Healthcare-associated infections (HAIs) remain a major threat to patient safety. A significant proportion of such infections are likely preventable through the application of infection prevention measures,Reference Harbarth, Sax and Gastmeier 1 – Reference Storr, Twyman and Zingg 4 such as those aiming to reduce the transmission of pathogens that may lead to patient colonization or infection.Reference Siegel, Rhinehart, Jackson and Chiarello 5 Hand hygiene, for example, is widely recognized as one of the most effective practices to reduce infection rates and patient colonization with multidrug-resistant bacteria by reducing the transmission of microorganisms.Reference Allegranzi and Pittet 6 Strong evidence also suggests that environmental contamination of surfaces and objects contribute to HAI,Reference Boyce 7 – Reference Fawley, Parnell, Verity, Freeman and Wilcox 12 yet the behavioral focus of such studies is often limited to hand hygiene and environmental cleaning. While the practice of hand hygiene has been increasingly studied over the last decade for its role in infection prevention, considerably less knowledge exists regarding other important infection-related behaviors.
A growing body of evidence suggests that practices beyond those addressed by hand hygiene may be relevant in the transmission of microorganisms that results in patient colonization and infection, such as handling of mobile objects,Reference Clack, Schmutz, Manser and Sax 13 , Reference Livshiz-Riven, Borer, Nativ, Eskira and Larson 14 healthcare worker (HCW) privateReference Lopez, Ron, Parthasarathy, Soothill and Spitz 15 and professional attire,Reference Wiener-Well, Galuty, Rudensky, Schlesinger, Attias and Yinnon 16 , Reference Treakle, Thom, Furuno, Strauss, Harris and Perencevich 17 and medical devices.Reference Schultsz, Meester and Kranenburg 11 , Reference Livshiz-Riven, Borer, Nativ, Eskira and Larson 14 , Reference Birnbach, Rosen, Fitzpatrick, Carling and Munoz-Price 18 Therefore, we hypothesize that an important portion of infectious risks lie in infectious risk moments (IRM), defined as seemingly innocuous, yet frequently occurring care manipulations that potentially result in the transfer of pathogens. Such IRM include yet go beyond existing indications for hand hygiene.Reference Clack, Schmutz, Manser and Sax 13
The design of infection prevention strategies that consider a broad range of infectious risks must begin with systematic identification and classification of IRMs. In a 2-part project, we conducted (1) exploratory observations to establish a comprehensive inventory of potential IRMs, which served as a basis for developing a taxonomy for structured observations and (2) structured observations to quantify the frequency and nature of IRMs in 3 distinct typical healthcare settings.
METHODS
Design
We conducted a prospective observational study in 2 parts. First, we conducted live exploratory observations to identify a wide range of potential IRM and to establish a structured taxonomy called INFORM (INFectiOus Risk Moment) for identifying and classifying IRMs. Second, we conducted live structured observations based on the INFORM taxonomy. Parts of this methodology have been pilot tested previously.Reference Clack, Schmutz, Manser and Sax 13 The observations reported in the current manuscript do not include the pilot observations.
Setting
An intensive care unit (ICU), general medical ward, and emergency ward, including trauma unit, located at a 900-bed, university-affiliated, tertiary-care hospital were purposefully sampled to represent a broad range of care activities and potential infectious risks. All healthcare workers (HCWs) from the participating wards were included in the study. The study hospital has a well-established infection prevention and control (IPC) group with extensive state-of-the-art, written IPC standard operating procedures, weekly IPC rounds, and a designated IPC nurse consultant for each hospital ward.
Exploratory Observations
Observers with backgrounds in nursing (C.D.A. and V.G.) and human factors/psychology (L.C.) and extensive experience conducting observations for patient safety research carried out exploratory observations in all 3 settings. Field notes documented the care processes observed and any potential IRMs, which were operationally defined as behaviors potentially resulting in the transmission of pathogens that may result in patient colonization or infection. The observers discussed all identified potential IRMs regularly throughout the exploratory observation period together with a senior infection prevention physician (H.S.) and all potential IRM were collected in a database.
Based on the definition of IRMs and following the hand hygiene literature, IRMs were limited to moments resulting in potential transfer of pathogens to patients and their immediate surroundings (eg, bedding), rather than the larger translocation of microorganisms throughout the healthcare environment. For example, an HCW entering a patient room then, without doing hand hygiene, touching the patient’s bedside monitor to silence an alarm (a behavior that occurs often and may introduce nonpatient flora to the patient environment) was not considered an IRM. Only behaviors that resulted in potential transfer of pathogens directly to the patient were considered. We distinguished between noncritical patient sites (eg, intact skin, intact dressings, patient clothing), critical patient sites, defined as “body sites or medical devices that have to be protected against microorganisms potentially leading to HAI”Reference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 19 (eg, mucous membranes, catheter insertion sites, or open wounds), and patient bedding. Exploratory observations were conducted until saturation was achieved in each setting, that is, until no new IRMs were observed.
Structured Observation Taxonomy and Mobile Observation Tool Validation
Following exploratory observations, all IRMs were extracted from field notes and were systematically coded according to the source, vector, and endpoint from, through, and to which pathogens were transferred, respectively. This structure was used to establish the INFORM classification taxonomy, on which structured observations were based (Figure 1). A mobile observation tool based on the INFORM taxonomy was programmed with Filemaker 14 (FileMaker, Santa Clara, CA). To ensure the quality of observations, 2 observers (L.C. and S.P.) validated the mobile observation tool during a 1-month test period. The percentage of agreement between the 2 observers was calculated to measure sensitivity (detection of the same IRM) and Cohen’s κ was calculated to determine interobserver agreement (ie, consistent classification of IRM) using STATA version 14 software (StataCorp, College Station, TX).
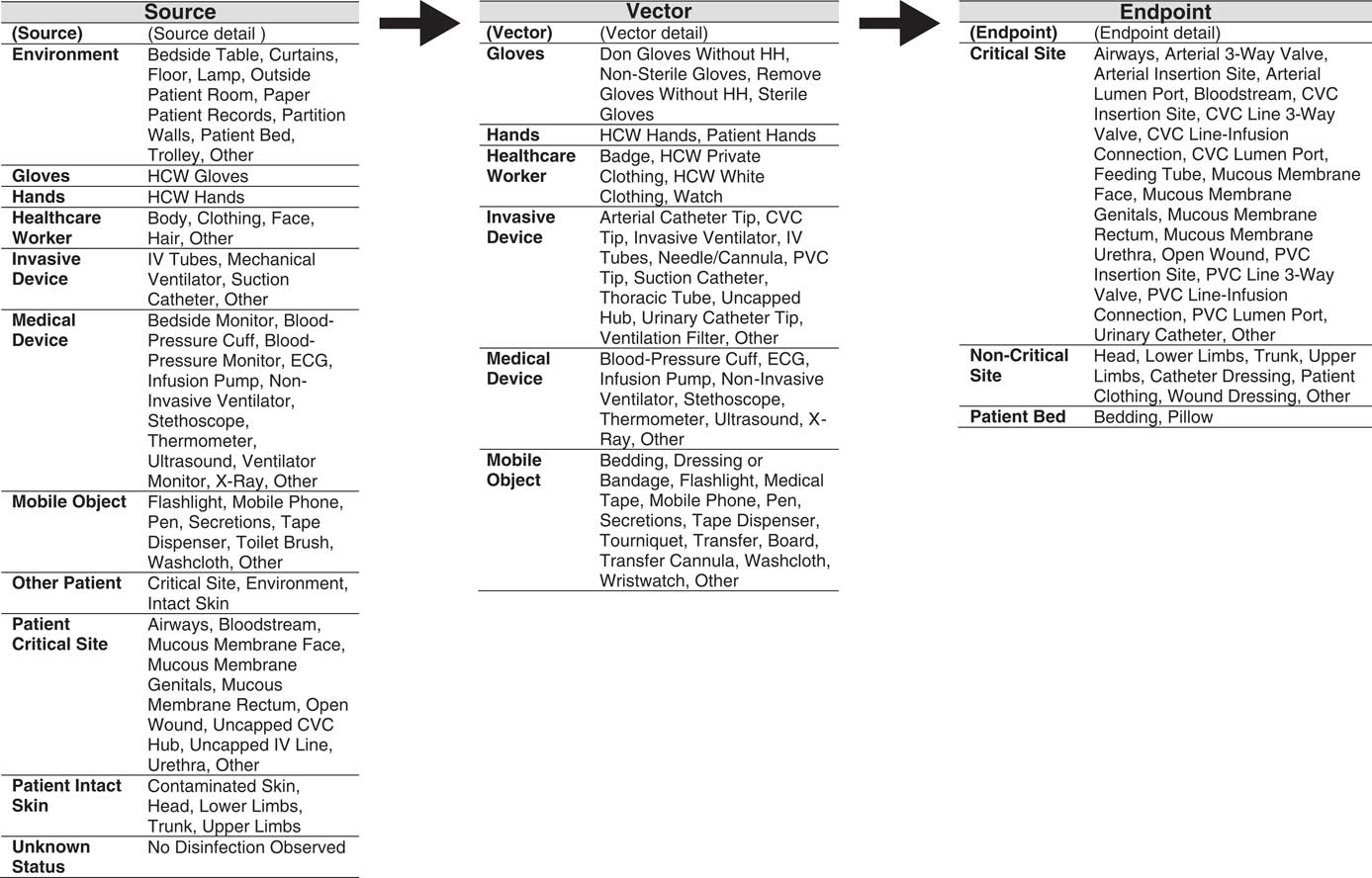
FIGURE 1 The INFORM (INFectious Risk Moment) structured taxonomy used to classify surfaces involved in the observed infectious risk moment according to source, vector, and endpoint of potential pathogen transfer. Note: HCW, Healthcare worker; IV, Intravenous; ECG, electrocardiography; CVC, Central-venous catheter; PVC, Peripheral-venous catheter
Structured Observations
Structured observations were carried out in the same 3 clinical settings using the mobile observation tool. Two observers (L.C. and S.P.) conducted live, structured observations in parallel to ensure systematic documentation of all IRMs. Structured observations targeted periods of active patient care, and both observers focused on the same HCW at once. Observation sessions of 30–60 minutes were deliberately conducted at different times throughout the workday to include many different HCWs who performed a diverse range of care tasks for multiple patients during each session. During live observations, both observers independently noted the source, vector, and endpoint of pathogens for each IRM according to the observational taxonomy as well as demographic information about the HCW being observed (ie, gender and professional category) and contextual information (ie, date, time, ward name, and patient isolation status) using the mobile observation tool (Appendix 1). No identifying patient or HCW data were collected during observations. For each observation period, we recorded the total amount of observation time, as well as the amount of active patient care time to calculate the density of IRMs per setting. Following each structured observation session, all observed IRMs were compared between the 2 observers, and any discrepancies were discussed until a consensus agreement was achieved. Frequent discussion among researchers to achieve consensus after each observation period was maintained throughout the study to ensure quality and to avoid drift between observers.
Ethics
The Cantonal Ethics Committee of Zurich formally waived the ethics requirement for this study (KEK-StV-Nr.73/14). Participation in observations was voluntary, and HCWs were free to opt out or stop observations at any time without providing justification.
RESULTS
Exploratory Observations
A total of 129.17 hours of exploratory observations resulted in the identification of 292 unique IRMs. Identified IRMs included moments of potential direct contact transmission (potentially infected or colonized HCW to patient) as well as potential indirect contact transmission via vectors such as care devices, mobile objects, and HCW clothing and accessories. Following exploratory observations, IRMs were systematically coded according to the source, vector, and endpoint of potential pathogen transfer, and these codes formed the basis of the INFORM structured taxonomy (Figure 1).
Structured Observation Taxonomy and Mobile Observation Tool Validation
The 3-level taxonomy begins with classification of surfaces (loci) involved in the observed IRM according to source, vector, or endpoint of potential pathogen transfer (level 1: locus), then assigns each source, vector, and endpoint to a main category (level 2: surface), and specifies the exact nature (level 3: surface detail). Each observed IRM is then represented as a transmission chain composed of 3 loci (source, vector, and endpoint), with each locus having 2 levels of detail (surface and surface detail). Table 1 lists examples of archetypal observed and classified IRMs for each of the observed vectors.
TABLE 1 Example Coding of Archetypal Infectious Risk Moments Using the INFORM Structured Taxonomy

During the 1-month test of the taxonomy using the mobile observation tool (5.5 hours of active patient care), observers 1 and 2 detected 123 (78.9%) and 118 (75.6%) of all observed IRMs, respectively. Based on this detection rate, the decision was made to have 2 observers present for all structured observations to ensure the highest possible sensitivity. For moments identified by both observers during the pilot test, the Cohen’s κ measure of interobserver agreement was 0.75, indicating substantial agreement between individual observers.Reference Landis and Koch 20
Structured Observations
Following validation of the taxonomy using the mobile observation tool, 53.77 hours of structured observations (31.25 hours of active care) were conducted, during which 1,338 IRM were identified. The average densities of IRMs per active care hour were 42.8 overall, and 34.9, 36.8, and 56.3 in the intensive care, medical, and emergency wards, respectively. We identified 566 unique IRMs, which fell into 71 main categories according to level 2 of the structured taxonomy. A comprehensive inventory of observed IRMs appears in Table 2.
TABLE 2 Inventory and Observed Frequency of All Infectious Risk Moments per Care Setting by (A) Critical Site and (B) Noncritical Site

NOTE. ICU, intensive care unit; MED, general medical ward; ER, emergency ward; HCW, healthcare worker.
The vectors in the identified IRMs included hands (n=596; 44.54%), gloves (n=457; 34.16%), medical devices (n=115; 8.59%), mobile objects (n=102; 7.62%), invasive devices (n=53; 3.96%), and HCW clothing and accessories (n=15; 1.12%). Overall, 25.8% of IRM concerned moments of potential transmission of pathogens to a critical site, described in detail in Table 2A. Among the 217 IRMs dealing with medical devices and mobile objects as vectors, 143 IRMs (65.90%) involved the lack of disinfection of a device or object prior to patient contact. The 3 most frequently occurring IRMs per clinical setting are described in detail in Table 3.
TABLE 3 Three Most Frequently Occurring Infectious Risk Moments (IRM) per Clinical Setting

NOTE. This table presents the 3 most frequently occurring main categories of infectious risk moments (IRMs) based on level 2 of the structured taxonomy.
a Number of times the IRM was observed in the indicated setting.
b Frequency per hour of active patient care in the indicated setting.
DISCUSSION
Hands and gloves continue to be among the most important contributors to the transfer of pathogens in the healthcare setting. Nonetheless, we identified moments dealing with other vectors such as medical devices, mobile objects, invasive devices, and HCW clothing and accessories, which may also contribute to patient colonization and/or infection. While previous studies have shown that indications for hand hygiene occur between 8 per hour in pediatric wards and 30 per hour in ICUs,Reference Pittet, Mourouga and Perneger 21 , Reference Hugonnet, Perneger and Pittet 22 we found that IRMs occurred with a frequency of 42.8 IRM per active care hour overall and up to 56.3 IRM per active care hour in emergency settings. Similar to opportunities for hand hygiene, the high frequency with which IRMs occur suggests that the cumulative risk of negative patient outcomes due to IRMs may be significant, although the risk of patient infection or colonization with multiresistant pathogens at any single IRM may be low. The fact that 25.8% of IRMs concerned moments of potential pathogen transfer to critical patient sites further highlights the clinical relevance of IRM for infection prevention.
The structured observations in this study were targeted to moments resulting in potential pathogen transfer to the patient, as opposed to movement of pathogens around the larger healthcare environment. Our exploratory observations nonetheless revealed that pathogen transfer from outside to inside the patient zone likely occurred, for example when coming from one patient to silence an alarm on another patient’s monitor without hand hygiene, or when transporting mobile objects that come into contact with multiple consecutive patients during clinical rounds. These findings are consistent with other studies demonstrating that HCW hand hygiene compliance prior to initial contact with the patient or the patient environment is suboptimal.Reference Erasmus, Daha and Brug 23 Our results also challenge the “patient zone” concept, which defines the patient and his/her immediate surroundings (eg, bed rails, bedside table, and medical equipment) and frequently touched surfaces (eg, monitors, knobs, and buttons) as the patient zone and assumes that surfaces within the patient zone are colonized by patient flora.Reference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 19 When disinfection is omitted prior to contact with the patient or patient environment,Reference Erasmus, Daha and Brug 23 it is likely that pathogens from the healthcare environment are introduced to these surfaces. Such ambiguity is a major challenge to safe behavior.Reference Sax and Clack 24 For this reason, during observations, we considered that environmental surfaces could potentially harbor pathogenic bacteria regardless of their location inside or outside of the patient zone.
Similarly, our findings are consistent with multiple systematic reviews demonstrating that the frequent movement of healthcare equipmentReference Schabrun and Chipchase 25 and care itemsReference Livshiz-Riven, Borer, Nativ, Eskira and Larson 14 between patients, together with suboptimal or missing disinfection of such items, result in the transfer of pathogens between patients. Potential contamination or missing disinfection of medical devices and mobile objects (classified as source=“unknown status” and source detail=“no disinfection observed”) accounted for 16.2% of IRMs observed in this study (Table 2).
The transmission-based observational approach employed in this study, which sought to identify all behaviors potentially resulting in transmission pathogen, differs from traditional rule-based observations that measure compliance with existing local or national guidelines. Observations using the INFORM taxonomy could hence be employed in additional settings, regardless of local guidelines, to identify the most frequently occurring IRMs and to establish local infection prevention priorities.
This study has several limitations. It is possible that being observed influenced HCW behavior during this study.Reference Parsons 26 It is unlikely, however, that this resulted in systematic bias because HCWs were not aware of exactly what was being observed. Observations were limited to contact transmission (ie, the most common mode of transmissionReference Siegel, Rhinehart, Jackson and Chiarello 5 ) and did not consider airborne and droplet transmission. Furthermore, our observations did not consider other behaviors that may also impact infectious risks, such as those interfering with the patient’s defense system against infectious risks (eg, immune status, skin integrity, cough reflex, etc) because the associated HCW behavior rarely occurs at the bedside. Moreover, these observations were conducted in a single university hospital located in a high-income setting, which limits the generalizability of our findings. Further exploration of the nature and frequency of IRMs using the INFORM structured observational taxonomy is warranted to assess local priorities for infection prevention efforts in additional care settings. Finally, the risk of transmission during each type of IRM remains unknown. We aimed to bridge this gap through a modified Delphi survey with an international panel of experts in infectious diseases, infection prevention and control, and microbiology, in which experts rated the likelihood of infectious outcomes (eg, colonization, infection) following archetypical IRM.Reference Clack, Passerini, Manser and Sax 27
Despite these limitations, the combination of methods employed in this study was well suited to identify a wide range of potential IRMs and to systematically observe their frequency and nature in multiple healthcare settings. The resulting mobile observation tool featuring the INFORM taxonomy of source, vector, and endpoint of pathogens was useful for the systematic documentation and categorization of IRMs. Further observations based on the INFORM taxonomy may prove useful in other settings to identify the most frequently occurring IRMs, to establish educational content, and to prioritize targeted infection prevention strategies.
ACKNOWLEDGMENTS
We would like to wholeheartedly thank all healthcare workers for their participation in this study. We also gratefully acknowledge Claudia Dell Apollonia and Verena Gabler for their contributions to data collection during exploratory observations. We also thank Jasmina Bogdanovic and Sabine Greschek for their critical feedback on an advanced draft of this paper.
Financial support: This study was funded by the Swiss National Science Foundation (grant no. 32003B_149474).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.326








