters were sent out and the women were thereafter contacted for a structured telephone interview. The women were asked to participate if they were categorized as an MZ twin during the interview.
The structured interview began by assessing the mothers’ zygosity using classical questions on physical similarity, which have been widely applied in twin research and shown high validity (Johansson & Rasmussen, Reference Johansson and Rasmussen2001; Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006; Silventoinen et al., Reference Silventoinen, Magnusson, Tynelius, Kaprio and Rasmussen2008). These questions were based on childhood resemblance and whether the mothers’ teachers had had difficulties distinguishing between them in school. Only the mothers who answered ‘as like as two peas in a pod’ or ‘always or nearly always’ to these questions were categorized as an MZ twin and were subsequently asked to participate in the study. The co-twin was also contacted and asked for participation (in order to match the twins into pairs) and underwent the same interview process. All of the twins answered that they were reared together, which was confirmed by data from the Swedish Twin Registry (STR). Ethical approval of this study was granted by the Stockholm Regional Ethical Review Board (Ref no 2011/666-31/3) and all the participants (mothers and children above the age of 15 years) in this cohort have given informed written consent.
Data Collection
Data on the mothers’ early-pregnancy BMI and smoking status (measured and recorded at the first antenatal visit, on average around gestational week 10), parity, GWG, complications and diseases during pregnancy (gestational diabetes and pre-eclampsia), and age at delivery was gathered from antenatal and maternal healthcare medical records. For the children, growth charts from birth to their current age at recruitment, as well as breast-feeding duration, were retrieved from child health centers, school health services, county, and municipal archives. For our main analyses, data on GWG for both mothers in the twin pair as well as data on the children's BW were acquired for a total of 82 twin mother-pairs. In terms of the outcome variables, data for weight at 1 year and BMI of the children at the ages of 5 and 10 years were available for 71, 69 and 57 twin pairs, respectively. We excluded subjects whose infants were born weighing <2500 g (n = 12) and <37 weeks gestation (n = 6) as this could impact later growth development. Details on the number of women who declined or dropped out, or were DZ twins, at each stage of the recruitment process, are shown in Figure 1.
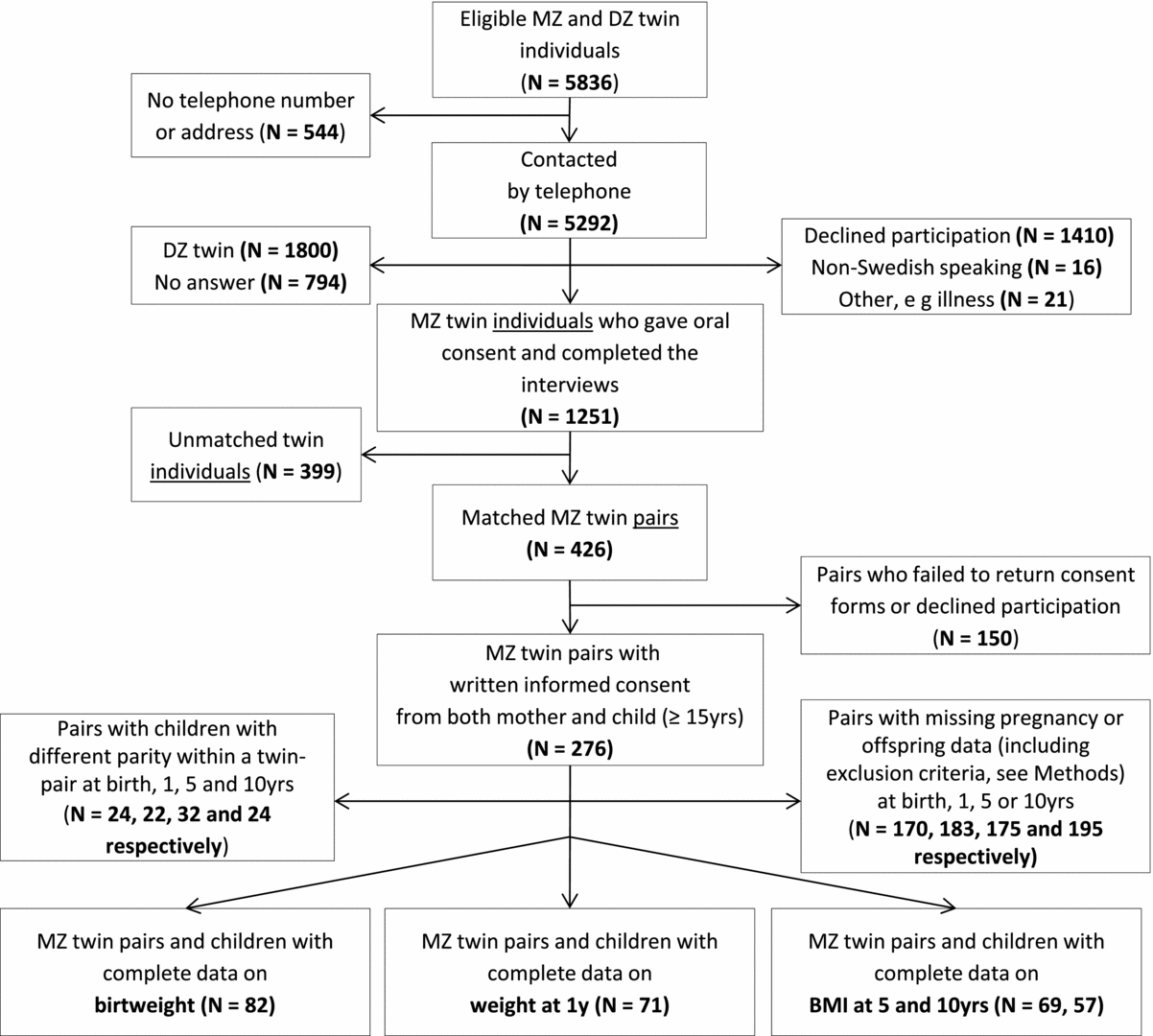
FIGURE 1 Recruitment tree.
Measurements of GWG and Outcome Variables
The exposure variable, GWG, was calculated as the difference between the mothers’ delivery weight and her early-pregnancy weight. To make sure that the twin mothers were followed for the same time during pregnancy for both children within a pair (same gestational week at delivery), we used the weight for each mother at the time of birth of the first child. The average weight gain for each trimester (kg/week) was also calculated for the first (10–14 gestational weeks), second (14–27 gestational weeks), and third (27 gestational weeks to birth) trimester. In terms of the outcome measures in the children, sex-specific and gestational-age-adjusted BW z-scores were calculated using nationwide data from the MBR (which covers 99% of all births in Sweden (Cnattingius et al., Reference Cnattingius, Ericson, Gunnarskog and Kallen1990) as a reference. One BW z-score unit corresponded to approximately 480 g. Internally standardized sex-specific z-scores were calculated from predicted growth curves for infant and childhood weight and BMI for the ages of 1, 5, and 10 years.
Statistical Analysis
With the purpose of predicting weight and BMI values at the specific time points for the ages 1, 5, and 10 years in the offspring, as well as maternal GWG, we adopted a non-parametric regression method, so called kernel-smoothing using the ‘lokern’ package in the R-software (http://www.r-project.org;
Gasser et al., Reference Gasser, Gervini, Molinari, Hauspie, Cameron and Molinari2004). To analyze if potential associations could be explained by shared genetic or common environmental factors (within-pair effects) or by non-shared factors (between-pair effects), we applied generalized estimating equations (GEE) with robust variance using the xtgee command in Stata 12.1 (Stata Corp, College Station, Texas, USA). The between-pair association was based on the means of the GWG (both total and trimester-specific) of the twin pair and the means of the offspring BW or infant/childhood weight/BMI. On the contrary, the within-pair association was based on the difference from the mean of the exposure and outcome variables. The following regression equation was used to calculate these between- and within-pair associations:
![]() $\ E( {{Y_{ij\ }}} ) = \ {{\rm{\beta }}_0} + \ {{\rm{\beta }}_{{\rm{w\ }}}}( {{X_{ij}} - {{\bar X}_i}} )\ + \ {{\rm{\beta }}_B}{\bar X_i}\ $
, where
$\ E( {{Y_{ij\ }}} ) = \ {{\rm{\beta }}_0} + \ {{\rm{\beta }}_{{\rm{w\ }}}}( {{X_{ij}} - {{\bar X}_i}} )\ + \ {{\rm{\beta }}_B}{\bar X_i}\ $
, where
![]() ${\bar X_i}$
depicts the mean value of X for twin pair i and Xij
represents the value of X (GWG) for each twin, j in the pair, i. β
w
and β
B
represent the within- and between-pair regression coefficients, respectively. Using this analytical approach, all potential confounding factors (measured and unmeasured) that are shared by the twins within the pair are effectively controlled for. Wald tests were applied to test for differences between the within- and between-pair effects.
${\bar X_i}$
depicts the mean value of X for twin pair i and Xij
represents the value of X (GWG) for each twin, j in the pair, i. β
w
and β
B
represent the within- and between-pair regression coefficients, respectively. Using this analytical approach, all potential confounding factors (measured and unmeasured) that are shared by the twins within the pair are effectively controlled for. Wald tests were applied to test for differences between the within- and between-pair effects.
Additional potential confounding, identified a priori from previous studies, was addressed in two different models: (1) adjustments were made for maternal age at birth, birth year, and parity (only included in the between-pair model as the children within each twin pair had the same parity) and (2) additional adjustments were made for early-pregnancy BMI and maternal education. Due to a large percentage of missing data on breastfeeding (38%), this variable could not be included as a covariate in the analyses. With regards to the covariates smoking and diseases during pregnancy, there were only 15 mothers who reported to be smoking 3 months before pregnancy and no mothers who reported to be smoking during pregnancy. According to the medical records, no mothers were diagnosed with gestational diabetes and only one mother was diagnosed with pre-eclampsia. These potential confounding variables were therefore not included in the models.
Results
The twin sisters’ self-reported zygosity from our interviews was validated against the zygosity recorded in the STR (described elsewhere; Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006; Pedersen & Lichtenstein, Reference Pedersen, Lichtenstein, Smedby and Sorensen2000). In our final analytical cohort (with complete data on GWG and BW, see Figure 1), only around 12% had unknown zygosity and almost 21% (17 out of 82 pairs) of the information on zygosity had been confirmed by DNA analysis by the STR. However, as previously stated, these pairs were deemed MZ according to the questions from our own interviews.
Descriptive Results
In Table 1, maternal and infant characteristics (at the different time points) of the study population of 164 children, for whom we had data on BW, are displayed. The mothers’ mean early-pregnancy BMI was 22.5 kg/m2 (SD = 2.8 kg/m2), and 17% were categorized as overweight or obese at the beginning of their pregnancy. On average, the mothers gained approximately 14 kg (SD = 4.4 kg) during the pregnancy. In terms of the offspring, the mean BW was about 3600 g (SD = 479 g) and only 8.7% and 11.4% of the girls and 5.8% and 6.2% of the boys at 5 and 10 years, respectively, were categorized as overweight or obese.
TABLE 1 Characteristics of the MZ Twin Mothers and Their Children

GWG = gestational weight gain; BMI = body mass index; SD = standard deviation; 1Based on a study sample of 142 twin mothers (71 pairs); 2Based on a study sample of 138 twin mothers (69 pairs); 3Based on a study sample of 114 twin mothers (57 pairs).
Total GWG and All Outcomes
As displayed in Table 2, each kg of total GWG was associated with an increase of 0.08 (95% CI: 0.001, 0.17) z-score units in BW (corresponding to approximately 43 g) within the twin pairs in the fully adjusted model. In terms of offspring weight at 1 year (Table 3), we found similar effects both within (β = 0.04, 95% CI: -0.06, 0.15) and between (β = 0.04, 95% CI: -0.01, 0.09) the twin pairs in the fully adjusted models. Similar, although statistically weak, results were also seen for the later outcome variables BMI at 5 and 10 years of age within twin pairs (Table 4; fully adjusted models): 0.02 z-score units for every 1-kg increase in GWG (95% CI: -0.06, 0.10) at both ages.
TABLE 2 Within- and Between-Pair Effects of 1-kg Greater Gestational Weight Gain and Mean Growth Rate (kg/Week) During Each Trimester on Children's Birth Weight (z-Scores) (n = 164 children, 82 Twin Pairs)
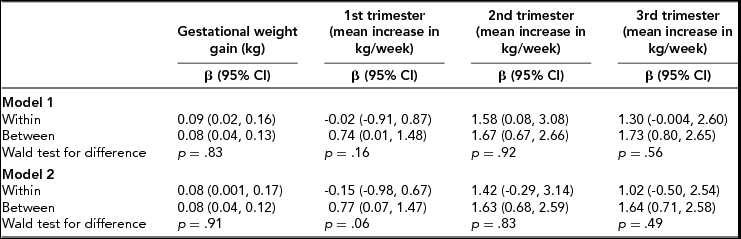
Model 1: Adjusted for maternal age at birth, birth year, and parity (only included in the between model); Model 2: Adjusted as per model 1 plus early-pregnancy BMI and maternal education.
TABLE 3 Within- and Between-Pair Effects of 1-kg Greater Gestational Weight Gain and Mean Growth Rate (kg/week) During Each Trimester on Children's Weight at 1 year (z-Scores) (n = 142 Children, 71 Twin Pairs)
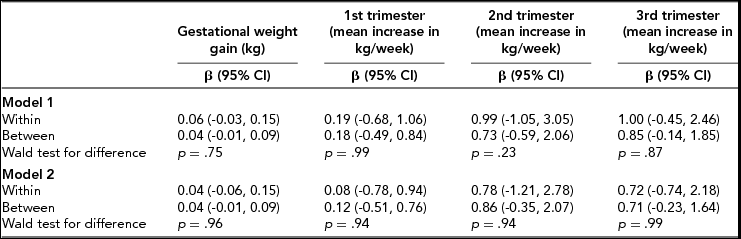
Model 1: Adjusted for maternal age at birth, birth year, and parity (only included in the between model), Model 2: Adjusted as per model 1 plus early-pregnancy BMI and maternal education.
TABLE 4 Within- and Between-Pair Effects of 1-Kg Greater Gestational Weight Gain and Mean Growth Rate (Kg/Week) During Each Trimester on Children's z-Score BMI at 5 and 10 Years

Model 1: Adjusted for maternal age at birth, birth year, and parity (only included in the between model); Model 2: Adjusted as per model 1 plus early-pregnancy BMI and maternal education.
Trimester-Specific GWG and All Outcomes
With regards to trimester-specific GWG and BW, statistically significant associations were found across all trimesters between twin mothers in the fully adjusted models, with the strongest effects seen in the second and third trimester: β = 1.63 (95% CI: 0.68, 2.59) and β = 1.64 (95% CI: 0.71, 2.58) z-score units, respectively, (corresponding to approximately 782 g and 776 g, respectively) per 1-kg mean weight increase per week (Table 2). When taking genetic and shared environment into account (within twin pairs), the results became non-significant. However, the results might indicate that the second and third trimester were associated with offspring BW due to the magnitude of their respective coefficients (β = 1.42 z-score units (95% CI: -0.29, 3.14) and β = 1.02 z-score units (95% CI: -0.50, 2.54) corresponding to approximately 682 g and 490 g increase in BW per 1-kg mean weight increase per week). As seen in Table 3, we found no associations between GWG in the first trimester and weight at 1 year in the offspring. In terms of the second and third trimester, the beta coefficients were overall larger compared to the first trimester; however, the confidence intervals were rather broad. As for offspring BMI at 5 and 10 years, similar statistically weak results were found across all trimesters (Table 4).
Sensitivity Analyses
We repeated the analyses using alternative measures of the outcome variables at ages 1, 5, and 10 years, namely BMI instead of weight at 1 year and weight instead of BMI at 5 and 10 years in the offspring. We also analyzed the potential association between the weight increase during the first year (weight at 1 year subtracted by the BW) and GWG, using the same models for adjustment as previously explained. In order to test whether parity could influence the results, we also repeated the analyses restricting the study cohort to first born children. As we used means and standard deviations from our own study cohort to create sex-specific z-scores for weight and BMI at the ages of 1, 5, and 10, we also repeated the analyses using external means and standard deviations from a larger Swedish population-based cohort (N ≈ 1,400 children; described elsewhere, Kark et al., Reference Kark, Tynelius and Rasmussen2009). Nevertheless, none of the results from these additional analyses differed substantially from those derived from the main analyses (data not shown).
Discussion
GWG, both total and within each trimester, was significantly associated with weight at birth in the offspring between twin pairs. When taking shared genetic and environmental factors within the twin pairs into account there was still a small significant effect for total GWG on BW; however, in terms of trimester-specific effects, the results were all non-significant. Nonetheless, the rather large effect sizes could indicate that GWG, specifically during the second and third trimesters, contributed to offspring BW. Given these findings, it seems that environmental and/or genetic factors shared between the twin pairs explain the association observed between GWG in the first trimester and offspring BW, as when these factors were taken into account in the within-pair analysis, no effects remained. These unknown environmental risk factors can perhaps include common lifestyle factors (e.g., physical activity and dietary habits) which the MZ twins shared and established during their upbringing (as they were all reared together). These could in turn influence both the GWG and their children's BW (as these types of obesity-related lifestyle patterns tend to cluster within families (Cameron et al., Reference Cameron, Crawford, Salmon, Campbell, McNaughton, Mishra and Ball2011). In contrast, intrauterine mechanisms (perhaps through an in utero programming of the fetus) seemed to drive the association between total and trimester-specific GWG during the second and third trimester (as we observed potential within-pair effects). These possible mechanisms are nonetheless speculative, and due to the uncertainty in our estimates, the results should be interpreted with caution. With regards to later infant and childhood weight or BMI outcomes, we did not find any associations with total or trimester-specific GWG, neither within nor between twin pairs.
Our results confirm previous research which found that both total and trimester-specific GWG were significantly associated with offspring BW in unrelated individuals (comparable to our between-pair effects; Abrams & Selvin, Reference Abrams and Selvin1995; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012; Starling et al., Reference Starling, Brinton, Glueck, Shapiro, Harrod, Lynch and Dabelea2015; Wander et al., Reference Wander, Sitlani, Badon, Siscovick, Williams and Enquobahrie2015), although some studies found that the association with offspring BW was limited to early and/or mid-pregnancy (Abrams & Selvin, Reference Abrams and Selvin1995; Brown et al., Reference Brown, Murtaugh, Jacobs and Margellos2002; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012; Sekiya et al., Reference Sekiya, Anai, Matsubara and Miyazaki2007; Starling et al., Reference Starling, Brinton, Glueck, Shapiro, Harrod, Lynch and Dabelea2015; Wander et al., Reference Wander, Sitlani, Badon, Siscovick, Williams and Enquobahrie2015). Once controlling for shared genes and environment in the within-pair model, we found an effect of total GWG on offspring BW, which is in line with earlier studies (using a similar study design with sibling-pairs; Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014; Lawlor et al., Reference Lawlor, Lichtenstein, Fraser and Langstrom2011; Ludwig & Currie, Reference Ludwig and Currie2010). One previous study of siblings born before and after maternal weight loss induced by bariatric surgery also used a within- and between-comparison design to look at the trimester-specific effects of GWG on offspring BW. They found evidence for a stronger association specifically during the second trimester within sibling pairs (β = 0.93 z-score units, 95% CI: 0.26, 1.59; Berglind et al., Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014). In accordance with the latter study, we found stronger within-pair effects during the second trimester, although the estimate was not significant.
As mentioned in the introduction, several studies on unrelated women and their offspring have found positive associations between GWG and infant and childhood weight or BMI (Guo et al., Reference Guo, Liu, Ye, Zhuang and Ren2015; Margerison-Zilko et al., Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams2012; Oken et al., Reference Oken, Taveras, Kleinman, Rich-Edwards and Gillman2007; Schack-Nielsen et al., Reference Schack-Nielsen, Michaelsen, Gamborg, Mortensen and Sorensen2010). However, studies that have adjusted for shared mother–child genetic and environmental influences are few and their findings are conflicting (Branum et al., Reference Branum, Parker, Keim and Schempf2011; Ludwig et al., Reference Ludwig, Rouse and Currie2013). Ludwig et al. (Reference Ludwig, Rouse and Currie2013) detected a positive within-sibling pair association of GWG and offspring BMI at the mean age of 11.9 years, whereas Branum et al. (Reference Branum, Parker, Keim and Schempf2011) found that GWG was not related to child BMI at 4 years of age, after controlling for shared genetic and environmental factors. Berglind et al. (Reference Berglind, Willmer, Naslund, Tynelius, Sorensen and Rasmussen2014) did not find any significant associations between total GWG and childhood BMI at ages 4 and 6 years, neither within nor between sibling pairs. The implications of the positive association from Ludwig et al. (Reference Ludwig, Rouse and Currie2013) can, however, be discussed, as the effect size was very small (β = 0.02 kg/m2 for every 1-kg increase in GWG, 95% CI: 0.01, 0.03) and could possibly be explained by residual confounding from unmeasured non-shared factors. Although the results from our study (which show no significant associations between total or trimester-specific GWG and infant and childhood weight/BMI) point in the same direction as the study by Branum et al. (Reference Branum, Parker, Keim and Schempf2011), we cannot confirm those findings due to the fact that we had similar non-significant results in the between-pair analyses.
The major strength of our study was the ability to reduce bias from shared genetic and environmental factors which are held constant within the twin pair (fixed effects). However, it is important to point out that even though the MZ twin sisters share the same genotype, their children are genetically like half-siblings, that is, they share only 25% of their genotype, which is why we cannot fully eliminate genetic confounding. It should also be highlighted that this data set of MZ twin pairs gave us the unique opportunity to study GWG in singleton pregnancies of twin sisters. In comparison, studying early anthropometric measurements in twin pregnancies is not feasible as they differ in terms of BW compared to singleton pregnancies (Kaprio & Silventoinen, Reference Kaprio and Silventoinen2011).
Maternal age and parity are two factors which are known to confound the potential association between GWG and childhood weight (Paulino et al., Reference Paulino, Surita, Peres, Nascimento and Morais2015; Prysak et al., Reference Prysak, Lorenz and Kisly1995; Reynolds et al., Reference Reynolds, Osmond, Phillips and Godfrey2010). We were able to control for parity (or birth order) by restricting the analyses to children of the same parity in the twin pair, which is also an advantage when studying offspring of MZ twins compared to full-sibling pairs. With respect to maternal age, the mean age difference of the mothers within the pairs was rather small (0.95 years). Additionally, due to the fact that we extracted our data from medical records, we had access to detailed measurements of GWG, which enabled us to disentangle the effects of GWG in the different trimesters. Although this type of routinely collected data may include measurement errors, it is a strength to have objectively measured data, as many previous studies relied on self-reported data.
A limitation of the present study was the relatively small sample size, which implied low statistical power. This was particularly apparent for the later weight outcomes at ages 1, 5, and 10 years, where we did not reach a statistical power of 50% in any of the within-pair analyses. To achieve a statistical power of at least 80% in terms of these outcome variables, the effect size needed to be approximately 1.5 times stronger than the strongest observed within-pair association. Further large family-based studies, with trimester-specific GWG measurements and longer follow-up in the children, are therefore needed. Moreover, even though we were able to adjust for various potential confounding factors, we cannot rule out the influence of residual confounding from other unique environmental factors. In terms of generalizing our results to non-twin (singleton) mothers, adult twins have previously been shown to be highly representative of the general population (Kaprio & Silventoinen, Reference Kaprio and Silventoinen2011). However, as our participants were pre-dominantly white Caucasian, we cannot assert that our findings are generalizable to other ethnic groups, as a recent study found that the effect of GWG on offspring BW differed by ethnicity (Lin et al., Reference Lin, Aris, Tint, Soh, Godfrey, Yeo and Lee2015).
In conclusion, our findings suggest that total, and possibly also second and third trimester, GWG are associated with offspring BW when taking shared genetic and environmental factors within twin mother pairs into account. No associations were found between total or trimester-specific GWG and offspring weight at 1 year or BMI at ages 5 or 10 years. Due to our rather limited sample size, our findings need replication in future large family-based studies that can account for confounding from genetic and shared environment.
Acknowledgments
The authors kindly thank the twin mothers and their children, as well as the Swedish archives and antenatal clinics and schools for their generous collaboration. Information on zygosity was kindly provided by the Swedish Twin Registry, which was supported by a grant from the Ministry for Higher Education. This study was funded by a grant to Finn Rasmussen from the Swedish Research Council for Health, Working Life and Welfare (2010-0671).
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









