The core research focus of the Wisconsin Twin Project is human affective development, and the primary outcome for many analyses is clinical symptoms. Presently, the project covers development from the prenatal period to early adulthood. The Wisconsin Twin Project’s signature approach is its depth and breadth of phenotyping.
Our research foci include the etiology and developmental course of early emotions, temperament, childhood anxiety and impulsivity, the autism spectrum, auditory and tactile sensory sensitivity, and related neural, psychobiological and behavioral phenotypes. We employ a range of research methods to assess behavior (structured batteries of vignettes with affective incentives, interviews, observer ratings, child self-report, co-twin report, parent-report questionnaires, and computer-based cognitive and affective performance tests), experiences (observations and interviews), hormones (assays of basal and reactive cortisol, puberty-related hormones), brain structure and function (multimodal magnetic resonance imaging [MRI] and electroencephalogram [EEG]), genes (quantitative variance partitioning methods, genotyping) and other topics (e.g., pregnancy, birth and medical reports, self-reports of health).
We did not design the Wisconsin Twin Project to test any single overarching theory of development. Rather, the panel is meant to be a resource for individuals with potentially differing theoretical perspectives. Still, a general framework beginning with the development of temperament from birth underlies our design. Various aspects of temperament are regarded as predictors of later symptoms (e.g., lack of inhibitory control for later attention-deficit/hyperactivity disorder [ADHD] symptoms); moreover, temperament is expected to moderate the influence of experiences and exposures on the development of symptoms (e.g., behavioral inhibition may moderate the impact of overprotective parenting on later social anxiety). Figure 1 is a highly conceptual diagram of this framework.

Fig. 1. Longitudinal framework for generating hypotheses in the Wisconsin Twin Project.
We regard the neural underpinnings of symptoms not only as mediators of the effects of experience and temperament on symptoms but also as an interesting outcome of experience. The genetic features of this conceptual diagram are not depicted, but the twin design allows the analyses to be genetically informative. None of our published reports include every component of Figure 1. Rather, we specify components and test parts of the framework. For example, ‘temperament’ might be specified as ‘behavioral inhibition in early childhood’, ‘experiences and exposures’ as an ‘adverse childhood events index’, ‘neural structure and function’ as ‘resting state connectivity of amygdala with the dorsolateral prefrontal cortex’ and ‘anxiety’ as ‘social anxiety symptoms in late adolescence’. Then, this specific model can be tested with our data.
Wisconsin Twin Project birth cohorts spanned 1989–2004. After nearly 30 years, the research program encompasses a series of longitudinal studies that span infancy to early adulthood. Not every study uses the entire panel. In some cases, subsamples of panel members are selected to create an enriched sample for a phenotype of interest or due to cost considerations. The research is conducted at the University of Wisconsin–Madison’s Waisman Center and the Department of Psychology (https://goldsmithtwins.waisman.wisc.edu/). Procedures in studies under the Wisconsin Twin Project were approved by University of Wisconsin–Madison Internal Review Boards and comply with the Helsinki Accords of 1975, as revised in 2008. Twin family recruitment and early results were covered in prior overviews of the project (Goldsmith et al., Reference Goldsmith, Lemery-Chalfant, Schmidt, Arneson and Schmidt2007; Schmidt et al., Reference Schmidt, Van Hulle, Brooker, Meyer, Lemery-Chalfant and Goldsmith2013) and our recent description of the project (Schmidt et al., in press). Here, we summarize research results from the past few years.
Twin Neuroimaging
During early 2019, we completed data collection for a set of twin neuroimaging studies with approximately 600 adolescents and young adult twins. These studies assessed brain structure and function with multimodal MRI. We use T1- and T2-weighted structural scans, diffusion-weighted imaging, arterial spin labeling, resting state functional MRI (fMRI) and task-related fMRI. Concurrent with neuroimaging, we also include diverse measures of emotion, behavior, hormones and experience. The neuroimaging studies have incorporated Research Domain Criteria themes that emphasize dimensional approaches and neurobiological measurement for improved nosology (Cuthbert, Reference Cuthbert2014; Insel, Reference Insel2014). Monozygotic (MZ) twin differences are interrogated in some detail. In the MRI analyses, we focused more on white matter microstructure and on resting state and task-related functional measures (i.e., circuitry and networks) than on grey matter structure. Here, we describe three empirical results.
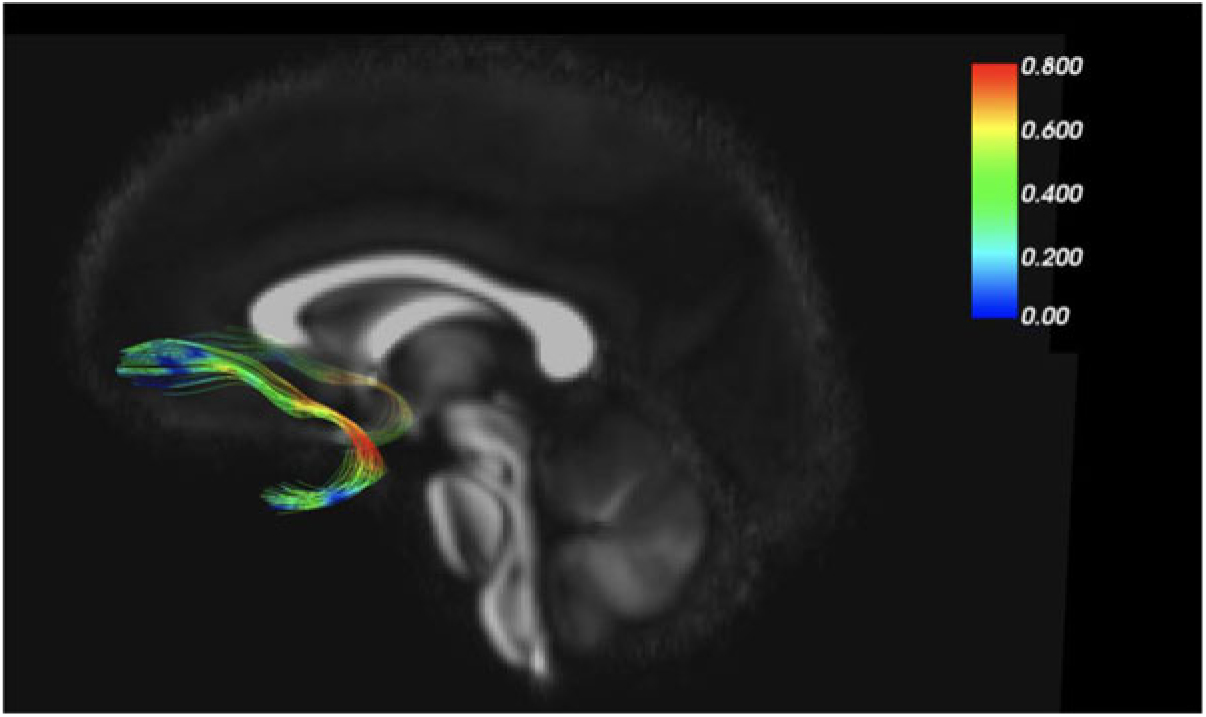
As an example of our findings, Figure 2 depicts the uncinate fasciculus, a prominent white matter tract that connects limbic regions with prefrontal cortex, which is likely involved in cortical regulation of emotional reactivity. We are interested in the white matter microstructure of adolescent uncinate fasciculus, measured by fractional anisotropy (FA) using diffusion tensor imaging (DTI) as a putative measure of the integrity (and thus functional properties) of that tract. Figure 2 color codes the degree of MZ co-twin similarity (i.e., MZ intraclass correlations [ICCs]) for FA along the uncinate fasciculus, from the frontal to the temporal segment. As the figure shows, adolescent MZ co-twins are substantially more similar in FA in the intermediary/insular segment of the uncinate fasciculus than in other segments. The mean MZ ICC of 0.42 thus obscures substantial variation and suggests that a question such as ‘How heritable is FA in the uncinate fasciculus?’ may be formulated at too general a level to be optimally informative.
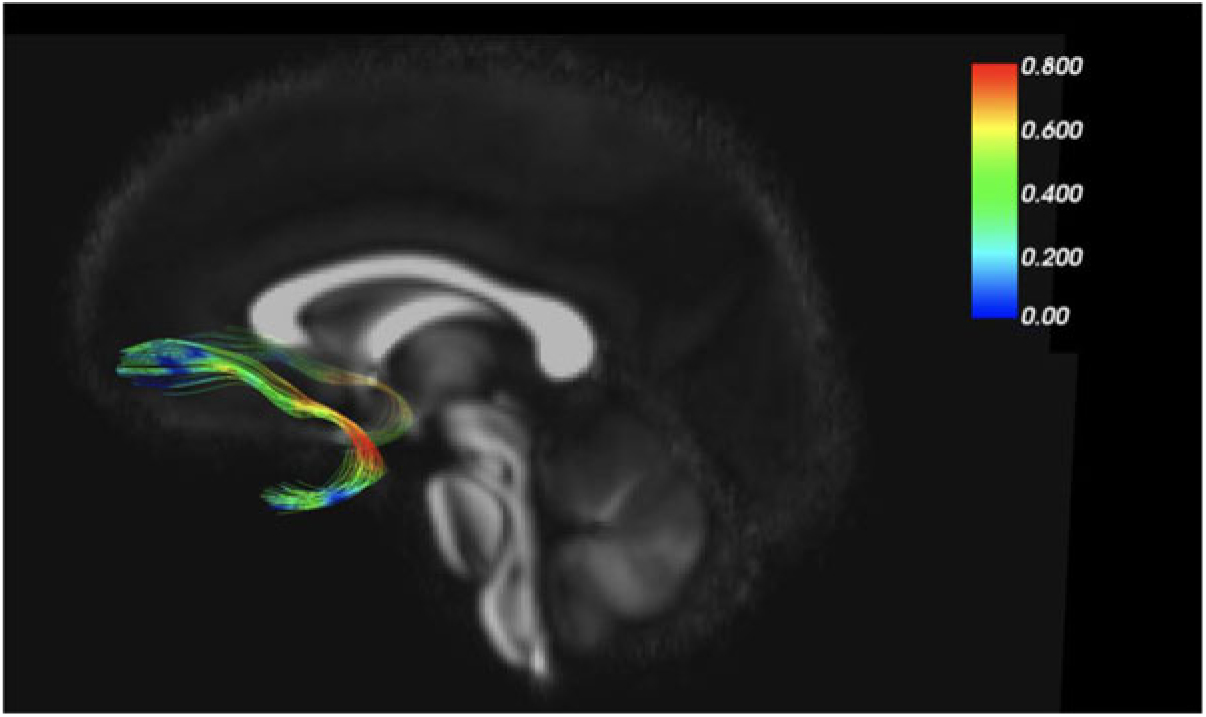
Fig. 2. MZ twin similarity in FA varies along the uncinate fasciculus.
Another example of our neuroimaging research uses the MZ difference design. In DTI analyses, we again focus on the uncinate fasciculus (Adluru et al., Reference Adluru, Luo, Van Hulle, Schoen, Davidson, Alexander and Goldsmith2017). MZ co-twins are similar for FA values in the left and right uncinate fasciculus (ICCs = .66 and .75, respectively). MZ co-twins are also similar for our anxiety measure, ICC = .67. When we shift from twin similarity to analyze (nongenetic) MZ twin differences, we detected an environmentally based relationship between FA in the uncinate fasciculus and concurrent anxiety symptoms. That is, the co-twin with more anxiety symptoms had lower FA (interpreted as lower white matter integrity) in the uncinate fasciculus. This white matter–anxiety relationship was not apparent when twins were treated as individuals, thus highlighting the importance of the control over confounding variables, such as gross brain morphology, afforded by the MZ difference design.
A final neuroimaging example is our implementation of novel brain structural network analysis using a classic twin design (Chung et al., Reference Chung, Luo, Adluru, Alexander, Davidson and Goldsmith2018). In a standard brain network analysis, the resolution of structural network connections is limited by the number of parcellations defined in a standard brain atlas. In this study, we proposed a hierarchical parcellation scheme that iteratively divides discrete brain structure into smaller subregions, even down to the voxel level. With the ability to choose the scale of the network under investigation, we demonstrated that the heritability of white matter structural connectivity patterns was robust and consistent across multiple network scales (including after five iterations that subdivided the whole brain into 3712 parcellations).
Psychophysiology and Endocrine Studies
Measures of psychophysiology and of hormones play key roles in the literature on temperament, emotion and stress. A subset of twins completed two sessions each at ages 6 and 12 months that included physiological measures (e.g., EEG and cortisol) along with behavioral episodes. We found differences in the stability of alpha-based frontal asymmetry in infants across baseline and emotion-eliciting contexts (Brooker et al., Reference Brooker, Canen, Davidson and Hill Goldsmith2017). Frontal asymmetry during baseline, or resting, conditions is viewed as an index of individual differences in emotional behavior (Brooker, Davidson et al., Reference Brooker, Davidson and Goldsmith2016); frontal asymmetry during emotion provocation is thought to index a readiness to respond to the contextual demands of one’s environment (Coan et al., Reference Coan, Allen and McKnight2006). Our work showed relatively high levels of stability in frontal asymmetry baseline conditions in infants. In contrast, we found lower levels of stability in frontal asymmetry during emotion elicitation, perhaps reflective of the rapid changes in emotional development occurring during infancy. This work thus holds relevance for developmental scientists seeking guidance with study design and procedure as well as those looking to refine current theory regarding the temporal unfolding of emotion during infancy.
We have used EEG along with other physiological measures in multimethod investigations of the interplay between children’s developmental context and biological function, particularly as they relate to risk for psychopathology. Specifically, we found that heightened levels of maternal negativity during infancy predict longitudinal disruptions in the normative patterns of change in diurnal cortisol and cross-context EEG asymmetry measures reflecting readiness to cope with environmental challenge (Brooker, Davidson et al., Reference Brooker, Davidson and Goldsmith2016). Disrupted cortisol, in particular, appeared to be a risk factor for emerging symptoms of anxiety by age 7. Moreover, heightened cortisol was linked to increased neural coupling, a putative index of neural efforts at regulation (Knyazev, Reference Knyazev2007) in response to nonpositive laboratory episodes (Brooker, Phelps, et al., Reference Brooker, Phelps, Davidson and Goldsmith2016).
Individual differences in cortisol are linked to a broad range of health outcomes (Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013). Twins provided salivary samples (3× per day) on three consecutive days during childhood (ages 7–10 years) and adolescence (ages 12–16 years). Samples were assayed for cortisol at both ages and for dehydroepiandrosterone (DHEA) and testosterone at adolescence only. We examined the genetic architecture of these hormonal measures and their relationship to behavior and brain function. Shared and nonshared environmental factors accounted for the majority of variation in cortisol level and diurnal slope, whereas genetic factors accounted for a modest proportion of the variation in morning cortisol level and morning to afternoon diurnal slope (Van Hulle et al., Reference Van Hulle, Shirtcliff, Lemery-Chalfant and Goldsmith2012). We also examined the contribution of these latent biometric factors to the coupling of DHEA and testosterone in adolescence. Hormone coupling (or covariation) impacts behavior problems beyond the effects of either hormone alone (Johnson et al., Reference Johnson, Dismukes, Vitacco, Breiman, Fleury and Shirtcliff2014). Testosterone–DHEA covariation, independent of pubertal status, was due to correlated shared and nonshared environmental factors (Van Hulle et al., Reference Van Hulle, Lemery-Chalfant and Goldsmith2015). Hypothalamic pituitary adrenal (HPA) axis function is shaped by early life experience and impacts later behavior. We showed that the infants who exhibited a steep increase in fear of strangers from ages 6 to 36 months had flatter diurnal cortisol slope (girls only) and more anxiety symptoms at age 7 years than infants who followed a more typical pattern of stranger fear development (Van Hulle, Moore et al., Reference Van Hulle, Moore, Lemery-Chalfant, Goldsmith and Brooker2017). MZ twin intrapair differences in childhood afternoon cortisol level were in turn associated with intrapair differences in resting state connectivity between the amygdala and perigenual prefrontal cortex (PFC), and with intrapair differences in amygdala recovery following unpleasant images in adolescence (Burghy et al., Reference Burghy, Fox, Cornejo, Stodola, Sommerfeldt, Westbrook and Birn2016). The co-twin with higher childhood cortisol evinced relatively lower resting state connectivity and poorer amygdala recovery in adolescence. Together, these studies highlight the importance of nongenetic factors in contributing to HPA function and its effects on emotion regulation and behavior. Overall, products from this work include contributions to basic developmental science as well as theory-driven empirical tests of the interplay between experience and the development of biological systems.
Studies of the Genetics of Temperamental Development
A consistent theme of our longitudinal work concerns the etiology of temperamental traits, their developmental trajectories and their functional significance. The age range in papers extends from infancy to early adolescence. Our temperament assessment batteries are modified to be age-appropriate but are still aligned to allow testing the same construct over time (Goldsmith et al., Reference Goldsmith, Lemery, Schmidt, Schmidt, Chow and Justen2010; Goldsmith & Rothbart, Reference Goldsmith and Rothbart1996). For example, we have used multiple laboratory temperament assessment episodes designed to measure child fear, anger, sadness and positive affect in infancy, toddlerhood, preschool and middle childhood. We also transformed what was initially a lab-based infant/toddler temperament battery to become a middle childhood battery administered in the home setting (Goldsmith et al., Reference Goldsmith, Lemery, Schmidt, Schmidt, Chow and Justen2010). Measurement properties and scale validation are established (Gagne et al., Reference Gagne, Van Hulle, Aksan, Essex and Goldsmith2011; Planalp, Van Hulle, Gagne et al., Reference Planalp, Van Hulle, Gagne and Goldsmith2017), and the measures are conceptualized in terms of theories of emotion (Planalp, Van Hulle, Gagne et al., Reference Planalp, Van Hulle, Gagne and Goldsmith2017).
Here, we recount recent findings. We identified distinct trajectories of stranger fear during the first 3 years of life (Brooker et al., Reference Brooker, Buss, Lemery-Chalfant, Aksan, Davidson and Goldsmith2013) and noted associations with later diurnal cortisol and anxious behavior (Van Hulle, Moore et al., Reference Van Hulle, Moore, Lemery-Chalfant, Goldsmith and Brooker2017). We found that self-conscious shyness is etiologically distinct from fearful shyness during early childhood (Eggum-Wilkens et al., Reference Eggum-Wilkens, Lemery-Chalfant, Aksan and Goldsmith2015), with strong genetic contributions to both varieties of shyness. Genetic factors were particularly strong for developmental growth in self-conscious shyness (Eggum-Wilkens et al., Reference Eggum-Wilkens, Lemery-Chalfant, Aksan and Goldsmith2015). In contrast, strong environmental factors were observed for infant positive affect; environmental factors also contributed to positive affect development through the first year of life (Planalp, Van Hulle, Lemery-Chalfant et al., Reference Planalp, Van Hulle, Lemery-Chalfant and Goldsmith2017). When examining parent- and lab-assessed anger and inhibitory control in 3-year-old twins, we found significant genetic influences on parent-rated anger and inhibitory control, and lab-assessed anger (shared environmental influences contributed to twin similarity on lab assessments of inhibitory control). Twins with higher levels of anger had lower inhibitory control, but genetic covariance was only present for parent-rated anger and inhibitory control (Gagne & Goldsmith, Reference Gagne and Goldsmith2011).
Modest gender differences in temperament were observed at 3 years of age, with girls scoring somewhat higher in shyness and inhibitory control and boys scoring higher in activity level. Similar patterns were observed in both same-sex and opposite-sex twins; opposite-sex twin girls were not significantly different to same-sex twin girls (Gagne et al., Reference Gagne, Miller and Goldsmith2013). Shyer children had higher scores on inhibitory control and lower scores on activity level.
In a study of negative emotionality at approximately 7 years of age, genetic contributions were observed for parent-rated anger, sadness and fear. Estimates of additive genetic influences were comparable for anger and sadness but nearly twice as strong for fear; standardized estimates of additive genetic variance were .45, .45 and .74, respectively (Clifford et al., Reference Clifford, Lemery-Chalfant and Goldsmith2015). Furthermore, genetic influences on anger were not shared with fear.
Two recent papers identified profiles of temperament across multiple domains. Profile analysis of the Children’s Behavior Questionnaire revealed four groupings of 7- to 8-year-old children based on differences in temperamental reactivity and regulation, and positive versus negative emotionality (Scott et al., Reference Scott, Lemery-Chalfant, Clifford, Tein, Stoll and Goldsmith2016). Genetic factors influenced all four profiles, but were strongest for the Well-regulated, Positive Reactive and Regulated, Surgent profiles. Shared environmental factors were strongest for the Regulated, Typical Reactive profile and were also present for the Dysregulated, Negative Reactive profile (Scott et al., Reference Scott, Lemery-Chalfant, Clifford, Tein, Stoll and Goldsmith2016). In a profile analysis of observed behavior from the Lab-TAB at 6 and 12 months of age, we again identified four profiles, with the strongest stability and genetic contribution in the Withdrawn/Inhibited profile (Planalp & Goldsmith, Reference Planalp and Goldsmith2019). Thus, our project views dimensional and categorical (profile) approaches as complementary and examines both types of measures with biometric approaches.
Our twin data help address pressing questions concerning pediatric comorbidity. A latent class approach applied to item-level symptoms with a sample of >3200 children at mean age 7.5 years revealed nine classes for both boys and girls, ranging from low symptoms to moderately internalizing and severe externalizing (Vendlinski et al., Reference Vendlinski, Javaras, Van Hulle, Lemery-Chalfant, Maier, Davidson and Goldsmith2014). Although the classes for girls and boys were largely similar, a unique class for boys was characterized by severely impulsive and inattentive symptoms. A unique class for girls was characterized by moderate anxiety symptoms (Vendlinski et al., Reference Vendlinski, Javaras, Van Hulle, Lemery-Chalfant, Maier, Davidson and Goldsmith2014). Univariate ACE models fit to probability of class membership in a combined-gender analysis are shown in Table 1.
Table 1. Univariate biometric analyses of the probability of latent class membership for symptoms in childhood (adapted from Vendlinski et al., Reference Vendlinski, Javaras, Van Hulle, Lemery-Chalfant, Maier, Davidson and Goldsmith2014)

Note: ***p<.001, **p<.01; A, C and E refer to Additive, Common (shared) environmental and unique (nonshared) Environmental sources of variation. N = 3223 individual twins; mean age = 7.5 years; 51% female.
Notable features of this analysis include the frequency of combined internalizing and externalizing symptoms in three of the more severely impaired classes (C8, C6, C5), the temperament-like nature of the less severe classes (C1, C2, C3) and the importance of shared environmental factors for both the low symptom class (C4) and the three most symptomatic classes (C6, C7, C8).
Roles for Environmental Factors and Gene–Environment Interplay
Our longitudinal research captures a wealth of environmental contexts, including prenatal risks, parenting, marital quality, sibling relationships and peer relationships. Most measures are repeated over time and include perspectives from multiple family members, including co-twin ratings of twin peer experiences. Here, we describe six recent papers that characterize the role of environmental factors as moderators of development.
We considered the role of early life stress as a moderator of the link between early anger and preschool behavior problems. Family-level measures of cumulative life events interacted with early laboratory-derived anger profiles at 6 and 12 months of age to predict behavior problems at age 3 years. Children who exhibited atypical anger in infancy showed more behavior problems than children who exhibited increasing anger, though only when family stress was low (Brooker et al., Reference Brooker, Buss, Lemery-Chalfant, Aksan, Davidson and Goldsmith2014). In a second study identifying temperament profiles at 6 and 12 months, fathers reported higher stress levels when infants were more Withdrawn/Inhibited, and less stress when infants were identified as Low Negative (Planalp & Goldsmith, Reference Planalp and Goldsmith2019). In the same sample, at 3 years of age, correlates of mother and father parenting behaviors during a dyadic interaction differed depending on parent factors: fathers were more sensitive with their children when marital quality was higher, and mothers were more sensitive when her own positive affect was higher. Further, fathers were more responsive toward children rated higher in inhibitory control (Planalp et al., Reference Planalp, Van Hulle and Goldsmith2019). Thus, different levels of parental stress and different types of parenting behaviors provide varying experiential contexts for child development (Planalp et al., Reference Planalp, Van Hulle and Goldsmith2019).
In the fourth paper, we showed that both child effortful control and extraversion/surgency at age 8 years were modestly more heritable under more chaotic home environment conditions (Lemery-Chalfant et al., Reference Lemery-Chalfant, Kao, Swann and Goldsmith2013). The overall variance in effortful control was greater in more chaotic home environments, but variance in extraversion/surgency was minimally different in more chaotic environments (Lemery-Chalfant et al., Reference Lemery-Chalfant, Kao, Swann and Goldsmith2013). Furthermore, home environments were less chaotic for children with higher levels of effortful control and more chaotic for children with high extraversion/surgency, and both associations were genetically mediated. Parents provide genes that contribute to variation in both home life and in their children’s temperament. Heritability of negative affectivity increased in more crowded or unsafe home environments, and overall variance was higher in crowded or unsafe environments.
We further explored the interaction of genetic risk and experience for anxiety and depression symptoms at age 8 years. Although independent main effects of genetic risk and environmental risk accounted for most of the variance in child behavior symptoms, we found G × E interactions that supported both the diathesis-stress and bioecological models of development (Vendlinski et al., Reference Vendlinski, Lemery, Essex and Goldsmith2011). Associations with parent psychopathology symptoms and child anxiety and depression symptoms were higher for those at elevated environmental risk (e.g., cumulative risk that considered socioeconomic status, single-parent household, two or more stressful life events in the past year, greater perceived negative impact of life events and observed maternal negativity). Consistent with the bioecological model, higher stress environments led to greater risk for anxiety and depression, regardless of genetic risk as inferred from co-twin mental health. Heritability estimates for anxiety and depression symptoms were higher for children in low stress environments.
One epigenetic analysis using the MZ difference design supplements our genetic research on inhibited temperament and anxiety. We examined whole genome sequence data on a pilot sample of three early-adult MZ pairs to generate DNA methylation profiles. These three pairs were chosen to be discordant for age 15 anxiety diagnosis, afternoon cortisol levels across 3 days at age 8 years and amygdala-modulated blood-oxygen-level-dependent signal recovery at age 15 years in an fMRI task. Both of these biological measures are linked to behavioral anxiety. There were 230 significantly differentially methylated sites, each with a minimum of 8 contiguous CpG dinucleotides. These sites could be annotated to 183 genes (often located in the promoter or transcription factor regions), including known stress-related genes such as NAV1, IGF2, GNAS and CRTC1. Importantly, the differential methylation levels in these 230 regions consistently differentiated the more anxious co-twins from the less anxious member in each of the three pairs (Alisch et al., Reference Alisch, Van Hulle, Chopra, Bhattacharyya, Zhang, Davidson and Goldsmith2017).
Studies of Other Aspects of Mental and Physical Health
Rumination
Cognitive processes such as rumination and brooding are associated with risk for depression. In an early adolescent sample, we observed phenotypic associations among depressed mood, rumination and distraction. Heritability was moderate for depression (54%), and lower for brooding (21%) and distraction (30%) (Moore et al., Reference Moore, Salk, Van Hulle, Abramson, Hyde, Lemery-Chalfant and Goldsmith2013). Bivariate genetic analyses demonstrated that the association between brooding and depression symptoms was primarily due to shared genetic factors.
In a partially successful attempt to replicate two candidate gene results in the literature, we first examined a well-known single nucleotide polymorphism (SNP) at codon 66 in the BDNF gene, which specifies a Val/Met amino acid substitution. We found higher brooding among BDNF Met carriers, and the genetic effect was carried by more physically mature adolescents. Moreover, status for two CRHR1 SNPs and maternal depression interacted, such that the homozygous minor genotype was protective against brooding when adolescents’ mothers had more symptoms of depression (Van Hulle, Clifford et al., Reference Van Hulle, Clifford, Moore, Lemery-Chalfant and Goldsmith2017). Pending further replication, these findings provide partial support for the influence of candidate genes in two environmentally sensitive systems on a putative endophenotype for depression.
Suicidal Ideation
Following national trends, a distressing portion of our sample screens positive for suicidal ideation, which is a strong predictor of suicide attempts (Nock et al., Reference Nock, Borges, Bromet, Alonso, Angermeyer, Beautrais and Williams2008). Comparing twins with suicidal ideation (probands) with matched nontwin controls, a brooding style of rumination, inattention, impulsivity and current depression all increased the odds of experiencing suicidal thoughts (odds ratios [ORs] 2.1–3.0). When probands were compared with their discordant co-twins, only inattention remained a significant predictor of suicidal ideation (OR = 1.9). Crucially, current depression did not distinguish probands from their co-twins who did not experience suicidal thoughts (Sarkisian et al., Reference Sarkisian, Van Hulle and Goldsmith2019), suggesting that nondiagnostic cognitive factors are a useful addition to risk prediction.
In follow-up research (Sarkisian, Reference Sarkisian2019), we showed that the association of attentional problems and related problem-solving behaviors with suicidal ideation holds when these predictors are measured 7 years earlier. That is, twins who had more attentional problems and exhibited less persistence during behavioral problem-solving tasks than their co-twins during childhood were more likely to experience suicidal ideation as adolescents. Given the key role that attentional processes play in anxiety disorders, we hypothesize that anxious behavioral patterns and anxiety-relevant circuitry are similarly influenced longitudinally and concurrently by nonshared environmental effects. Thus, our twin difference approach identifies attentional problems as a novel predictor of suicidal ideation, and we are now analyzing the neural basis and developmental antecedents of this association.
Obesity
Obesity is a global public health crisis. We investigated whether temperament measures of control and reward-related behavior predict early adolescent adiposity (body mass index ≥ 85th sex- and age-percentile). Higher inhibitory control ratings on behavioral ratings from the ‘Tower of Patience’ task at age 7 years almost halved the odds of age 12 overweight/obesity in females (OR = 0.52, p = .10), but not in males (OR = 1.27, p = .63; Javaras, Reference Javaras2012). Similarly, higher ‘virtue’ measures (no bold cheating during the ‘Throwing Game’) almost halved the odds of age 12 overweight/obesity in females (OR = 0.56, p = .06), but not in males (OR = 0.96, p = .91). Controlling for socioeconomic status, IQ and parent adiposity had little effect on the relationships. Parents’ ratings at age 7 of lower inhibitory control, lower attentional focusing and higher impulsivity also predicted higher adiposity in females at age 12 (Javaras, Reference Javaras2012).
Peer Victimization
Peer victimization is a widespread social stressor impacting the lives of many children and adolescents. Despite its well-documented associations with adverse outcomes, mechanisms linking peer victimization to these outcomes remain largely uninvestigated. We considered the experience of peer victimization — the experience of being bullied — during early adolescence and concurrent anxiety and depression symptoms and attentional response bias during an affective go/no-go task from the Cambridge Neuropsychological Test Automated Battery (Cambridge Cognition, 2019; Robbins et al., Reference Robbins, James, Owen, Sahakian, McInnes and Rabbitt1994). We used the MZ difference design, which controls for genetic factors, gender, age, socioeconomic status and other confounders, and a signal detection approach. Twins who experienced more severe peer victimization were biased toward detecting goal relevant stimuli during the go/no-go task (Carroll et al., Reference Carroll, Planalp, Van Hulle and Goldsmith2019). The finding held when anxiety and depression were statistically controlled. Thus, our findings suggest an environmentally salient relation between peer victimization and goal-oriented selective attention. Our work identifies selective attention as a candidate for future mechanistic investigation linking peer victimization to adverse outcomes.
Sensory Over-Responsiveness
Sensory over-responsivity, characterized by highly aversive or painful responses to everyday sensory experiences, has been a major theme of our work. Among the understudied sensory-related behavioral phenotypes, we generally focus on auditory and tactile over-responsiveness. Children with sensory over-responsivity may find the school gymnasium or cafeteria environment too loud, or they may experience an extremely negative reaction to subtle noise from certain light fixtures. Similarly, children may have an extremely negative reaction to certain food or clothing textures, or dislike going barefoot at home or outdoors. Our recent work on sensory issues focuses on chronicity and genetic transmission from parents. Our longitudinal data identified chronicity for sensory challenges from ages 2 to 7 years, with a trend toward greater severity at age 7 years (Van Hulle et al., Reference Van Hulle, Lemery-Chalfant and Goldsmith2015). Prematurity and low birth weight were more common in the chronic symptom group. Chronicity was also predicted by features of temperament such that greater fearfulness and less soothability during toddlerhood predicted greater symptoms of tactile over-responsiveness (Van Hulle et al., Reference Van Hulle, Lemery-Chalfant and Goldsmith2015). Similar results were found for symptoms of auditory over-responsiveness.
Parents also completed sensory profiles and diagnostic interviews. Mothers with a diagnosis of anxiety or depression reported more frequent sensory symptoms (Van Hulle et al., Reference Van Hulle, Lemery-Chalfant and Goldsmith2018). Interestingly, children of parents with affective disorders were at greater risk for sensory over-responsivity. Specifically, parent depression uniquely predicted adolescent sensory symptoms, even after accounting for parent sensory challenges. Moreover, father alcohol abuse/dependency predicted offspring sensory over-responsivity symptoms (Van Hulle et al., Reference Van Hulle, Lemery-Chalfant and Goldsmith2018). In summary, sensory over-responsiveness both accompanies recognized clinical conditions and occurs independently of these conditions. Thus, sensory over-responsiveness appears as an under-recognized transdiagnostic phenotype with partial genetic underpinnings, and the recent addition of the sensorimotor domain to National Institute of Mental Health’s Research Domain Criteria appears well-justified.
Comorbidity of Attention Measures with ADHD and Anxiety
In investigating comorbidity during adolescence (N ≥ 1000 twins), we found that the association between attentional control, ADHD and anxiety symptoms was due to shared genetic and nonshared environmental factors (Brooker et al., Reference Brooker, Moore, Van Hulle, Beekman, Begnoche, Lemery-Chalfant and Goldsmith2019). Shared genetic factors also accounted for co-occurring anxiety and ADHD symptoms, even after controlling for attention. Also, shared genetic factors explained the association between adolescent attentional control and four subtypes of anxiety (e.g., general anxiety, separation anxiety, social anxiety and obsessive-compulsive anxiety), with some specificity and distinct etiology for anxiety subtypes (Gagne et al., Reference Gagne, O’Sullivan, Schmidt, Spann and Goldsmith2017).
Future Directions
This abbreviated account of our recent results suggests many avenues for investigation. The sample sizes afford the possibility to examine selected subsamples (e.g., subsamples enriched for symptoms) and for many more longitudinal analyses. To date, we have focused more on anxiety and depression than on disinhibitory symptoms, although the extant data allow for extensive examination of ADHD and conduct problems. We plan new assessments with early adulthood outcomes, deeper assessment of experiential differences using ecological momentary assessment and a greater focus on general health outcomes. We welcome interest in collaboration.
Acknowledgments
This work was supported by the National Institute of Mental Health (R01-MH101504, R01-MH059785, R01-MH069793 and R37-MH050560), the Wisconsin Center for Affective Science (P50-MH069315), two Conte Neuroscience Centers (P50-MH084051 and P50-MH100031), the Wallace Research Foundation and the National Alliance for Autism Research. Infrastructure support was provided by the Waisman Center via core support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P30-HD003352 and U54-HD090256). The authors owe special gratitude to Wisconsin twins and their families for their generous time for research participation. We also thank a large team of scientific collaborators, graduate students, postdoctoral trainees, research staff and undergraduate students. Over 450 undergraduate students at the University of Wisconsin–Madison received training in research and directly supported Wisconsin Twin Project research activities.