Introduction
Asian elephants Elephas maximus prefer forest–grassland and forest–agricultural ecotones over large expanses of continuous habitats such as primary forests (Fernando et al., Reference Fernando, Wikramanayake, Janaka, Jayasinghe, Gunawardena, Kotagama, Weerakoon and Pastorini2008). These preferred habitats are often embedded within human-dominated landscapes and outside protected area networks (Fernando et al., Reference Fernando, Janaaka, Prasad and Pastorini2010, Reference Fernando, De Silva, Jayasinghe, Janaka and Pastorini2019). In shared landscapes, elephants often exploit anthropogenic food sources such as crops, leading to negative human–elephant interactions (Sukumar, Reference Sukumar1989, Reference Sukumar1990; Hoare, Reference Hoare1999; Corea, Reference Corea2001, Reference Corea2006; Sitati et al., Reference Sitati, Walpole, Smith and Leader-Williams2003; Fernando et al., Reference Fernando, Wikramanayake, Weerakoon, Jayasinghe, Gunawardene and Janaka2005, Reference Fernando, De Silva, Jayasinghe, Janaka and Pastorini2019). Such negative interactions are a major conservation and social issue, leading to food insecurity, emotional distress and restricted mobility in affected human communities (Desai & Heidi, Reference Desai and Heidi2015; Mayberry et al., Reference Mayberry, Hovorkab and Evans2017), and causing fatalities among people and elephants (Desai, Reference Desai1998; Desai & Heidi, Reference Desai and Heidi2015). These issues are increasing throughout the range of the Asian elephant (Sampson et al., Reference Sampson, McEvoy, Oo, Chit, Chan and Tonkyn2018). In rural areas of Sri Lanka, elephants frequently consume and damage crops (Fisher, Reference Fisher2016); as a result, farmers are becoming less tolerant of elephants and regard them as pests (Fernando et al., Reference Fernando, Wikramanayake, Weerakoon, Jayasinghe, Gunawardene and Janaka2005).
The main contributing factor leading to increasing human–elephant interactions is habitat loss and fragmentation because of infrastructure development and conversion to agricultural land (Corea, Reference Corea2001, Reference Corea2006; Koirala et al., Reference Koirala, Ji, Aryal, Rothman and Raubenheimer2015; Sampson et al., Reference Sampson, McEvoy, Oo, Chit, Chan and Tonkyn2018). This decreases the availability of natural food resources for elephants and increases their need to enter human-dominated landscapes. Expanding agricultural areas also provide a diversity of cultivated crops (Koirala et al., Reference Koirala, Ji, Aryal, Rothman and Raubenheimer2015) that elephants have learnt to exploit as a food source (Sukumar, Reference Sukumar1989; Hoare, Reference Hoare1999). Farmers, to protect their livelihoods, employ a variety of strategies to disrupt crop use by elephants, sometimes leading to injury or death of the animals (Koirala et al., Reference Koirala, Ji, Aryal, Rothman and Raubenheimer2015). Thus, elephants live in a mosaic of increasingly fragmented natural habitats interspersed with resource-rich but risky human-dominated agricultural lands (Fernando et al., Reference Fernando, Wikramanayake, Weerakoon, Jayasinghe, Gunawardene and Janaka2005; Koirala et al., Reference Koirala, Ji, Aryal, Rothman and Raubenheimer2015).
Navigating such environments involves managing risks by minimizing potential costs (e.g. experiencing harassment) and maximizing benefits (e.g. food acquisition; Cooper & Blumstein, Reference Cooper and Blumstein2015). Elephants have adapted to human-dominated landscapes (Fernando & Leimgruber, Reference Fernando, Leimgruber, McShea, Davies and Bhumpakphan2011) and appear to have changed their behaviour based on their perception of the risk posed by people (Wittemyer et al., Reference Wittemyer, Keating, Vollrath and Douglas-Hamilton2017). Most notably, elephants adapt the temporal structure of their activities to avoid human activity and persecution (Cook et al., Reference Cook, Henley and Parrini2015). For example, both Asian and African elephants Loxodontata africana tend to exploit risky anthropogenic food sources such as crops at night (Sitati et al., Reference Sitati, Walpole, Smith and Leader-Williams2003; Graham et al., Reference Graham, Douglas-Hamilton, Adams and Lee2009), when people are less active and less able to detect and deter elephants than during the day (Nichols & Powers, Reference Nichols and Powers1964). It has been suggested that elephant movements may be influenced by moon phases as lunar illumination affects the level of darkness and may subsequently influence elephant behaviour. Studies on predator–prey interactions have shown effects of lunar illumination on nocturnal behaviour and movement patterns of prey and predators (Rockhill et al., Reference Rockhill, DePerno and Powell2013; Huck et al., Reference Huck, Juárez and Fernández-Duque2017; Linley et al., Reference Linley, Pauligk, Marneweck and Ritchie2020), and there have been reports that elephants cause less damage to crops during bright moonlit nights, when they are more likely to be harassed by people (Barnes et al., Reference Barnes, Dubiure, Danquah, Boafo, Nandjui, Hema and Manford2006; Gunn et al., Reference Gunn, Hawkins, Barnes, Mofulu, Grant and Norton2014). However, it is not known whether these observations can be generalized, and there have been no studies on activity patterns of elephants in Sri Lanka, where many rural communities have little artificial light and protect their crops from watch towers at night (Koirala et al., Reference Koirala, Ji, Aryal, Rothman and Raubenheimer2015).
Many studies investigating human–elephant interactions report incidences of crop damage within human-dominated landscapes (Chiyo et al., Reference Chiyo, Cochrane, Naughton and Basuta2005), but few consider the ecological drivers behind elephant movements (Srinivasaiah et al., Reference Srinivasaiah, Anand, Vaidyanathan and Sinha2012). It is often assumed that elephants move into human-dominated landscapes only for the purpose of exploiting crops for food. However, their movements may also be influenced by other factors, including seasonality, rainfall and availability of anthropogenic water sources (Weerakoon et al., Reference Weerakoon, Gunawardene, Janaka, Jayasinghe, Perera, Fernando and Wickramanayake2003; Loarie et al., Reference Loarie, van Aarde and Pimm2009; Birkett et al., Reference Birkett, Vanak, Muggeo, Ferreira and Slotow2012). If elephants manage risks, seeking to maximize energy gains, more incursions into agricultural areas are expected to occur when cultivated crops are mature and thus more resources are available (Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011; Ngama et al., Reference Ngama, Bindelle, Poulsen, Hornick, Linden, Korte, Doucet and Vermeulen2019), and when food supplies in natural areas are insufficient or more difficult to access (Cook et al., Reference Cook, Henley and Parrini2015). In Sri Lanka, there is a seasonal pattern of crop damage by elephants (Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011) that may reflect availability of crops and/or variation in natural food supplies, but little is known as to whether elephant incursions into human-dominated landscapes always result in crop damage.
Social factors such as caring for dependent offspring probably influence risk management in animals (Cooper & Blumstein, Reference Cooper and Blumstein2015). Elephants typically occur either as solitary males or in family groups consisting of adult females and their calves (Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011; De Silva & Wittemyer, Reference De Silva and Wittemyer2012), but males sometimes also form male-only groups (Srinivasaiah et al., Reference Srinivasaiah, Kumar, Vaidyanathan, Sukumar and Sinha2019). The potential costs and benefits of exploiting human-dominated landscapes are expected to differ between these social groupings, as has been shown for African elephants, with males interacting with areas in and around villages more regularly (Cook et al., Reference Cook, Henley and Parrini2015). Mature adult males probably require more food than females, and are more able and inclined to defend themselves against people. In Sri Lanka, male elephants are causing the majority of crop damage (Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011). In contrast, females in family groups may require less food and be less tolerant of harassment by people, especially when calves are present (Cook et al., Reference Cook, Henley and Parrini2015), and are thus less likely to use human-dominated landscapes.
In Sri Lanka, to our knowledge, there have been no studies on the correlation of elephant movements and crop damage events. Here, we examine the movements of elephants across an ecotone of natural to human-dominated landscapes in Sri Lanka. We expect that elephants use such landscapes only when benefits outweigh costs. Specifically, we make four predictions: (1) elephants make incursions into agricultural areas at times of the year when available resources are plentiful, (2) elephants avoid interactions with people by exploiting these areas by night, (3) increased nocturnal illumination reduces use of agricultural areas by elephants because they mitigate the risk of encountering people, and (4) given that human-dominated environments are risky, we expect that males, with higher metabolic requirements and defensive capacity, make greater use of anthropogenic food sources. Unlike previous studies (Chiyo et al., Reference Chiyo, Cochrane, Naughton and Basuta2005; Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011; Wilson et al., Reference Wilson, Davies, Hazarika and Zimmermann2013) that focussed solely on the prevalence of crop damage, we quantified elephant movements between natural and human-dominated areas irrespective of any crop use, using camera traps on transitional pathways, to examine temporal patterns of elephant incursions into human-dominated landscapes.
Study area
We conducted this study in a transitional area between natural and human-dominated landscapes near the south-western boundary of Wasgamuwa National Park in Wasgamuwa, Matale District, Central Province, Sri Lanka. Wasgamuwa has a tropical climate with distinct wet (November–February) and dry (March–October) seasons. Dawn and dusk times are consistent throughout the year, with sunrise around 6.00 and sunset around 18.00. Mean annual rainfall is 2,250 mm and the mean annual temperature is 32 °C.
The villages of Weheragala and Himbiliyakada are embedded in agricultural landscapes outside the Park. Between them is the Weheragala–Himbiliyakada Forest Reserve, comprising secondary forest and encroaching human development (Fig. 1). This is the main corridor that elephants use throughout the year to move between Wasgamuwa National Park and human-dominated areas (Fig. 1). The landscape around the villages is a mosaic of agricultural fields, home gardens, human-made lakes and lake beds, scrublands and secondary forest. Artificial lakes, especially Weheragala Tank, attract elephants that use them as water sources. Elephants accessing Weheragala Tank typically spend several days within the surrounding secondary forest and foraging in agricultural fields and home gardens.
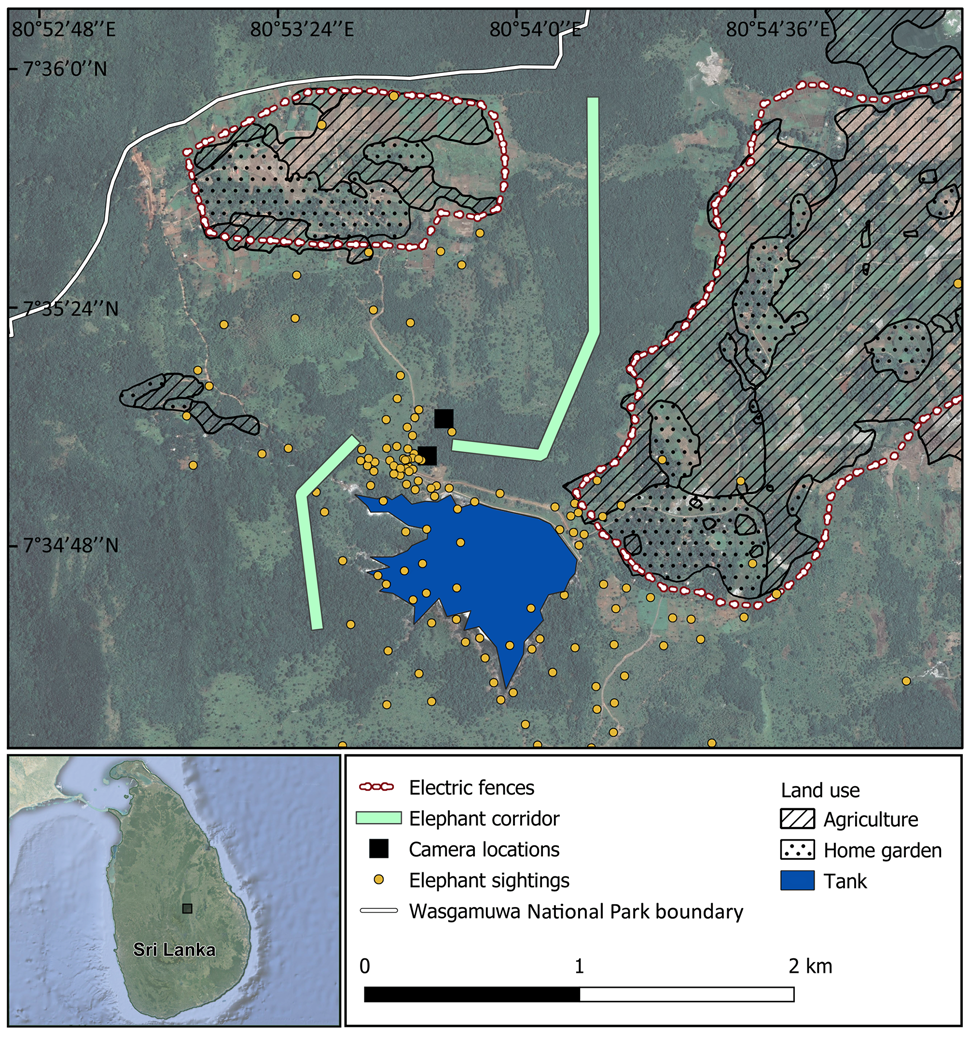
Fig. 1 Study area near the south-western boundary of Wasgamuwa National Park in Wasgamuwa, Matale District, Central Province, Sri Lanka. The map shows the Weheragala–Himbiliyakada Forest Reserve corridor used by elephants Elephas maximus to travel through this area, and the Weheragala Tank, an artificial lake that elephants access as a water source. Elephants often remain in the small secondary forest patches near the Weheragala Tank for days at a time, foraging in surrounding human-dominated areas before returning to the Park. Elephant sightings (CF, unpubl. data, 2016–2018) were recorded during regular patrols by the Sri Lankan Wildlife Conservation Society, and reported by local people. These sightings are biased towards open, human-dominated areas, and thus do not illustrate the use of forested areas as corridors.
Human–elephant interactions are prevalent throughout the region (Corea, Reference Corea2001, Reference Corea2007) but have not previously been quantified or formally assessed. Farmers adopt a range of mitigation measures, including monitoring their fields from tree-based huts throughout the evening and night to detect intruding elephants, and deterring them with thunder flashes, firecrackers, shouting and sometimes shooting. Farmers in both villages also use conventional electric fences and single-wire fences to prevent elephants from entering their crop fields and home gardens (Fig. 1).
Methods
Data collection
We identified the two main paths that elephants use to move in and out of the forest, based on our long-term (> 15 years) experience of research on elephants in the study area and our connections with the local farming communities (Corea, Reference Corea2001, Reference Corea2007). In addition, a pilot study (January– May 2016) demonstrated that our camera sites were in the most frequently used transition zones between forested and human-dominated landscapes.
Cameras triggered by animal movement and heat have been shown to facilitate the analysis of elephant movement patterns (Adams et al., Reference Adams, Chase, Rogers and Leggett2015). Therefore, along the elephant trails, we established two permanent camera stations that operated continuously during July 2016–November 2018. We used Xenon flash ScoutGuard cameras (model SG 565FV; HCO, Guangdong, China), set on high sensitivity and long-range illumination, with no delay between images. These white-flash cameras did not deter elephants, thereby reducing detection rates, as has been found in other studies (Fernando & Corea, Reference Fernando and Corea2019). Cameras were enclosed in secure metal boxes with metal spikes to prevent them being damaged by elephants. The cameras were checked at least once every 3 weeks for the duration of the study to ensure they were functioning, and to download images.
We used unique characteristics (e.g. differences in ear folds, nicks and holes in ears, tail characteristics, scars, warts and pigmentation patterns) to identify individual elephants and treated images of the same individual or family group as independent captures (for either entering or exiting) only if they were separated by at least 1 day. Each independent detection was classified as either male-only (single male or male-only groups) or family group (one or more adult females and their female offspring of all age classes and younger, dependent male offspring). For each detection, we assessed the direction of movement based on the orientation of the individual: cameras were positioned facing the Park, so that images of elephants facing the camera represented movement into (entering) human-dominated landscapes, and elephants facing away from the camera represented movements out of (exiting) human-dominated landscapes. If the direction of movement was ambiguous, we excluded the images from the analysis.
We categorized moon phases as full moon, waning moon, new moon or waxing moon. Following Gunn et al. (Reference Gunn, Hawkins, Barnes, Mofulu, Grant and Norton2014), we defined the full moon period as the night of the full moon and the three nights before and after that night, and accordingly for the new moon period. The nights between a full and new moon were considered the waning moon phase, and the time between a new and full moon the waxing moon phase.
Data analysis
To assess the annual pattern of elephant movements and the effect of moon phase, we ran generalized additive models using the mgcv package in R 3.5.1 (Wood, Reference Wood2011; R Development Core Team, 2018), with separate models for males and family groups as their movements may differ (Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011). We calculated the frequency of elephant incursions into human-dominated landscapes as the number of detections per moon phase per month across the 3-year study period. An offset was incorporated into the model to account for differing sampling periods within moon phases, and the frequency of elephant crossings and the offset were log10 transformed to normalize the data. We examined whether elephant movements differed between the four moon phases (new moon, waxing moon, full moon, and waning moon) and the months of the year (treated as a continuous variable). We used a cyclic cubic regression spline against the month to account for expected non-linear patterns in elephant movements. A variance structure was included in the models for male elephants in relation to month of movement, to account for heterogenous patterns between months. Models were validated through visual inspection of residuals compared to fitted values, and residual values compared to each parameter in the model.
To investigate the daily timing of elephant movements, we converted the time stamps of camera-trap images to radians (as time of day is circular). We then created activity curves based on kernel densities to compare differences in activity between moon phases for both males and family groups. We considered how activity periods differed for males and family groups and the timing of when they entered and exited human-dominated landscapes. We used the overlap package in R (Ridout & Linkie, Reference Ridout and Linkie2009; R Development Core Team, 2018) to calculate the level of overlap between two activity curves (e.g. male activity during full moon vs male activity during a new moon). We ran 10,000 bootstrap samples to estimate the precision of this estimate. The level of overlap ranges from zero to one, where zero represents no overlap and one suggests complete overlap. We tested the level of overlap for statistical significance using the non-parametric Mardia–Watson–Wheeler test (Frey et al., Reference Frey, Fisher, Burton and Volpe2017) in the circular package in R (Agostinelli & Lund, Reference Agostinelli and Lund2017; R Development Core Team, 2018).
Results
A total of 993 elephant detections were recorded during July 2016–November 2018. Males were detected more frequently than family groups (males: 79% of detections; family groups: 21%), and more detections were of elephants entering (56% of detections) than exiting (44%) human-dominated landscapes.
We found the frequency of elephant movements per month varied throughout the year for males (F = 3.569, df = 9, P < 0.001), with a similar pattern for family groups (F = 1.381, df = 9, P = 0.021). There was no effect of moon phase on elephant movements for either males or family groups (all comparisons: P > 0.05). Movements of males and family groups had two distinct annual peaks: the first in February–March, and the second in August–September (Fig. 2). These patterns had moderate explanatory power for males (R 2 = 0.171), but less for family groups (R 2 = 0.084).
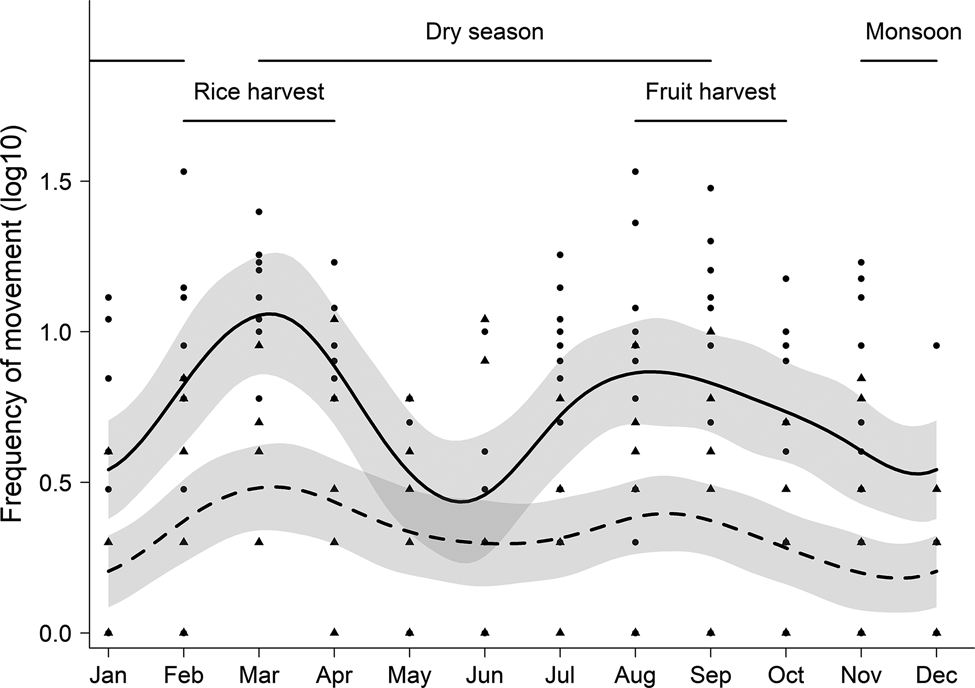
Fig. 2 The frequency of elephant movements between natural and human-dominated landscapes for males (circles, upper curve) and family groups (triangles, lower curve) in Wasgamuwa, Central Province, Sri Lanka, over the course of the year, recorded during July 2016–November 2018.
Elephant movements coincided with crop cycles, with the majority of rice harvested during February–March and fruits such as watermelons, corn, pumpkins and long beans harvested throughout August and September (Fig. 2). These anthropogenic food sources are utilized by elephants (RC & CF, unpubl. data, 2021).
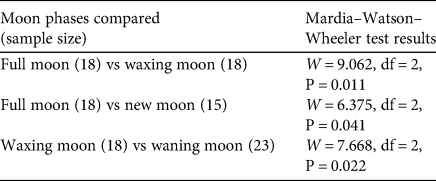
Elephants entered human-dominated landscapes most frequently between dusk and midnight, with a peak around 18.00. Movements exiting human-dominated landscapes peaked at 06.00 (Fig. 3). The timing of the movements of family groups differed from that of males (entering: W = 5.510, df = 2, P = 0.060; exiting: W = 6.03, df = 2, P = 0.049), which may be a result of different sample sizes for the two groups, rather than actual differences in behaviour (Supplementary Fig. 1). Comparison of movement overlap between moon phases showed few differences, but movements of elephants exiting human-dominated landscapes differed between waxing and waning moons (W = 7.702, df = 2, P = 0.021) and between waning and new moons (W = 6.342, df = 2, P = 0.042). When considering males and family groups separately, no differences in movements were observed for males between different moon phases (Supplementary Table 1), but there were some differences for family groups (Table 1). This needs to be interpreted with caution because of limited data (N < 30 detections per group) within each moon phase for family groups. However, our findings suggest the timing of family groups leaving human-dominated landscapes varies more during moon phases with more lunar illumination (Fig. 4). During darker moon phases, family groups appear to have more distinct timings of their movements, whereas during phases of higher lunar illumination, movements occur throughout the day and night, without a clear peak.
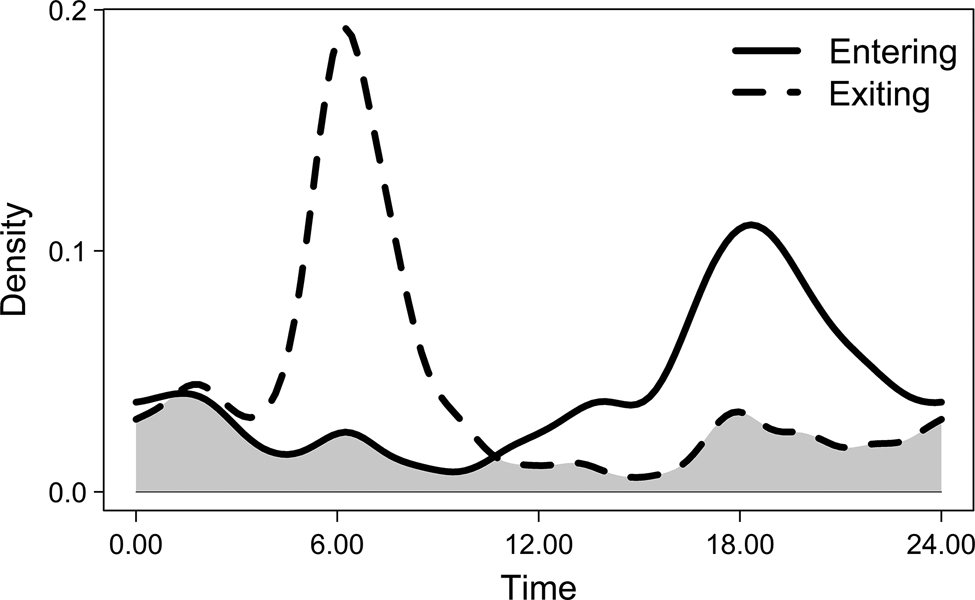
Fig. 3 Timing of elephant movements entering and exiting human-dominated landscapes by time of day, shown as relative density of recorded times within the sample. The grey area represents the level of overlap between entering and exiting movements.
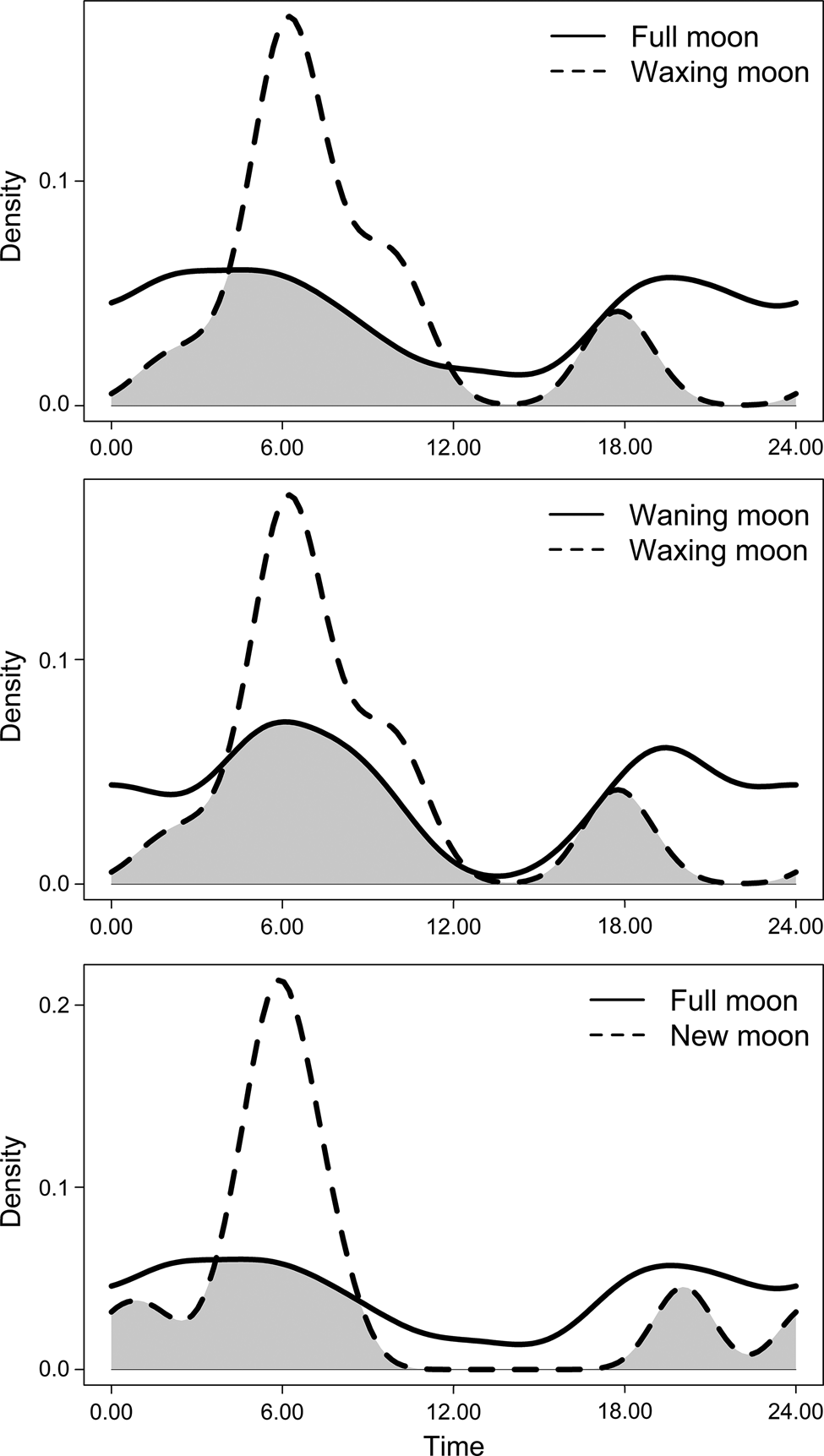
Fig. 4 The timing of elephant family group movements exiting human-dominated landscapes in relation to different moon phases, shown as relative density of records within the sample. Grey areas represent the level of overlap between moon phases.
Table 1 Movements of family groups of Asian elephants Elephas maximus exiting human-dominated areas in Sri Lanka, during July 2016–November 2018. The table shows the temporal overlap of these movements between moon phases, with the results of Mardia–Watson–Wheeler tests.
Discussion
We found that Asian elephants in Wasgamuwa make incursions into agricultural areas at times of the year when available resources are plentiful. Such incursions are most frequent during the harvest periods of rice and other crops such as watermelons, corn and pumpkins, as has been reported elsewhere (Wilson et al., Reference Wilson, Davies, Hazarika and Zimmermann2013; Neupane et al., Reference Neupane, Johnson and Risch2017). In south-eastern Sri Lanka, crop damage, mostly by males, peaks around February and again around August (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009; Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011); this corresponds to the seasonal movement pattern observed in Wasgamuwa. Elephants moved less frequently into human-dominated landscapes during periods when such resources were unavailable and when presumably the costs outweighed the benefits of visiting human-dominated areas. They may also rely less on crops when more natural food resources are available (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009). Crop damage by African savannah elephants peaks during the late wet season, coinciding with maturing crops (Hoare, Reference Hoare1995). Similar patterns of crop damage have been observed throughout the range of the African forest elephant Loxonta cyclotis (Lahm, Reference Lahm1996). However, in Sri Lanka, one of the two peaks of crop damage (in February–March) occurs immediately after the north-eastern monsoon (the wet season in Wasgamuwa), and it has been suggested that it is the availability of crops, not a lack of natural food, that draws elephants into agricultural areas (Sukumar, Reference Sukumar1990; Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011). Alternatively, the main driver of seasonality in the use of human-dominated landscapes may be rainfall, which influences both crop production and the availability of natural fodder (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009; Birkett et al., Reference Birkett, Vanak, Muggeo, Ferreira and Slotow2012). The fact that the seasonal frequency of movements into human-dominated landscapes corresponds with the seasonal patterns of reported crop damage suggest that when elephants are in human-dominated landscapes, they use crops.
Our prediction that elephants minimize the risk of encountering people by exploiting these areas by night was well supported, for males and family groups. Elephants moved into human-dominated landscapes around or after dusk and retreated into forested areas before or around dawn. In some areas, such as Assam, India, most crop and property damage occurs in the early hours of the night (Wilson et al., Reference Wilson, Davies, Hazarika and Zimmermann2013), but in our study area and elsewhere in Sri Lanka (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009) damage has been reported to occur at any time of night. Other reasons for this pattern of nocturnal intrusions may include the need for thermoregulation, avoiding hot, exposed areas during the heat of the day, especially given that human-dominated landscapes often lack shade (Wilson et al., Reference Wilson, Davies, Hazarika and Zimmermann2013). People and elephants also often share water resources, and elephants may thus enter human-dominated areas more frequently during the dry season to access water.
Nocturnal illumination appeared to have a marginal effect on elephant movements in this study, and the effects we report are weak, perhaps because of limited sample size. There was no difference in the frequency of movements between the different moon phases, but the timing of family group movements back into natural landscapes appeared to be more scattered during moon phases with more illumination. There was no effect of moon phase on the movements of males, suggesting they are largely indifferent to levels of nocturnal illumination. Previous studies have reported significant effects of moonlight on the usage of human-dominated environments by African elephants (Barnes et al., Reference Barnes, Dubiure, Danquah, Boafo, Nandjui, Hema and Manford2006; Gunn et al., Reference Gunn, Hawkins, Barnes, Mofulu, Grant and Norton2014). To our knowlegde, this has not been previously examined for Asian elephants, and the two species may differ in this respect. Additionally, we did not directly measure nocturnal illumination, which varies depending on cloud cover (there is little anthropogenic light in the study area).
The annual pattern of movements of males and family groups differed. Males showed two distinct peaks in movements, which correspond to the seasonal pattern of reported crop damage, mostly by male elephants, in south-eastern Sri Lanka (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009; Ekanyaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011). Movements of family groups varied only modestly by season, as does reported crop damage by family groups in south-east Sri Lanka (Campos-Arceiz et al., Reference Campos-Arceiz, Takatsuki, Ekanayaka and Hasegawa2009; Ekanayaka et al., Reference Ekanayaka, Campos-Arceiz, Rupasinghe, Pastorini and Fernando2011). This corroborates suggestions that family groups are more risk-averse, less capable or inclined to defend themselves or less dependent on human food sources.
Our findings confirm that male Asian elephants in Wasgamuwa move into human-dominated landscapes predominantly during peak times of crop availability, whereas family groups showed a less pronounced seasonal pattern. All elephants visit human-dominated areas by night, with a possible influence of nocturnal illumination on family groups. The close temporal association between elephant movements and crop damage suggests elephant visitation to human-dominated environments is driven by crop availability. Substantive efforts are directed at mitigating human–elephant interactions, including technological advances that could provide early warning of elephants using specific trails (e.g. Anni & Sangaiah, Reference Anni and Sangaiah2015). Such systems may help increase the effectiveness of crop guarding efforts by farmers and thereby reduce the time and effort they have to spend on deterring elephants (Mayberry et al., Reference Mayberry, Hovorkab and Evans2017). To develop effective solutions for this ongoing and intensifying problem, an enhanced understanding of the role of human-dominated landscapes in elephant ecology is needed. Long-term planning that integrates effective land management and land use, combined with targeted conflict mitigation (e.g. guarding crops, fencing villages, planting orange/citrus fences) and compensation schemes, may de-escalate human–elephant interactions and facilitate coexistence (Corea, Reference Corea2001, Reference Corea2006).
Acknowledgements
We thank Indika Sampath and Asitha Lakmal for support with the collection of camera-trap data, the staff of the Department of Wildlife Conservation for their support, and S.P.E.C.I.E.S. for providing remote cameras. The School of Life and Environmental Sciences (Deakin University, International Collaboration Grant) and the Sri Lankan Wildlife Conservation Society supported a visit by ARR and MAW to its field station at Wasgamuwa to analyse the data and collaborate on this article.
Author contributions
Study design: CF, MAW, ARR; data collection: CF; facilitation of camera trapping: KP; data analysis: ARR; writing: CF, MAW, ARR. RC initiated a community-based initiative in 1998 to mitigate human–elephant conflict, established a field station, engaged local communities in the study area, continues to lead the Sri Lankan Wildlife Conservation Society, and was involved in establishing this particular project.
Conflict of interest
None.
Ethical standards
This study abided by the Oryx guidelines on ethical standards. Methods were non-invasive, and permission to deploy camera traps was obtained from the Department of Wildlife Conservation Sri Lanka (permit number: WL/3/2/51/16). Deakin University Animal Ethics Committee did not require specific approval for this project.
Data availability
Requests for access to the data should be directed to the Sri Lankan Wildlife Conservation Society.







