The WHO considered iron deficiency (ID) as one of the most common forms of nutritional deficiencies, especially among females affecting 33 % of non-pregnant women, 40 % of pregnant women and 42 % of children worldwide(1). ID during pregnancy can cause widespread and significant consequences for both the mother and her infant’s physiological and mental health(Reference McArdle, Gambling and Kennedy2).
Iron has several vital functions in the body including the functions of the iron-containing enzymes, the synthesis of bile acid and steroid hormones, and signal -controlling in some neurotransmitters, such as the serotonin and dopamine systems in brain(Reference Gupta3). ID has adverse effects on areas of women’s mental health such as mood, short-term memory, verbal learning, attention, concentration and intelligence. It can also lead to depression(Reference Vahdat Shariatpanaahi, Vahdat Shariatpanaahi and Moshtaaghi4).
Various physiological variables related to ID that contribute to postpartum depression (PPD) such as fatigue that have been considered as significant predictor of PPD(Reference Bozoky and Corwin5). The next variable is anaemia which contributes to fatigue and is associated with additional symptoms such as irritability, apathy and an inability to concentrate(Reference Turkoski6). Furthermore, ID has been found to have a role in altering thyroid hormone metabolism(Reference Ashraf, De Sanctis and Yassin7). All of these symptoms (fatigue, anaemia and abnormal thyroid status) are probably interrelated in certain individuals; if they occur during the postpartum period, they could affect maternal psychological and health outcome.
PPD is a major public health concern that may lead to the most common complications of childbearing and represents a serious health problem affecting mothers and their families, and thus the whole society, as it affects about 10–15 % of new mothers(Reference Vigod, Villegas and Dennis8). However, depression in women during the postpartum period may start during pregnancy or may have onset beyond the first postpartum month(Reference Pearlstein, Howard and Salisbury9). The exact aetiology of PPD is still unclear; however, it is generally thought to be linked to genetic, biological, hormonal, psychosocial, environmental and nutritional factors(Reference Yan, Liu and Cao10).
Generally, balanced nutrition plays an important role in the model of thinking and behaviour, and affects the cognition and memory capacity, and has direct or indirect effects on brain health; as the intake of different foods is directly involved in the synthesis and metabolism of related neurotransmitters(Reference Huang, Liu and Suzuki11).
During pregnancy and postpartum periods, mothers face continuous nutritional vulnerability, since malabsorption, food choice, food preparation and other socio-economic factors collectively may have a reverse effect on the micronutrients level including iron(Reference Hogg-Kollars, Mortimore and Snow12). Moreover, due to the physiological changes during pregnancy, ID often occurs towards the end of pregnancy even among women who enter pregnancy with some iron stores, which extends to the postpartum period that is associated with the development of maternal PPD(Reference Brown, Isaacs and Krinke13).
Generally, there are discrepancies in the literature concerning the association between iron and PPD. The direct relationship between ID biomarkers and PPD has been confirmed in different studies(Reference Albacar, Sans and Martín-Santos14–Reference Wassef, Nguyen and St-André16). This relation stems from the fact that iron is critical for adequate myelination, neurotransmitter metabolism and function, and neuronal cellular and oxidative processes(Reference Chandrasekaran, De Souza and Urquia17). In contrast, a study established in China found that there is no relationship between maternal iron status and PPD(Reference Armony-Sivan, Shao and Li18).
This study was conducted in Gaza City, Palestine, due to some perturbing factors. Gaza had been insecure since 2009, due to the dramatic series of events in the region(Reference Jalambo, Karim and Naser19). In addition to the socio-economic situation and food insecurity, the Palestinians are exposed to political violence that makes them at heightened risk for major depression and post-traumatic stress disorder. This makes depression one of the top five causes of disability in the occupied Palestinian territory(Reference Canetti, Hall and Greene20). It leads to low socio-economic status resulting in micronutrients deficiencies. This study aims to investigate the association between iron body status and PPD among mothers during the postpartum period in Gaza city.
Methods
Study design and sampling
The study population was obtained from the immunisation electronic records of the newly registered neonates in postnatal care. The study involved postpartum mothers, a month (30 d) after delivery, who visited the randomly selected (five out of ten) governmental primary health care centres with their infants for Polio vaccines.
A case–control study was conducted in the period from January 2020 to August 2020 in which 1100 postpartum mothers were screened for PPD by using a validated Edinburgh Postnatal Depression Scale (EPDS). The cases were postpartum mothers with depression who scored EPDS (≥ 10), while mothers who scored EPDS (< 10) were not diagnosed with PPD disorder and considered as controls. The controls were paired with the cases according to age and place of residency. Participants who were taking psychiatric drugs during the pregnancy period were excluded from the study. The additional exclusion criteria included mothers who had a history of postpartum blood transfusion, had a postpartum hemorrhage, smokers – as smoking may alter the Hb ratio and increase the oxidative stress – and mothers who refused to sign the consent form.
The study involved 300 mothers, as the sample size was calculated using Power Statistics program for sample size calculation (PS) and by using two-proportion method in Power Statistics software. P0 is the possibility of exposure in the control group = 23·3 % based on the prevalence of ID among non-PPD mothers according to Albacar and his colleagues, while P1 = 38·8 % indicating that 38·8 of the PPD had ID(Reference Albacar, Sans and Martín-Santos14). The calculated sample size according to PS software was 144 in each group. After considering the non-response rate, the number of the subjects in each group was increased to 150.
Questionnaire
A face-to-face interview was conducted to complete a questionnaire designed to meet the needs of the case and control groups. During the interview, the researcher clarified questions for participants. Most questions were closed-ended questions. The questionnaire is divided into three parts: the first part deals with socio-demographic and socio-economic characteristics, the second part contains obstetric and gynecological history, the third part includes the biochemical section.
Assessment of PPD
The translated copy of EPDS used in this study was established by the Department of Health Government of Western Australia. The cut-off point, recommended by the author, was 9/10, which means a score of 10 or higher indicates that depressive symptoms have been reported. The validation study of this translated questionnaire on Arabic mothers showed that the sensitivity and specificity for the selected cut-off point 9/10 were 91 % and 84 %, respectively(21). Thus, the mothers who scored EPDS (≥ 10) were diagnosed with PPD disorder, while mothers who scored EPDS (<10) were not diagnosed with PPD disorder.
Blood samples and biochemical measurements
The blood sample collection from the mothers occurred at the newborn’s vaccination section in the selected clinics. The samples were drawn carefully to avoid haemolysis. A 5-ml venous blood sample was drawn from each participant. About 1 and a 1/2 ml were discharged in EDTA vacutainer tubes and mixed gently for the complete blood count test (CBC). The rest of the blood was discharged into the vacutainer plain tube for the biochemical tests and was left to clot then centrifuged at a speed of 3000 rpm for 10 min under room temperature. Then the serum was placed in plain tubes, and in order to avoid loss of bioactivity and contamination, the tubes were sealed and stored at −20°C until the test performed. The biochemical tests that were done from the serum sample are ferritin, hs-CRP and soluble transferrin receptors (sTfR). Each device was calibrated, and controls were performed before each run of samples. All the blood tests were conducted at the Palestinian Medical Relief Society (PMRS) laboratory by the researcher herself.
Assessment of body iron
In this study, instead of performing the serum iron test, body iron stores were calculated. According to the report of a joint WHO and CDC on the assessment of iron status at the population level, the following equation was used to represent the body iron stores(22):
According to Cogswell and her colleagues, after applying the previous equation, the estimate of the prevalence of ID defined as body iron < 0 mg/kg; moreover, they compared these estimates with ID based on the ferritin model among females aged between 12 and 49 years. This means that body iron is expressed as iron excess in stores if the result of sTfR/log ferritin index was ≥ 0 mg/kg. However, if the result was <0 mg/kg, then it indicates iron deficit in the body(Reference Cogswell, Looker and Pfeiffer23).
Assessment of serum transferrin receptor levels has been used to distinguish ID anaemia from anaemia of chronic disease because the receptors are generally unaffected by concurrent infection or inflammation(24). Combining the use of serum transferrin receptor concentrations with serum ferritin concentrations as the serum transferrin receptor/log ferritin ratio has also been proposed to increase the diagnostic sensitivity and specificity for diagnosing ID(Reference Infusino, Braga and Dolci25,Reference López, Molinos and Carmona26) .
Statistical analysis
Following data collection, the questionnaires and anthropometric data were reviewed before being entered into the study database. Keyed data were checked for missing or unclear responses following completion of the interview. Respondents were contacted by phone to inquire about unclear and missing data.
Categorical variables like the socio-demographic and obstetric variables were presented as percentages and frequencies, while the quantitative continuous variables, like the biochemical results, were presented as means and standard deviations. The χ 2 test was used to compare the distribution of categorical qualitative variables across the PPD and non-PPD groups. Independent t-test and Mann–Whitney test were used to compare between means and ranks, respectively. Multiple logistic regression was performed to investigate the association between body iron status and PPD in present of other confounding factors as shown in Fig. 1. The level of significance was set at 0·05.
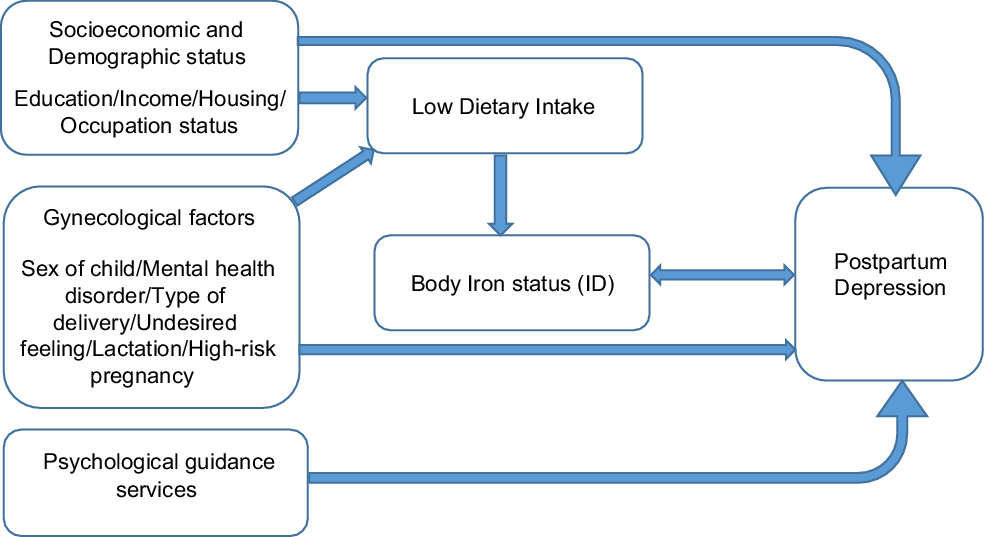
Fig. 1 Conceptual frame of the study
Results
The 300 mothers who participated in this study were categorised into 2 groups: the case group that included 150 mothers who were diagnosed with PPD by the EPDS (≥ 10) with a mean score of 15·2 and sd (± 4·2) and the control group included 150 mothers who were not diagnosed with PPD by the EPDS (<10) with mean score of 5·26 and sd (± 2·65).
Table 1 shows that 85 % of cases and 89·3 % of controls were homeowners and the rest of both groups home renters, thus the type of housing (P = 0·856) was not significantly different. Neither the educational level nor the occupational status of the mother showed any significant different as P-values were 1·00 and 0·289, respectively. Regarding the total family monthly income for the participant mothers, there was no significant difference between cases and controls (P = 0·388). The results showed no statistically significant associations between the PPD and the sex of the child (P = 0·298). There were no significant differences in proportions between the two groups regarding the worries about the embryo gender (P = 1·000), having the first baby (P = 0·250) and planning for the pregnancy (P = 0·468). As for the type of delivery, most of the cases and controls had a normal delivery 78·7 % and 76·7 %, respectively, while 21·3 % of cases and 23·3 % of controls had caesarean section (C.S.) delivery, which indicates the absence of the significant differences between PPD and the type of delivery (P = 0·782). Despite that the mothers in the control group were breast-feeding 60·0 % compared to the cases group 50·7 %, the results in this study, no statistical association between PPD and the lactation (P = 0·212). The results showed statistically significant associations between PPD and being classified as a high-risk pregnancy (P = 0·003), in which 6·0 % of the cases mothers faced gestational hypertension, 3·3 % faced gestational diabetes and 2·0 % had pre-term labour. Previous use of oral or injection contraceptive methods did not indicate any significant differences between the two groups (P = 0·178).
Table 1 Comparison between cases and control

Table 1 also shows that the mothers who had an undesired and uncomfortable feeling during the last pregnancy period were more susceptible to develop PPD after the baby delivery, as for 58·0 % of the mothers in the cases group were suffering from this such issues, and thus there were statistically significant differences between the two groups (P = 0·008). The relationship between the PPD and attending psychological guidance services was highly statistically significant (P < 0·001), since 20·7 % of the controls attended such services, while 98·0 % of the cases did not attend any psychological guidance services before or during the pregnancy. Moreover, there was a highly significant association between the PPD and having one of the mental health disorder in the last pregnancy (P < 0·001), 12·7 % of the mothers in the cases group were suffering from major depression during the last pregnancy, 22·0 % were suffering from eating disorders and 23·3 % were suffering from either anxiety, insomnia or panic.
Table 2 presents a highly significant differences between the two groups regarding the following CBC parameters that have a role in screening and diagnosis of the body iron status namely, Hb (P < 0·001), haematocrit (P < 0·001) and mean corpuscular volume (P = 0·013). The table shows that most of the participants were not having inflammation since 69·3 % of them had a normal hs-CRP level (<5 mg/l) and the rest of the participants had high hs-CRP level (>5 mg/l).
Table 2 Haematological and biochemical characteristics of cases and controls
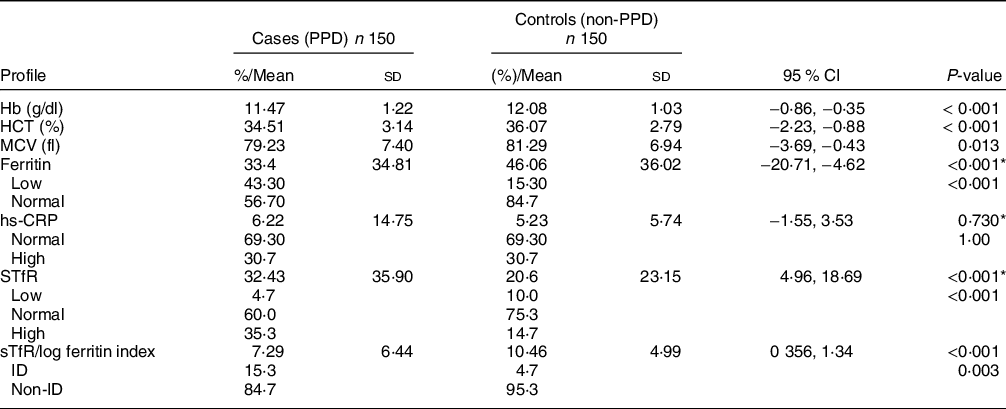
HCT, haematocrit; MCV, mean corpuscular volume; sTfR, soluble transferrin receptors; ID, iron deficiency.
* Mann–Whitney test for non-parametric test.
The mean difference in the sTfR between the two groups was statistically significant and as shown in the table. The results show that cases have almost 12 units of sTfR higher than that of the controls. Moreover, the mean difference in the sTfR/log ferritin index was statistically significant and it was in favour of the control group. The sTfR/log ferritin index determined that most (84·7 %) of the mothers in the cases group were not categorised with ID and 23 mothers (15·3 %) were categorised with ID, while only 7 (4·7 %) mothers in the controls group were categorised with ID, and 95·3 % were not categorised with ID. Thus, there was a statistical significant differences in ID between the two groups (P = 0·003).
Based on the results of simple logistic regression (SLgR), independent variables with a P-value < 0·25 or any other important associated factors from previous studies were included in the multiple logistic regression (MLgR). Table 3 presents all the determinants appearing in the final model that remained significantly associated with the PPD status, namely, the psychological guidance services, the undesired feeling during the last pregnancy, the mental health disorder in the last pregnancy and the body iron status. The results of MLgR showed that the mothers who did not attend any psychological guidance services were 8·54 times more likely to develop PPD (ORadj 8·54, (95 % CI 2·41, 30·21); P = 0·001), and that the mothers who had undesired feeling in the last pregnancy were 1·77 times more susceptible to suffer from PPD than the mothers who did not have that feeling (ORadj 1·77, (95 % CI 1·04, 3·03); P = 0·034).
Table 3 Factors associated with postpartum depression from multiple logistic regression analysis (n 300)

ORadj: OR adjusted.
The findings of the MLgR reveal that there was a statistically significant association between PPD and mental health disorders in the last pregnancy (P = 0·001), as the mothers who suffered from major depression or eating disorders were three times more likely to be PPD (ORadj 3·56, (95 % CI 1·36, 9·34); P = 0·010) and (ORadj 3·33, (95 % CI 1·57, 7·06); P = 0·002), respectively, while mothers who faced anxiety, insomnia or panic disorder were two times more likely to develop PPD than the mothers who did not suffer from mental health disorders (ORadj 2·54, (95 % CI, 1·3, 4·99); P = 0·007). In investigating the association between PPD and body iron status, findings of this study imply that a significant association between PPD and body iron status existed (p = 0·015). The mothers who suffered from ID were three times more likely to have PPD (ORadj 3·25, (95 % CI 1·26, 8·40); P = 0·015).
Discussion
The recurrent wars on Gaza exacerbated the humanitarian crisis, unemployed in Palestine reached 31 %; of which 52 % in Gaza while the poverty percentage among Palestinian individuals according to consumption patterns was 29 %, of which 53 % of which was in Gaza, and almost three in four people are food-insecure(27,28) . As mentioned earlier, the degraded socio-economic situation and food-insecure and the political violence makes depression one of the top five causes of disability in the occupied Palestinian territory. The intended aims of the present study were to investigate the role of body iron status role in PPD, in addition to other factors.
The results of a prospective observational study by Chandrasekaran and his team showed that there was no difference in Hb or iron levels in women who had PPD compared to those without(Reference Chandrasekaran, De Souza and Urquia17). This contradicts the results of our study, since the results showed a highly significant association between PPD and Hb and haematocrit. In addition to a significant association with mean corpuscular volume (P = 0·013), which could be due to the small sample size in Chandrasekaran et al. study that included only 103 mothers. On the other hand, there were many studies that correspond to the results of our study regarding the significant association between PPD and Hb, as in the multivariate analysis of a study by Beard et al. which showed that the mother’s scores on the EPDS were significantly correlated with Hb and mean corpuscular volume(Reference Beard, Hendricks and Perez29). In addition, another study that involved 352 Saudi postpartum mothers depends only on Hb testing, as its low level detect of anaemia among the delivered mothers. The results of the study suggested that early postpartum anaemia evident by low Hb level is a significant risk factor for PPD(Reference Alharbi and Abdulghani30).
In the current study, the ferritin level in mothers with PPD was lower than its level in mothers without PPD. Also, the results of this study showed a highly significant association between PPD and serum ferritin level of the postpartum mothers. The same significant differences were detected between mothers with PPD and mothers without PPD regarding sTfR and body iron status (sTfR/log ferritin index). The results of the study did not detect any significant association between PPD and the hs-CRP level (P = 1·00). This significant relationship can be explained by the biological role of iron in maintaining the brain and cognition health since iron is critical for adequate myelination, neurotransmitter metabolism and function, and neuronal cellular and oxidative processes(Reference Chandrasekaran, De Souza and Urquia17). Thus low iron level will lead to a deterioration of these functions and develop the clinical depression in mothers. Likewise, there were studies in which results are consistent with the results of our study as the Albacara et al. study that supports the role of iron in the aetiology of PPD. The study observed a strong association between ferritin and PPD(Reference Albacar, Sans and Martín-Santos14), while there was no significant association between CRP and PPD (P = 0·51). The association between PPD and iron was also detected in a randomised double-blind placebo-controlled trial by Sheikh et al. which concluded that providing early iron supplementation to the delivered mothers, who suffer from PPD, can significantly improve the iron stores and lead to significant improvement in PPD with a 42·8 % improvement rate during 6 weeks and that the persistence of PPD may be relevant to the lower postnatal ferritin levels in untreated mothers(Reference Sheikh, Hantoushzadeh and Shariat15). In contrast, results of study by Armony-Sivan et al. who investigated this association among Chinese mothers demonstrated that even in this large sample, no correlation reached statistical significance, as the correlations between the mothers iron biochemical tests (Hb, mean corpuscular volume, ferritin, sTfR and sTfR Index) during mid- or late-pregnancy or 3 d postpartum. EPDS scores shortly after delivery or at 6 weeks were also low. Besides that, EPDS scores in anaemic and non-anaemic mothers did not differ from the results of Armony-Sivan et al. study, which showed similarity in iron status in both groups (women with or without PPD)(Reference Armony-Sivan, Shao and Li18). Thus, there was no relationship between the maternal iron status and PPD. Moreover, there was an exception for sTfR at mid- and late-pregnancy and EPDS at 6 weeks, as higher sTfR was related to lower depressive symptoms, which was opposite from what would be expected.
The current study found that the mothers who suffered from ID were three times more likely to have PPD. However, the discrepancy between our results and the results of the other studies regarding the biochemical part could be due to the differences in the methodologies of performing the test including the device and the kits that been used to assess the biochemical markers since some normal ranges could differ from kit to another. Furthermore, we could not find any study that depends on the sTfR/log ferritin index to assess body iron status and its association with PPD.
The study detected a highly significant association between PPD and having one of the mental health disorders in the last pregnancy including major depression, eating disorders, anxiety, insomnia or panic. Our results are consistent with previous studies, as in the retrospective controlled study by Morgan et al., which showed that, during pregnancy, active bulimia (one of the eating disorders) is associated with postnatal depression, miscarriage and pre-term delivery. In addition, bulimia may be a treatable cause of the adverse obstetric outcome(Reference Morgan, Lacey and Chung31). While in Stewart work that investigated the depression and anxiety showed that most PPD and anxiety were preceded by antenatal depression and anxiety. Furthermore, the antenatal anxiety also predicted PPD, even after controlling for antenatal depression(Reference Stewart32).
On the other hand, the results of our study showed a significant difference between PPD mothers and non-PPD mothers regarding the classification of the mother as high-risk pregnancy during the last pregnancy. This corresponds to the results of a study by Zadeh et al. which found that the prevalence of moderate-to-severe symptoms of depression and anxiety was higher among mothers who had a high-risk pregnancy(Reference Zadeh, Khajehei and Sharif33).
Overall, there was a discrepancy between the results of this study and the results of the numerous previous studies that investigated those factors. This could be due to many reasons as the variation in studies environment, habits, concepts and beliefs, in addition to the diversity regarding the methodology that has been used. Furthermore, the variation between the studies in selecting the cut-off point in the EPDS could be one of the most important reasons.
The results of the current study indicate that undesired feeling in the last pregnancy, mental health disorder and high-risk pregnancy are some of the PPD factors. These factors combined together can also worsen the condition of PPD in the affected mothers, as these factors are usually combined with affecting mother’s appetite for food. This will lead to a deficiency of some basic elements such as ID, which in turn, will aggravate the situation.
Strengths
Choosing the mothers after a month of postpartum helped prevent overlapping with other postpartum psychiatric disorders including the postpartum blues and psychosis. Furthermore, the translated copy of the EPDS questionnaire including the used cut-off point (scoring of 10 and higher) was valid and pre-tested, whereas the framing of the questionnaire questions and their answers in addition to the selection of the cut-off point are considered critical in detecting the presence of PPD among the mothers.
Regarding the detection of ID, the effect of inflammation (high CRP concentration) on the ferritin was avoided, by the use of sTfR that reflects the functional iron compartment, and is not acute phase reactant. Moreover, the equation that was used (sTfR/log ferritin index) in detecting the body iron stores has been shown to be valid and superior to routine tests.
Limitations
Despite all the precautions that were taken to make the participants feel safe and comfortable while answering the questions, it is believed that some of them were embarrassed to answer some questions correctly.
Using sTfR/log ferritin index as a proxy for iron status can be considered an addition point, but on the other hand, it can be considered a negative point, as it deprived the researchers of comparisons with other studies.
Case–control studies are an efficient method for the study of rare outcomes but suffer various limitations, including susceptibility to bias in recollection about exposure, and reverse causality
Due to the financial barriers, the biochemical tests were restricted to the selected tests. Other complementary tests could not be performed, as well as the anthropometric measurements could not be performed due to minimising interview times out of consideration for the circumstances of the participating mothers.
Conclusion
The results showed that mothers who have ID are at high risk of developing PPD, as the mothers who suffered from ID were three times more likely to have PPD.
Recommendations
The mothers and their families need to pay more attention to the mother’s food consumption before, during and after pregnancy. Primarily, ensure diversity in her consumed food and focus on foods rich in iron so she can meet her recommended daily intake. Furthermore, the mother must be aware of her feelings and psychological state and should not hesitate to attend the available psychological guidance services before, during or after the postpartum period. Routine physical and psychological check-ups during pregnancy period are highly recommended particularly for mental health disorders.
Acknowledgements
Acknowledgements: We thank the mothers who gave us their time, the Palestinian Ministry of Health for guaranteed access to Primary Health Care centres. Our gratitude is extended to Al-Azhar University-Gaza academic staff for their continued support and cooperation. Our sincere thanks to ECNAD team for their guidance assistance in accomplishing this work. This is to indicate that this study has not received any funds from any party. Also our thanks extended to Jennifer Kastner for or her effort in English language editing. Financial support: The authors have not received any financial support from anybody. Conflict of interest: There are no conflicts of interest. Authorship: S.A.H. designed and implemented the research, conducted data analysis and wrote the paper. I.A.N. and M.A.G were involved in designing and refining the study protocol and M.S.L. did the data analysis and reviewing the article. Ethics of human subject participation: The present study was approved by the Helsinki Committee (PHRC/HC/633/19), the Deanship of the Faculty of Pharmacy at Al-Azhar University-Gaza and the Palestinian ministry of Health. Written consent was obtained from the parents of children and they were informed about their rights to reject or to withdraw from the study at any time.









