Prebiotics are non-digestible dietary factors that provide a beneficial physiological effect for the host by selectively stimulating the growth or activity of a limited number of indigenous bacteria. A combination of prebiotics and probiotics known as synbiotics, as originally proposed by Gibson & Roberfroid( Reference Gibson and Roberfroid 1 ), shows a synergistic influence on obesity, diabetes, fatty liver and colitis( Reference Patel and DuPont 2 ). We have also demonstrated a synergistic effect of synbiotcs in combination with Bifidobacterium breve and raffinose on the intestinal epithelial proliferation in rats( Reference Ishizuka, Iwama and Dinoto 3 ). Orally introduced bacteria usually suffer because of the gastric acid, digestive enzymes and bile acids (BA) secreted from the gastrointestinal tissues. Prebiotics promote their viability and activity in the luminal environment of the gastrointestinal tract. Bacillus coagulans is one of the spore-forming lactic acid bacteria that tolerate heat, oxygen, acid and BA( Reference Ara, Meguro and Hase 4 ). Soya pulp, which was supplemented to the rats in this study, is a by-product after extraction of soya milk. Some types of oligosaccharides observed in soyabean can be included in the soya pulp as well( Reference Fan, Zang and Xing 5 ), indicating that soya pulp has potential as a prebiotic. B. coagulans lilac-01 was previously isolated and the synbiotics of the strain with soya pulp as a prebiotic are effective for improving bowel movement and faecal properties in functionally constipated patients in a double-blinded and randomised study( Reference Minamida, Nishimura and Miwa 6 ).
BA, which are steroids synthesised from cholesterol, are made in the liver as primary BA and are secreted into the luminal environment of the intestine to help digestion and absorption of dietary lipids and hydrophobic nutrients( Reference Uchida, Nomura and Takeuchi 7 ). Most of the BA are reabsorbed in the ileum and return to the liver through the blood stream in a process known as enterohepatic circulation( Reference Redinger 8 , Reference Shneider 9 ). Secondary BA are produced by some intestinal bacteria via the deconjugation and dehydroxylation of primary BA. There is a mutual relationship between the intestinal BA metabolism and the intestinal bacterial composition. Some bacteria influence BA composition via deconjugation, dehydorgenation, epimerisation and dehydroxylation( Reference Ridlon, Kang and Hylemon 10 ). Both aerobic and anaerobic bacteria, such as Lactobacillus ( Reference Dashkevicz and Feighner 11 – Reference Pereira, McCartney and Gibson 13 ), Bifidobacterium ( Reference Kim, Yi and Lee 14 , Reference Grill, Schneider and Crociani 15 ) and Enterococcus ( Reference Knarreborg, Engberg and Jensen 16 ), possess deconjugation activity. Dehydroxylation activity is detected in Clostridium ( Reference Ferrari and Beretta 17 ). Increased secondary BA can cause colorectal and hepatic carcinogenesis via the induction of necrosis with inflammation and DNA damage( Reference Degirolamo, Modica and Palasciano 18 ).
The consumption of a high-fat diet increases BA secretion, especially that of the secondary BA such as deoxycholic acid (DCA) and lithocholic acid (LCA)( Reference Reddy 19 ). Ageing raises the cholic acid (CA):chenodeoxycholic acid (CDCA) ratio of the BA composition in rodents( Reference Uchida, Chikai and Takase 20 ). In humans, BA secreted from the liver are unchanged by age( Reference Salemans, Nagengast and Tangerman 21 ). However, there is an increase in the serum DCA concentration in response to meal consumption, suggesting an increase in the production of secondary BA in the intestine. We used dietary CA supplementation at a moderate level in rats to mimic the increase in both BA secretion and the CA:CDCA ratio by ageing with a high-fat diet. As a result, in the CA-fed rats, an increase in the relative abundance of the phylum Firmicutes and a decrease in the phylum Bacteroidetes were observed( Reference Islam, Fukiya and Hagio 22 ). Such alterations of the gut bacterial composition are similar to that observed in obese individuals or with high-fat diet consumption. The results indicate that CA supplementation at a moderate level in the diet is involved in some aspects of metabolic disorders. In addition, probiotics, prebiotics and synbiotics might influence the CA-induced dysregulations in host homoeostasis.
The aim of this study was to investigate whether the combination of B. coagulans with soya pulp influences BA excretion and host homoeostasis under CA-supplemented conditions.
Methods
Animals
This study was approved by the Hokkaido University Animal Committee. Animal care and use were in accordance with the Hokkaido University guidelines for the care and use of laboratory animals. After acclimation of the male Wistar rats (4 weeks old; Japan SLC, Inc.) with an AIN-93G diet( Reference Reeves, Nielsen and Fahey 23 ) for 7 d, the rats were divided into five dietary groups and fed ad libitum a basal diet (B) or diets containing CA (0·5 g/kg diet) in combination with B. coagulans lilac-01 at 109 colony forming unit (CFU)/kg diet and soya pulp for 8 weeks, as shown in Table 1. The rats fed with the CA-supplemented diet were regarded as the control (Ct) among the CA-fed groups. The probiotic diet (Pro) and the synbiotic diet (Syn) supplemented with CA contained either B. coagulans lilac-01 (109 CFU/kg diet; Arterio Bio Co. Ltd) or a mixture of soya pulp and B. coagulans lilac-01 (109 CFU/kg diet), respectively. The prebiotic diet (Pre) supplemented with CA contained soya pulp as the fibre source. The rats were housed in an air-conditioned room at 22±2°C with 55±5 % humidity, which was in the light cycle from 08.00 to 20.00 hours automatically.
Table 1 Diet compositions (g/kg diet)
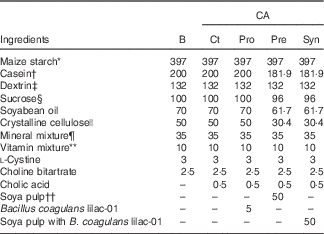
CA, cholic acid; B, basal diet; Pro, probiotic diet; Pre, prebiotic; Syn, synbiotic diet.
* Amylalpha; Chuo Shokuryou Co. Ltd.
† NZMP acid casein; Co-operative Group.
‡ TK16; Matsutani Chemical Industry Co., Ltd.
§ Nippon Beet Sugar Manufacturing Co., Ltd.
|| Ceolus PH-102; Asahi Kasei Chemicals Co.
¶ AIN-93 mineral mixture; MP Biomedicals, LLC.
** AIN-93 vitamin mixture; CLEA Japan Inc.
†† Kikkoman Soyfoods Co., Ltd.
After sampling the aorta and portal vein blood under anaesthesia with pentobarbital sodium salt (50 mg/kg body weight, Somnopentyl; Kyoritsu Seiyaku Corporation), organs including the liver, kidneys, whole caecum, epididymal adipose tissue and retroperitoneal adipose tissue were collected. The caecal contents were diluted with ultra-pure water, and the pH was measured. The plasma and liver samples were stored at −80°C. The caecal contents and faeces were stored at −40°C until the HPLC analysis.
Gut permeability
The rats were orally administered chromium (III) chloride hexahydrate (Wako Pure Chemical Industries, Ltd) solution with EDTA 2Na (Dojindo Laboratories) 2 d before being killed( Reference Suzuki and Hara 24 ). Urine samples were collected at 24 h after the oral administration, and urinary excretion of Cr was measured using an atomic absorption photometer (Z-5310; Hitachi High-Technologies Corporation).
Liver and plasma parameters
For aspartate aminotransferase (AST) and alanine aminotransaminase (ALT), aortic plasma was analysed using a transaminase CII test Wako kit (Wako Pure Chemical Industries, Ltd). NEFA were determined in aortic plasma using a NEFA C test Wako (Wako Pure Chemical Industries, Ltd). Adiponectin levels in the aortic plasma were measured using an adiponectin ELISA kit (Otsuka Pharmaceutical Co., Ltd). For lipid extraction, 100 mg of liver was immersed in lipid-extraction solution (chloroform–methanol=2:1)( Reference Folch, Lee and Solane-Stanley 25 ) and placed for 2 d. The solvent in the extracts was corrected and evaporated spontaneously in the fume hood. The extracted lipids were dissolved with 2-propanol for measurement. TAG and cholesterol were determined using the TAG E-test and cholesterol E-test from Wako (Wako Pure Chemical Industries, Ltd), respectively.
Bile acid analysis
The BA were measured by UPLC/ESI-MS (Waters) with 23-nor-5β-cholanic acid-3α,12α-diol (NDCA) as an internal standard according to our previous study( Reference Hagio, Matsumoto and Fukushima 26 ). The BA in the freeze-dried samples were extracted with ethanol and then purified with an HLB cartridge (Waters) according to the manufacturer’s instruction. The BA in each sample were separated using an Acquity UPLC system equipped with a BEH C18 column (1·7 μm, 100×2·0 mm i.d.) (Waters) and were analysed on a Quattro Premier XE quadrupole tandem MS (Waters). The BA measured in this study were as follows: 5β-cholanic acid-3α,7α,12α-triol (CA); 5β-cholanic acid-3α,6β,7α-triol (α-muricholic acid; αMCA); 5β-cholanic acid-3α,6β,7β-triol (β-muricholic acid; βMCA); 5β-cholanic acid-3α,6α,7β-triol (ω-muricholic acid; ωMCA); 5β-cholanic acid-3α,6α-diol (hyodeoxycholic acid; HDCA); 5β-cholanic acid-3α,7β-diol; 5β-cholanic acid-3α,7α-diol (CDCA); 5β-cholanic acid-3α,12α-diol (DCA); 5β-cholanic acid-3α-ol (LCA); 5β-cholanic acid-3α,6α,7α-triol; 5β-cholanic acid-3α,7β,12α-triol (ursocholic acid; UCA); 5β-cholanic acid-3α,12α-diol-7-one; 5β-cholanic acid-3α-ol-7-one; 5β-cholanic acid-3α-ol-12-one (12-oxo-lithocholic acid; 12oLCA); 5β-cholanic acid-12α-ol-3-one (3o12α); 5β-cholanic-3α,7α,12α-triol-N-(2-sulphoethyl)-amide (taurocholic acid; TCA); 5β-cholanic-3α,6β,7α-triol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,6β,7β-triol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,6α,7β-triol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,6α-diol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,7α-diol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,12α-diol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α-ol-N-(2-sulphoethyl)-amide; 5β-cholanic-3α,7α,12α-triol-N-(carboxymethl)-amide; 5β-cholanic-3α,6α-diol-N-(carboxymethl)-amide; 5β-cholanic-3α,7β-diol-N-(carboxymethl)-amide; 5β-cholanic-3α,7α-diol-N-(carboxymethl)-amide; 5β-cholanic-3α,12α-diol-N-(carboxymethl)-amide; and 5β-cholanic-3α-ol-N-(carboxymethl)-amide.
Organic acid analysis
Organic acids in the caecal contents were measured using HPLC (Shimadzu Corporation) with crotonic acid (Wako Pure Chemical Industries, Ltd) as an internal standard according to the method of Hoshi et al. ( Reference Hoshi, Sakata and Mikuni 27 ). The caecal contents were homogenised and neutralised with sodium hydroxide to prevent the extraction of SCFA. Fat-soluble substances in the supernatant were removed by chloroform, and the aqueous phase was passed through a membrane filter (cellulose acetate, 0·20 μm pore size; DISMIC-13cp; Toyo Roshi Kaisha, Ltd). The samples were analysed by HPLC (Shimadzu Corporation) equipped with a solvent delivery system (SCL-10 AVP; Shimadzu Corporation), a double ion-exchange column (Shim-Pack SCR-102H, 8×300 mm; Shimadzu) and an electro-conductivity detector (CDD-6A; Shimadzu Corporation). The mobile phase was 5 mm of p-toluenesulphonic acid, and the detection solution was 5 mm of p-toluenesulphonic acid containing 100 μm of EDTA and 20 mm of bis-tris.
Enumeration of the intestinal bacteria
Intestinal bacteria in the caecal contents were determined as described in the previous study( Reference Minamida, Ohashi and Hara 28 ) with some modifications. The diluted solutions of caecal contents were inoculated onto plates with XM-G agar (Nissui Pharmaceutical Co., Ltd) for Escherichia coli and with standard method agar (Nissui Pharmaceutical Co., Ltd) for B. coagulans. Colony counts were made after cultivation at 37°C for 20 h (E. coli) and 3 d (bifidobacteria and lactobacilli) in an anaerobic chamber and at 55°C for 2 d (B. coagulans). The CFU per gram of wet caecal contents were calculated.
Intraperitoneal glucose tolerance test
The intraperitoneal glucose tolerance (IPGTT) test was conducted according to the method of Higuchi et al. ( Reference Higuchi, Hira and Yamada 29 ). After fasting for 16 h, blood samples were collected for the controls from the tail vein with 50 IU/ml heparin and 200 kIU/ml aprotinin at 7 weeks, and the glucose solution was injected (1 g/kg body weight) into the intraperitoneal cavity. Plasma samples were obtained from the tail vein with heparin and aprotinin at 15, 30, 60 and 120 min after glucose administration. The blood glucose levels were analysed using a glucose CII test Wako kit (Wako Pure Chemical Industries, Ltd).
Statistics
All the results are expressed as mean values with their standard errors. Statistical significance for comparisons between the basal group and each CA-fed group was determined using Dunnett’s test. A two-way ANOVA was performed in the CA-fed groups for B. coagulans lilac-01 and soya pulp; probability <0·05 was considered statistically significant. JMP pro 12.0.1 (SAS institute Inc.) was used for the statistical analysis.
Results
Dietary supplementation of CA significantly promoted faecal BA excretion in various molecular species, such as DCA, 12oLCA, 3o12α and HDCA (Fig. 1). In particular, faecal excretion of DCA, one of the toxic secondary BA originating from CA, increased significantly in the CA-fed rats as expected (Dunnett’s test with basal, P<0·05). Moreover, DCA concentration was extremely higher in rats fed prebiotics in the CA-fed groups. An unexpected increase was found in faecal excretion of MCA and DCA by pre-ingestion. Interestingly, such an increase in secondary BA by soya pulp was almost abolished when the rats were fed the form of synbiotics with B. coagulans.

Fig. 1 Bile acid composition in wet faeces of rats fed basal (B) or cholic acid (CA)-supplemented diets in combination with Bacillus coagulans and soya pulp. Values are means (n 5–7), with standard errors represented by vertical bars. * Significant difference from the value observed in rats fed the basal diet (P<0·05, Dunnett’s test). P values of the two-way ANOVA are shown in the inserted table for B. coagulans (Bc) and soya pulp (Soy). αMCA, α-muricholic acid; βMCA, β-muricholic acid; ωMCA, ω-muricholic acid; HDCA, hyodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UCA, ursocholic acid; 12oLCA, 12-oxo-lithocholic acid; 3o12α, 5β-cholanic acid-12α-ol-3-one; ![]() , B; CA:
, B; CA: ![]() , Ct;
, Ct; ![]() , probiotic diet;
, probiotic diet; ![]() , prebiotic diet;
, prebiotic diet; ![]() , synbiotic diet.
, synbiotic diet.
In addition, no significant difference was found in body weight gain and total food intake among the groups. In CA-fed groups, ingestion of soya pulp decreased liver weight (two-way ANOVA, P=0·0145) (Table 2). Liver weights tended to increase in CA-fed rats compared with basal diet-fed rats. No differences were observed for the other tissue weights, such as the kidney, whole caecum, epididymal adipose tissue and retroperitoneal adipose tissue. As a liver damage marker, we determined plasma AST (Fig. 2(a)) and ALT (Fig. 2(b)). There was no significant difference in AST levels among CA-fed rats, although ALT levels increased slightly in Syn-fed rats compared with basal diet-fed rats (Dunnett’s test with basal, P<0·05). Plasma AST and ALT also tended to increase in CA-fed rats compared with basal diet-fed rats. No significant difference was found in NEFA among the groups (Fig. 2(c)). Plasma TAG concentration was reduced in CA-fed rats, with the exception of Pro-fed rats, compared with the level in rats fed the basal diet (Dunnett’s test with basal, P<0·05) (Fig. 2(d)). In addition, there was an interaction between the effect of prebiotics and probiotics on the plasma TAG concentration in CA-fed rats (two-way ANOVA, P=0·0252). No significant difference was observed in plasma cholesterol concentrations (Fig. 2(e)). The ingestion of soya pulp increased plasma adiponectin concentrations in CA-fed rats (two-way ANOVA, P=0·0023) (Fig. 2(f)). The CA treatment promoted urinary Cr excretion as a marker of gut permeability (Dunnett’s test with B, P<0·05) (Fig. 2(g)). Within CA-fed groups, gut permeability was alleviated by the ingestion of soya pulp (two-way ANOVA, P=0·0036). The ingestion of B. coagulans did not significantly influence gut permeability. There was no significant influence of the diets on plasma glucose concentration in the IPGTT study (Fig. 2(h)). Although there was no difference in liver TAG concentration among the groups (Fig. 2(i)), the ingestion of soya pulp significantly reduced liver cholesterol concentration in CA-fed rats (two-way ANOVA, P=0·0165) (Fig. 2(j)).

Fig. 2 Plasma parameters, urinary chromium excretion, intraperitoneal glucose tolerance test (IPGTT) and liver lipids of the rats fed basal (B) or cholic acid (CA)-supplemented diets in combination with Bacillus coagulans and soya pulp. Plasma concentrations of (a) aspartate aminotransferase (AST), (b) alanine aminotransaminase (ALT), (c) NEFA, (d) TAG, (e) cholesterol and (f) adiponectin. (g) Urinary chromium excretion as a marker for gut permeability at week 8. (h) Changes in plasma concentration during IPGTT at week 7. (i) Liver TAG accumulation, and (j) liver cholesterol accumulation at week 8. Values are means (n 5–7), with standard errors represented by vertical bars. * Significant difference from the value observed in rats fed the basal diet (P<0·05, Dunnett’s test). P values of the two-way ANOVA are shown in the inserted table for B. coagulans (Bc) and soya pulp (Soy). ![]() , B;
, B; ![]() , control (Ct);
, control (Ct); ![]() , probiotic diet (Pro);
, probiotic diet (Pro); ![]() , prebiotic diet (Pre);
, prebiotic diet (Pre); ![]() , synbiotic diet (Syn);
, synbiotic diet (Syn); ![]() , B;
, B; ![]() , Ct;
, Ct; ![]() , Pro;
, Pro; ![]() , Pre;
, Pre; ![]() , Syn.
, Syn.
Table 2 Growth parameters and tissue weights in rats fed experimental diets (Mean values with their standard errors)

CA, cholic acid; B, basal diet; Ct, control; Pro, probiotic diet; Pre, prebiotic; Syn, synbiotic diet; Bc, Bacillus coagulans; Soy, soya pulp; BW, body weight.
For caecal SCFA, there was a significant increase in the concentration of acetic acid in the Pre- and Syn-fed rats and in the concentration of succinic acid in Syn-fed rats compared with rats fed the basal diet (Dunnett’s test, P<0·05) (Fig. 3(a)). There were subtle differences in the levels of other SCFA, but the levels were quite low in all cases. In CA-fed rats, ingestion of soya pulp increased acetic acid levels (two-way ANOVA, P=0·0361). In addition, a reduction of pH in caecal contents was found in relation to the increase in acetic acid concentrations (Fig. 3(b)). In caecal contents, B. coagulans was detected in Pro- and Syn-fed rats (Fig. 3(c)). The number of bifidobacteria and coliform was unaffected by the dietary intervention. A significant increase in the number of lactobacilli was observed following the ingestion of soya pulp in CA-fed rats (two-way ANOVA, P=0·0236).

Fig. 3 Caecal parameters of rats fed basal (B) or the cholic acid (CA)-supplemented diets in combination with Bacillus coagulans and soya pulp at week 8. (a) Organic acid concentrations, (b) the pH of the caecal contents and (c) the number of bacteria in the caecal contents. Values are means (n 5–7), with standard errors represented by vertical bars. * Significant difference from the value observed in rats fed the basal diet (P<0·05, Dunnett’s test). P values of the two-way ANOVA are shown in the inserted table for B. coagulans (Bc) and soya pulp (Soy). CFU, colony forming unit; ![]() , B; CA:
, B; CA: ![]() , control (Ct);
, control (Ct); ![]() , probiotic diet;
, probiotic diet; ![]() , prebiotic diet;
, prebiotic diet; ![]() , synbiotic diet.
, synbiotic diet.
Discussion
MCA derived from CDCA are major molecular species of primary BA in rodents( Reference Botham and Boyd 30 ); however, two major primary BA – CA and CDCA – are reported in humans( Reference Ridlon, Kang and Hylemon 10 ). Ageing increases DCA production in rodents and humans( Reference Uchida, Chikai and Takase 20 , Reference Salemans, Nagengast and Tangerman 21 ). As ingesting a high-fat diet increases BA secretion( Reference Reddy 19 , Reference Stadler, Stern and Yeung 31 ), CA supplementation in enterohepatic circulation is reasonable to mimic the BA alteration in ageing and high-fat diet consumption. Generally, CA and taurocholic acid (TCA) are present at trace levels in the faeces( Reference Ridlon, Kang and Hylemon 10 , Reference Hagio, Matsumoto and Fukushima 26 ), but excess amounts of these primary or conjugated BA are found in rat faeces when the supplementation level of CA is 0·2 % in the diet( Reference Hagio, Shimizu and Joe 32 ). These observations suggest that CA supplementation to the diet beyond 0·2 % is obviously an overdose for BA deconjugation and conversion ability of the intestinal bacteria. In contrast, such an excess increase in CA and TCA are not observed when rats are fed a CA diet at 0·05 % or below( Reference Islam, Fukiya and Hagio 22 ). Considering the gut BA metabolism, there must be an obstruction of BA deconjugation and hydroxylation in rats fed a CA supplementation >0·2 %.
There is a report showing that dietary CA supplementation at 0·5 % promotes energy expenditure and abrogates diet-induced obesity in mice( Reference Watanabe, Houten and Mataki 33 ). In our previous study( Reference Islam, Fukiya and Hagio 22 ), we also observed a similar reduction in adipose tissue weight in rats fed a normal-fat diet supplemented with 0·2 % of CA. However, such a reduction in adipose tissue weight disappeared in rats fed a diet that was supplemented with 0·05 % of CA. In contrast, a microbiota analysis revealed an increase in Firmicutes in rats fed CA diets, regardless of the CA supplementation levels.
High-fat diet feeding is one of the practical models to reproduce diet-induced obesity and the metabolic syndrome. In preparation of a high-fat diet, we usually change the levels of two major ingredients at the same time, usually carbohydrates and lipids, which makes the interpretation of results more complicated because we need to consider the influence of two ingredients in the diet with regard to the results obtained. We can avoid such a complicated situation in case of the CA-supplementation study, because CA supplementation level at 0·5 g/kg diet does not influence the composition of the major nutrients in the diets. We consider that the CA supplementation level in this experiment successfully mimics the BA metabolism found in ageing and in high-fat diet consumption without changing the composition of the major nutrients, as determined by the BA composition in the faeces.
First, we investigated BA metabolism in rats fed the CA diet. No difference in CA and TCA was confirmed among the groups, whereas a selective increase was found for 12α-hydroxylated BA, such as DCA, 12oLCA and 3o12α, in rats fed the CA diet. This result indicates that the supplemented CA was completely converted to DCA via 7α-hydroxylation in the large intestine, which represents the BA composition observed both in ageing and in high-fat diet consumption. By using CA supplementation, we evaluated whether B. coagulans and soya pulp influence BA metabolism. Interestingly, the ingestion of soya pulp increased DCA excretion, whereas the combination of soya pulp with B. coagulans normalised BA metabolism.
We observed a prominent increase in both MCA and DCA in rats fed Pro. In rodents, CA and MCA are usually secreted as taurine-conjugated forms in the bile juice. The taurine-conjugates are preferentially absorbed in the ileal epithelia, whereas the unconjugates are less absorbed( Reference Liong and Shah 12 ). Many types of bifidobacteria and lactobacilli possess bile salt hydrolase (BSH) activity and are involved in BA deconjugation( Reference Liong and Shah 12 ). It is possible that these bacteria also increased in the intestinal contents of rats fed the Pre or Syn diet because soya pulp can be used as an energy source in the upper small intestine. The promotion of deconjugation and the inhibition of enterohepatic BA circulation by the increased population of endogenous lactic acid bacteria might be responsible for the increase in faecal DCA and MCA excretions. At an acidic pH, achieved by luminal fermentation, unconjugated BA are protonated and precipitated, whereas taurine-conjugates remain solubilised( Reference Dashkevicz and Feighner 11 ). Such unconjugated BA might be precipitated in the soya pulp and move into the large intestine, resulting in an increase in secondary BA. It is notable that excess increases in DCA and MCA were not observed in Syn-fed rats, regardless of soya pulp consumption. In general, BA deconjugation occurs gradually in the small intestine( Reference Tannock, Tangerman and Schaik 34 ), and almost no conjugated BA are found in the caecal contents( Reference Islam, Fukiya and Hagio 22 ). However, in case of rats fed Syn, a massive amount of B. coagulans might exist in the small intestine due to the existence of soya pulp. It is possible that a reduction in BSH activity in the small intestine enables the suppression of DCA production if the B. coagulans does not display BSH activity.
Secondary BA are highly hydrophobic and can be absorbed in the large intestine, and thereby increase the risk for cancer development in the liver and large intestine. In a separate study( Reference Shimizu, Hagio and Iwaya 35 ), we observed that DCA is responsible for the proliferation and migration of vascular smooth muscle cells, indicating that an increased DCA concentration is involved in the development of various disorders. DCA is also shown to disrupt barrier function in gut epithelia in vivo ( Reference Stenman, Holma and Eggert 36 ), and the penetration of exogenous substances is considered to induce unnecessary inflammatory responses in the liver and other gastrointestinal tissues. However, this study showed that soya pulp itself increased DCA production in the large intestine without increasing gut permeability. This result indicated that DCA itself might not necessarily be responsible for the CA-induced increase in gut permeability. In this study, we found that in rats fed the CA diet, ingesting soya pulp promoted barrier function. Acetate and butyrate suppressed colonic permeability in ex vivo and culture studies( Reference Suzuki, Yoshida and Hara 37 ). It is possible that the increase in acetic acid concentration in the luminal contents is involved in the normalisation of gut permeability by the ingestion of soya pulp. It could be advantageous to use a combination of soya pulp and B. coagulans to reduce the unnecessary increase in secondary BA production in the large intestine.
In the present study, the ingestion of soya pulp improved lipid metabolism under CA supplementation, although no positive effect was found on glucose tolerance. According to the manufacturer’s instruction, the soya pulp used in the study contained 31 g of proteins, 8 g of carbohydrates, 17 g of lipids and 36 g of dietary fibre( Reference Minamida, Nishimura and Miwa 6 )/100 g. The actual concentration of soya protein was approximately 1·5 % in the diet. Soya pulp or soya protein is known to decrease liver lipid concentrations and lower the incidence of atheroscrelotic lesions( Reference Lo, Evans and Philip 38 ), but the supplementation levels of soya pulp and soya protein were quite low in this study. We have already reported a decrease in adiponectin concentration by CA ingestion( Reference Islam, Fukiya and Hagio 22 ), but the mechanism is still unclear. However, such increases in plasma adiponectin concentrations by the ingestion of soya pulp under the consumption of the CA diet are considered to be protective against many aspects of the metabolic disorder( Reference Brochu-Gaudreau, Rehfeldt and Blouin 39 ).
We usually consider that prebiotics are effective for health maintenance. In this study, some promotional effects of prebiotics were actually found in intestinal permeability and plasma adiponectin concentrations. However, this study also indicates that consumption of prebiotics was not necessarily ‘almighty’ in health maintenance. As a probiotic, B. coagulans might not be active in Pro-fed rats as judged by the pH in the caecal contents and DCA concentration in the faeces, indicating that they need an accessible form of energy source. We suggest that synbiotics will be an option to avoid such unfavourable situations. At this moment, there is no gold standard of a specific combination of prebiotics and probiotics. Careful management is required to find such suitable combinations.
In conclusion, soya pulp increased BA excretion induced by the CA diet, but the increased BA excretion was abolished when rats were fed soya pulp with B. coagulans. The ingestion of soya pulp basically improved gut permeability and plasma adiponectin, as well as normalisation of liver weight induced by ingestion of the CA diet. These results suggest that a suitable combination of probiotics and prebiotics reduces the risk for the development of various gastrointestinal disorders provoked via an increase in secondary BA.
Acknowledgements
This study was supported by the Regional Innovation Strategy Support Program of the MEXT (Ministry of Education, Culture, Sports, Science and Technology) from the Japanese Government.
Y. L. and S. I. analysed the data and wrote the article; S. I. and K. Minamida designed the study; Y. L., R. Y., K. K., G.-H. J., M. T., H. S. and T. N. carried out the research; K. Minamida and K. Miwa provided essential materials; K. Minamida and H. H. formulated the questions.
There are no conflicts of interest.









