Chapter 2 Conceptual premises in experimental design and their bearing on the use of analogy: A critical example from experiments on cut marks
Experimental archaeology and actualistic research are integral parts of middle-range theory and thus of modern scientific archaeology, which is based on the testing of alternative hypotheses (Binford, Reference Binford1981; Gifford, Reference Gifford and Schiffer1981). Hypotheses are framed within specific referential analogs created by careful observation. These referential frameworks are elaborated by controlled documentation of processes, in which behaviors of independent agents are understood within their specific contexts and the resulting actions of these agents are diagnosed (Binford, Reference Binford1981; Gifford, Reference Gifford and Schiffer1981; Gifford-Gonzalez, Reference Gifford-Gonzalez1991; Gould, Reference Gould1965, 1979; Wylie, Reference Wylie1982, Reference Wylie1988, Reference Wylie, Pinsky and Wylie1989).
If there is a hierarchy of principles that can be applied to the components of actualistic research, it can be argued that the most important one is the adequate use of premises (see Wylie, Reference Wylie1988) in the elaboration of referential frameworks. Researchers create these analogs primarily to understand behaviors represented in and responsible for the archaeological record. The significance of analogy as a nonobjective entity was initially stressed by Richter (Reference Richter1928). It entails a series of assumptions, some of them selected by the researcher, in a dialectic dynamic between the ideas that researchers try to test and the way the testing is eventually carried out.
A systemic evolutionary taphonomic approach (innovated by Fernández-López, Reference Fernández-López2006), considering taphonomic entities as endowed with properties subjected to change according to their structure, behavior, and environment, also shows that the selection of criteria to be replicated in experiments is ultimately dependent on what has been called taphonomic redundancy.1
A widely accepted articulation of theoretical principles guiding actualism was outlined by Gifford-Gonzalez (Reference Gifford-Gonzalez1991). She differentiated between “formal” analogy and “relational analogy.” The former is obtained through observation and the latter through inference. She conceived that there was a continuum from one form of analogy to the other within a hierarchical conception of taphonomic processes defined by six nested analytical categories (i.e., trace, causal agent, effector, actor, and behavioral and ecological context). Formal analogies can be used in the first four categories, because actors can be observed, provided equifinality can be overcome (Lyman, Reference Lyman2004). In contrast, the behaviors and the ecological factors that determine them can never be directly reconstructed from the analysis of bones and have to be indirectly inferred. In this case, relational analogy applies. Gifford-Gonzalez (Reference Gifford-Gonzalez1991) argued that the six analytical categories were interdependent. Starting from the broadest categories, Gifford-Gonzalez argued that every single taphonomic process is primarily understood in specific ecological contexts. If ecology conditions behavior, then actors should react in a predictable way according to those conditions; in turn, any such actions should be reflected in the traces imprinted on bones. Any experiment that obviates the relationship of these nested categories would be conceptually flawed.
Every analog is in essence incomplete, because it only reproduces a selected and limited set of variables and can only control for a determined number of these. Similar processes in the past in which other nonexperimentally considered variables might have intervened make the application of analogs systematically imperfect. Given that researchers must be aware of the imperfect nature of analogy, the relevance of the correct use of premises and assumptions in experiment design cannot be overemphasized.
Some analogies in taphonomy can be defined as substantive because they reproduce general processes that are not subjected to a significant degree of variability. For instance, the patterns of bone breakage (i.e., notches, planes) resulting from experimenting with physical processes such as dynamic (hammerstone) or static (carnivore dentition) loading are more generally applicable as analogs than other processes subjected to greater contextual variability. In many studies involving controlled experimentation of physical processes limited to the actor-trace sequence, analogies can be justifiably used within generalized referential frameworks. In contrast, and more specifically in archaeology, analogies depending on ecological-behavioral factors are subjected to a higher degree of variability and can be used confidently only as referential frameworks of determined taphonomic problems; they are case specific and could be labeled methodological analogies. In this type of analogy, given the large array of variables at play, researchers must be aware of the list of assumptions that they are making, on how these assumptions translate into hypothesis premises (Wylie, Reference Wylie1988), and eventually, how these premises and the hypotheses that contain them are subjected to testing. Failure to do so will produce false equifinality scenarios, ambiguity in interpretation, and eventually fuel postprocessual criticism of the subjective nature of the scientific method.
The present work uses experimental studies of cut marks as an example of the variability of criteria used by researchers when conducting experiments and designing referential frameworks. It is argued that this variability is not always scientifically acceptable, either because some approaches to experiment design are conceptually flawed (incoherent use of assumptions and premises of what is supposed to be replicated) or in other cases, because comparisons across experimental data sets cannot be sustained when the premises of the tested hypotheses by different researchers are not the same.
Analogy, uniformitarianism, and the concept of regularity
The only way to “reconstruct” the past is to assume that there are certain regularities in the way in which the world works that are not subjected to time and are therefore observable in the present. Thus, these regularities can also be inferred for the past. The assumptions of uniform rates and the implication of slow and gradual change in substantive uniformitarianism, using Gould's (1965) term, have proved incorrect in many cases. The modern conception of uniformitarianism does not assume the constant rate of change and acknowledges that the agents of change cannot be verified empirically. The laws that govern these agents remain permanent, however. This new uniformitarianism is methodological and vital to scientific procedure. Spatial and temporal invariability in the laws that control processes is absolutely critical if any general conclusion about the past is to be made from observations in the present (Gould, Reference Gould1965). Methodological uniformitarianism does not directly inform on nature but provides an approach with which to understand it (Shea, Reference Shea1982). This approach, in assuming that natural laws are invariant in time and space, does not invoke unknown hypothetical processes if the observed results can be explained through modern processes (Gould, Reference Gould1965).2
Simpson (Reference Simpson, Hecht and Steere1970) further elaborated on this concept by separating those aspects of the world that remain unmodified in time and space (immanent properties) and those that are contingent on particular interactions in each moment and place (configurational properties). Immanent properties allow historical processes (or parts of them) to be interpreted precisely because they are not subject to variation in space and time; that is, they are universal. Configurational properties must be approached in a different way. Unlike universal immanent properties, configurational properties are based on regularities in the variables that regulate them. There are two types of configurational processes, however: (1) those that are highly variable and therefore difficult to predict; and (2) those that are highly regular and therefore predictable. Obviously, only the latter can be reliably applied to past dynamics.
I agree with Gould (1980) in that only those processes that the properties and range of variation of which can be measured should be used in scientific archaeology. Some researchers think that only geological/physical processes can be understood from such an approach (e.g., Nairn, Reference Nairn1965). Simpson (Reference Simpson, Hecht and Steere1970) stresses that for any process to be understood it needs only to be uniform, however; that is, it must exhibit regular properties. This is possible whether reconstructing the mechanic aspects of the world or biotic behaviors. Much misunderstanding emanates from the misconception that only universal, or immanent, laws apply to the past. Because even universal laws are never absolute (Popper, Reference Popper1956, 1972), however, we are left with heuristic explanations (Lakatos, Reference Lakatos1978) that are grounded in the predictability of their regularities.
Regularity is derived from probability. Simpson (Reference Simpson, Hecht and Steere1970) notes two difficulties with inferring historical processes, however: (1) multiple processes can have similar results (i.e., equifinality); and (2) configuration makes processes unpredictable. Simpson (Reference Simpson, Hecht and Steere1970) himself suggests a solution for the latter. Scientific prediction depends on what is periodical and repetitive. Although historical events are unique and therefore unpredictable on at least some level, there are different degrees of distinctiveness, and historical events can be considered predictable to the extent to which we understand their causes and the regularity of their behavior. This is how Simpson (Reference Simpson, Hecht and Steere1970) defines historical configurations: based on probabilities and with similar heuristic power (sensu Lakatos, Reference Lakatos1978) as natural laws. An historical event is determined by the immanent characteristics of the universe that act on it, but in a configurational way.
This leads us to the use of analogy. To understand past configurational processes, the context and variables that generate modern processes must also be understood. This understanding differentiates descriptive from formal analogies and from relational (dialectical) analogies (Gifford-Gonzalez, Reference Gifford-Gonzalez1991). Analogies play an important role because they can discern and document variability in observed regularities. Most important, analogies can be observed and replicated. To use an analogy as a referential framework properly (sensu Binford, Reference Binford1981) for interpreting taphonomic processes in the past, researchers must clearly be able to (1) differentiate whether the analogy is case specific or general; (2) in the former case, produce a list of assumptions using collected data from the assemblage where hypothesis testing will take place; (3) justify that the experimental premises match the set of assumptions made for the formulation of a hypothesis. This can be better explained with an example.
A practical example documenting conceptual variability in hypothesis testing: Experimental replication and interpretation of cut marks
The use of replication in experimental archaeology during the 1980s enabled a certain optimism that cut marks could be scientifically used to infer human butchery behaviors (Binford, Reference Binford1978, Reference Binford1981; Bunn, Reference Bunn1981, Reference Bunn1982; Bunn and Kroll, Reference Bunn and Kroll1986; Gifford, Reference Gifford1977; Lyman, Reference Lyman1987; Gifford-Gonzalez, Reference Gifford-Gonzalez, Bonnichsen and Sorg1989). Nowhere has this been more illustrative than in the hunting-scavenging debate of Plio-Pleistocene sites in East Africa (see review of this debate in Domínguez-Rodrigo, Reference Domínguez-Rodrigo2002). The possibility that cut marks could be equally linked with hunting and scavenging behaviors prompted the development of new experimental protocols of opposite-hypothesis testing to distinguish both behaviors (Domínguez-Rodrigo, Reference Domínguez-Rodrigo1997a, Reference Domínguez-Rodrigo1997b). Nilssen (Reference Nilssen2000) also contributed with new experimental protocols to differentiate diverse butchery behaviors. In the past ten years, however, the diverse array of experimentation on carcass butchery has yielded a varied interpretive repertoire comprising the following claims (Table 2.1):
1. Cut marks are of limited value to interpret butchery behaviors and the order of access to carcasses by hominids because they are subjected to equifinality, given that they could be the result of the removal of the scraps of flesh surviving carnivore consumption of their prey (Capaldo, Reference Capaldo1995, Reference Capaldo1997, Reference Capaldo1998).
2. Cut marks, when applied to early Plio-Pleistocene sites, support the hypothesis that they were the result of hominids butchering carnivore kills (Selvaggio, Reference Selvaggio1994).
3. Cut-mark patterns found in Plio-Pleistocene sites, when compared to those documented in modern foragers (e.g., Hadza, in Tanzania), support a mixed strategy of early, intermediate, and late access to variously fleshed carcasses (Lupo and O’Connell, Reference Lupo and O’Connell2002).
4. Actualistic referential frameworks are useful to interpret cut marks as resulting from primary access to fleshed carcasses by humans versus defleshed carcasses abandoned by carnivores, and support the hypothesis of primary access to fleshed carcasses by hominids (Domínguez-Rodrigo, Reference Domínguez-Rodrigo1997a, Reference Domínguez-Rodrigo1997b, Reference Domínguez-Rodrigo2002; Domínguez-Rodrigo and Pickering, Reference Domínguez-Rodrigo and Pickering2003).
5. Comparisons of different experimental sets of cut marks, which test primary or secondary access to carcasses, show that they do not provide resolution, because they are statistically indistinguishable (Lupo and O’Connell, Reference Lupo and O’Connell2002; see critique in Domínguez-Rodrigo, Reference Domínguez-Rodrigo2003).
6. Recent experiments have widened the degree of variability of flesh abandoned in carnivore kills, which suggests that cut-mark patterns previously derived from experiments that recorded flesh distribution in a more restrictive sample of kills are no longer valid, providing more evidence of the behavioral ambiguity of cut marks (Pobiner, Reference Pobiner2007).
7. The tremendous range of variation in frequencies and anatomical distributions of cut marks across multiple assemblages prompts skepticism that the behavioral meaning of cut marks could be effectively inferred from prehistoric assemblages (Lyman, Reference Lyman2005).
The obvious message is this: the ambiguity of cut marks hampers their resolution to understand butchery behaviors and therefore the order of access by hominids to carcasses. Most of the experiments and interpretation of cut marks in the previous points (six of seven) have been carried out and applied to a restricted number of Plio-Pleistocene sites in East Africa to understand the butchery behavior that these sites have preserved for our understanding of a crucial stage of human evolution. More specifically, most of those have been applied to one site: FLK Zinj. This clearly shows that, in principle, the focus of these experiments was case specific. In blatant contradiction to this, however, most statements on the meaning of cut marks (this author's included) were thought to be of universal applicability. This is wrong: cut-mark frequency and anatomical distribution result from processes that belong to the “ecological” and “behavioral” spheres of Gifford-Gonzalez's (1991) nested set of inferences and are therefore subject to variability. This prevents any experiment carried out to test the meaning of cut marks in the kind of “inferred” ecological and behavioral contexts to be applied anywhere else where both variables might have been different. As Lyman (Reference Lyman2005, p. 1722) recently admitted: “well-founded interpretations of frequencies of cut-marked remains may require unique kinds of contextual data.” FLK Zinj was formed in an alluvial “near-lake” habitat within an ecosystem where felids and hyenas seem to have been fairly abundant (Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Egeland and Barba2007). Given that resource availability for scavenging hominids is ecologically dependent (Blumenschine, Reference Blumenschine1986; Tappen, Reference Tappen1992), when modeling opposing hypotheses of access to carcasses, researchers have to elaborate their experimental premises trying to ensure maximal consistency between experiments and the inferred context.
I will use the example of the behavioral meaning of cut marks at FLK Zinj to illustrate how a specific set of assumptions, premises, and hypotheses can be designed and successfully tested. I will also use a comparison with currently available experimentation to explain why some researchers might be closer than others to accurately testing the hypotheses of primary or secondary access to carcasses by hominids, and indirectly to providing high-resolution (rather than ambiguous) referential frameworks. This comparative exercise can be graphically followed in Figure 2.1. The null hypothesis is that cut marks lack resolution to infer primary or secondary access to carcasses. A subsequent null hypothesis is that hominids were scavengers (secondary access hypothesis). Proving that both versions of the null hypothesis are wrong would imply that early hominids had primary access to carcasses and that this can be inferred by specific placement and frequencies of cut marks.
Assumption 1. The essence of any experimental study is control. The only way to effectively link actor-effector-causal agent-trace is by having as much control as possible of the complete experimental/observational process. In the case of the hypotheses under testing, one factor in which control is key is resource availability from carnivore kills as potential scavengeable resources for hominids. This is especially relevant in the case of flesh scraps. The assumption is that no data derived from uncontrolled experiments should be heuristically used in this regard, because we could be inferring the wrong actor, producing an equivocal diagnosis. The resulting premise is that the experiment has to be carried out with as much control as possible or otherwise discarded.
For the secondary access hypothesis, the experiments that are inadequate according to this premise are those made by Pobiner (Reference Pobiner2007) in the wild, who never witnessed a complete process of carcass consumption in her lion sample, because she documented hunts in the late evening–early night and evaluated carcass modification and resource availability the next morning. Her study lacks control and is based on inferences that cannot be empirically supported. The reported tooth mark damage from the carcasses that she collected in the wild also could be the result of other carnivores having access to carcass remains during the night. This could explain why the only controlled sample that she collected in captivity shows a more intense consumption of flesh than that reported in her wild “lion” sample, and in apparent contradiction, almost one half the tooth mark frequency.
Pobiner (Reference Pobiner2008) does not agree with this evaluation of her work and justifies her study by saying that the control in her sample is enough to support her interpretations. She collected data from observations that lacked causal knowledge of actor-trace, however; that is, the whole process of hunting and carcass consumption by lions was not observed and completely documented. Control would have been the only guarantee that the data collected could be attributed exclusively to any specific agent. Evaluating carcasses “as soon as possible” after carcass abandonment by carnivores, “or to the earliest possible time the next day,” cannot be used as an epistemologically valid argument, because the interval between carcass abandonment and data collection could span several hours, and therefore the possibility of intrusion of other nonobserved (nondocumented) agents is fairly plausible. Any research program based on the belief that “actualistic researchers presumably aim to exert as much control over their sample as possible, but we are only as successful as circumstances allow” (Pobiner, Reference Pobiner2008: 469) should be interpreted with caution, because that limited control can also be taken as the boundary that separates scientific hypothesis testing from speculation (Barnes et al., Reference Barnes, Brunschwing, Burnyeat and Schofield2005; Niiniluoto, Reference Niiniluoto1987, Reference Niiniluoto2002; Bunge, Reference Bunge2006).
Pobiner (Reference Pobiner2008) fails to show convincingly that lions were the only carnivores intervening in the long nighttime hours that the carcasses were exposed. If that were the case, it would certainly show the highly disturbed ecological nature of the setting where she undertook her research, because absence of carnivores at kills during the night would be odd in any African park or reserve lacking human impact. None of the arguments that she uses to infer exclusive lion authorship in carcass modification can be sustained without some degree of faith, something that no scientific testable hypothesis can allow (Popper, Reference Popper1956, 1972; Lakatos, Reference Lakatos1978; Niiniluoto, Reference Niiniluoto1987, Reference Niiniluoto2002).
Pobiner admits that the bone damage that she obtained working with lions in captivity is different from that of wild lions and is probably due to boredom chewing. The question therefore is: where is the epistemological bridge that allows the use of such experiments as useful analogs to be applied to the past? It is widely known that boredom chewing by felids is documented only in captivity. Not even in their dens (e.g., leopard lairs) do felids show this behavior (Domínguez-Rodrigo and Pickering, Reference Domínguez-Rodrigo and Pickering2010).
Assumption 2. Following a basic Popperian principle, hypotheses can be tested only when confronted with their opposite. Our whole understanding of the use of statistics in science is based on this principle: the null hypothesis. Experiments used to test a hypothesis must be able to test the opposite and reject one of them. Inferences drawn from unilateral testing are not scientifically reliable. The assumption made is that an opposite-testing hypothesis is only well founded when the same set of assumptions, premises, and analytical variables have been used. This happens most frequently when it is the same researcher who carries out the testing of both hypotheses. The scientific premise is that only in equally comparable analytical sets can opposite hypotheses be tested and compared.
In the comparative set of experiments, most researchers have unilaterally tested a hypothesis, relying on the results obtained by a different researcher for the opposite hypothesis. Because the set of variables used by every researcher is unique (see description in Domínguez-Rodrigo, Reference Domínguez-Rodrigo2003), however, the comparisons are not necessarily valid.
Assumption 3. The constraints of elementary taphonomic alteration (as defined by Fernández-López, Reference Fernández-López2006) are primarily determined by the ecological context where it takes place. FLK Zinj was formed in a near-lacustrine habitat where a large array of carnivores was present. Actualistic studies have shown that the interplay between felids and hyenids is the most determinant to understand modern bone modification and deposition and resource availability for scavengers in modern African savannas (Blumenschine, Reference Blumenschine1986; Domínguez-Rodrigo, Reference Domínguez-Rodrigo1996; Tappen, Reference Tappen1992). Competition conditions the way that each carnivore consumes prey remains and also conditions the way that terrestrial felids feed. When under pressure from either hyenas or human, felids tend to consume their carcasses hastily, leaving more scraps of flesh (Domínguez-Rodrigo, Reference Domínguez-Rodrigo1999). At Olduvai, both during Bed I and Bed II times, hyenas seem to have been using the alluvial habitats with even higher frequency than they do today in similar modern settings (Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Egeland and Barba2007; Monahan, Reference Monahan1996). This can be inferred from the intensity of hyenid-modified assemblages in these settings with no modern equivalence. This has specific relevance regarding the amount of scavengeable resources by hominids. The assumption and subsequent premise made from this inference is that experimental replication of cut marks has to be carried out (especially those on carcasses obtained from carnivore kills) in similarly competitive settings to guarantee comparability.
When applied to the compared experimental set (Figure 1.1), all researchers but one comply with this premise. Capaldo (Reference Capaldo1995, Reference Capaldo1998) and Selvaggio (Reference Selvaggio1994) made their experiments in the Serengeti. Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a) carried out his experiments in Maasai Mara, Tsavo, Galana, and Kulalu. Lupo and O’Connell (Reference Lupo and O’Connell2002) made their observations in Eyasi – with a much lower presence of carnivores than the national parks where the previous authors carried out their research, but similarly diverse in carnivore taxa. In contrast, Pobiner (Reference Pobiner2007) conducted her research in a Kenyan private ranch, where some carnivores were systematically chased. Most of the hyenas were either poisoned or shot at, and given their abundance, lions were also shot sometimes, prompting them to be mostly nocturnal (L. Frank, personal communication, 2006). In this human-altered ecosystem, lion behavior was conditioned by two variables: marginal interspecific competition owing to the removal of hyenas and the human impact on the demographics of lions. As a result, the amount and anatomical distribution of flesh that Pobiner documented in carcasses abandoned by lions differ (in some cases drastically) from the more consistent descriptions reported by Selvaggio (Reference Selvaggio1994) and Blumenschine (Reference Blumenschine1986) for the Serengeti and Ngorongoro ecosystems and Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a, 1997b, 1997c, Reference Domínguez-Rodrigo1999) for the Maasai Mara, Tsavo, Galana, and Kulalu ecosystems, which were more similar when compared to each other. Thus, it can be seen that diverse environments in different ecosystems in national parks with minimal anthropogenic impact in trophic dynamics yield very different results from those documented in a highly altered environment, such as the one used by Pobiner (Reference Pobiner2007) or in specific situations of human–lion interactions as documented by Domínguez-Rodrigo et al. (Reference Domínguez-Rodrigo, Egeland and Barba2007). (See later discussion.) This supports the claim that only experiments conducted in environments unmodified by humans reliably document variability in carnivore behavior. Indeed, although Tsavo, Galana, and Kulalu offered different ecological conditions from Maasai Mara, Serengeti, and Ngorongoro, the documented manner of lion consumption of wild game was very similar in the resulting flesh availability. This is being currently supported by similar studies in progress in Tarangire National Park (Tanzania).
Pobiner's (2007) study obviates the ecological impact of the altered environment where she conducted her study, and she claims that the results obtained are heuristically useful for discriminating the real utility of cut marks inferred from the amount and variation in the anatomical distribution of flesh abandoned by felids. From the experimental frameworks currently available to understand flesh abandoned by felids in the Zinj environment, Pobiner's is the least appropriate, given the drastic ecological differences documented between both types of environments.3
Pobiner (Reference Pobiner2008) disagrees with this evaluation of the context where her research was carried out and tries to justify its suitability for actualistic research. The ranch where Pobiner conducted her study is surrounded by other ranches, however, and the hyenas had been systematically killed for years before her arrival at the site. The hyena population was (and still is) extremely low. This is reflected in the fact that if Pobiner were right about carcasses being exposed all night without other carnivore intervention after abandonment by lions, it would certainly suggest that hyenas were not a meaningful ecological factor shaping competition and therefore carnivore (lion) behavior. Such circumstances would also be incompatible with the statement that jackals are fairly common in the reserve. If so, why would they skip the chance of a generous meal at abandoned lion kills during the night? That is not what is documented in protected national parks. According to Pobiner, ranchers in Laikipia “enthusiastically tolerate a healthy population of large carnivores” other than hyenas, but they also sometimes do not tolerate lions. (See later discussion; emphasis added.) The ranch includes forty-three lions and two to five leopards; no cheetahs are reported. Lions are therefore virtually free from competition. Many zoos have a larger representation of large African carnivores than that. In contrast, national parks have much higher counts, expressed in thousands of individual animals: for example, the Serengeti carnivore census reports 2,800 lions, 9,000 spotted hyenas, 800 to 1,000 leopards, 6,300 jackals, 200 to 250 cheetahs, and 50 wild dogs (Caro and Durant, Reference Caro, Durant, Sinclair and Arcese1995). The differences in the ecology of these ecosystems are obvious.
Pobiner (Reference Pobiner2008) argues that no lion shooting ever took place, that lions were not mostly nocturnal, and that no systematic hunting on the area has been documented. Lawrence Frank, a highly respected authority on local carnivores, argues otherwise:
[This is] a ranch where they have always shot hyenas, and there are very few, if any. There are places in Laikipia where there are a reasonable number, but probably nowhere to compare to the Mara or parts of Serengeti. Further, this is bush country, so visibility is poor, plus the carnivores are wary of people – not good for observational studies of the kind you describe. Lions are shot regularly for eating cattle, so they are totally nocturnal, and not easy to find or watch. Hyenas are equally nocturnal. (Lawrence Frank, written communication, October 4, 2006)
Of note here is not only the systematic shooting of the hyena population but also that shooting of lions took place, which makes carnivores (including lions) wary of people. In the lion research carried out in Galana and Kulalu (described in Domínguez-Rodrigo et al. [Reference Domínguez-Rodrigo, Egeland and Barba2007]) Domínguez-Rodrigo witnessed two patterns of carcass consumption by lions: one on wild game (where humans, given their small number, left lions undisturbed), resulting in utterly defleshed carcasses; and one on cattle, where lions fed very fast during part of the night and subsequently fled the spot because of fear of humans, abandoning the carcass when it was still partially or even very fleshed. The fact that population density in the Laikipia area is relatively high and that as a result carnivores are wary of humans could cause lions to abandon carcasses earlier than they normally would, which has an impact of the availability of resources for other carnivores. The question again is this: how do we relate this analog, produced under specific circumstances that did not exist in Plio-Pleistocene savannas (caused by modern humans and their twenty-first-century technology), to the past to reconstruct prehistoric butchery?
Pobiner (Reference Pobiner2008) trivializes the importance of hyenas in the feeding behavior of lions. She says that she does not know of any references showing that such interaction is reflected in the amount of flesh available after abandonment of carcass by lions. Blumenschine (Reference Blumenschine1986) argued that he mostly focused his actualistic research in the Serengeti because the high lion–hyena competition in Ngorongoro Crater did not allow him to find enough carcasses prior to hyena consumption. Schaller (Reference Schaller1972) shows that prey availability conditions the way that lions process their carcasses. He documents how lions can give up carcass remains when hyenas are very bold. He also reports that lions frequently fail to keep their kills during the night. Because of hyenas, 17% of kills of a sample monitored for 23 nights were abandoned after lions had eaten a portion of the carcass, and 39% were eaten thoroughly by lions in the absence of hyenas. In these interactions, Schaller (Reference Schaller1972: 273) reports that in the presence of hyenas, lions “begin to feed rapidly as if anticipating the loss of their kill.” In the same ecosystem where Blumenschine reported more than 200 defleshed carcasses, Schaller (Reference Schaller1972) shows that during the concentration of the migratory wildebeest in the rainy season, lions may engage in “mass killing,” eating prey only partially (this constitutes 4% of their kills). Hyenas also engage in this type of “surplus killing” (Kruuk, Reference Kruuk1972; Wambuguh, Reference Wambuguh2007). Therefore, the availability of prey resources (in nonmigratory ecosystems determined by carnivore competition) conditions the amount of flesh available on abandonment of carcasses. This has been reported by Domínguez-Rodrigo (Reference Domínguez-Rodrigo1999), who also showed that the amount of flesh scraps found in lion kills varied according to habitat, because of carnivore interaction and different degrees of competition.
Pobiner (Reference Pobiner2008: 472) argues that “since the lions at SGR were not under pressure from hyenas or humans, they should presumably leave fewer flesh scraps.” If one is considering the analogs from undisturbed reserves (whose trophic dynamics are very different from privately owned reserves and ranches and should be compared at different levels), it can be seen that in low-competition settings like the reserve where she conducted her research, the amount of prey is well above the needs of the lion population, producing an effect that could be compared to the surplus-killing behavior exhibited by lions and hyenas in periods of prey abundance. This would be reflected in a less thorough consumption of carcasses, as is the case. For a closer example, several years ago I conducted some studies on lions that were kept in captivity and well fed. They barely consumed the flesh of complete carcasses. Working with felids outside their ecological context, where competition and resource availability shape their feeding habits, calls for caution when using such experiments as analogs.
Pobiner (Reference Pobiner2008:473) uses Blumenschine's data to claim that lions abandon more flesh in the Serengeti than reported by Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a,Reference Domínguez-Rodrigo1997b, 1997c) in Maasai Mara. She uses as support Blumenschine's (1986:86–89) data, which refer to the short periods during which prey abundance (coinciding with mass killings) is higher. These partially eaten kills make up only a small portion of the sample reported by Blumenschine. He immediately acknowledges that “the relatively large amounts of flesh abandoned during periods of prey abundance will quickly and thoroughly be scavenged by vultures…relatively large lion feeding group sizes result in the infrequent abandonment of any flesh. Unattended lion kills of medium-sized adults will therefore provide little to no flesh at all times of the year in the Serengeti, with further feeding opportunities being restricted to tissues within bones” (Blumenschine, Reference Blumenschine1986: 87; emphasis added). That is exactly what Blumenschine discovered: secondary access to lion kills in the Serengeti generally allows no access to flesh. Most carcasses are defleshed, just as in Domínguez-Rodrigo's (1997a, 1997b, 1997c) Maasai Mara sample. Ongoing research in Tarangire National Park in Tanzania is yielding exactly the same results. If we had to sum up the available results of flesh availability after lion feeding in national parks and reserves where these kind of studies have been made until present, we could not support Pobiner's interpretation of variability of flesh availability at kills reflecting the variability of ecological contexts. On the contrary, all of these studies show a similar amount of available flesh and the same anatomical distribution when the data have been collected in controlled samples. The data collected by Pobiner in the privately owned and anthropogenically modified reserve remain anomalous. Until proved otherwise from studies conducted in undisturbed ecosystems, defleshed small and medium-sized carcasses with few scraps available are therefore the most common feeding pattern exhibited by lions.
Assumption 4. Adequacy of the sample. Experimental samples should replicate (as much as possible) the archaeological samples in terms of the range of animal size and the range of body parts represented. This can be further defined by two independent analytical variables described by Domínguez-Rodrigo (Reference Domínguez-Rodrigo2003): animal size used in butchery experiments (small versus large) and experiment type (using complete carcasses, all limbs, or only a few limb bones). Carcasses accumulated at Zinj comprise a large number of individual animals, documented (despite the abundance of limb bones) by all skeletal elements from small and large animals. The assumption is that an experiment replicating complete carcass consumption of small and large individuals would more accurately reflect what happened at Zinj than experiments based on a few bones from a single carcass size and from a single individual. Domínguez-Rodrigo and Barba (Reference Domínguez-Rodrigo and Barba2005) and Pobiner and Braun (Reference Pobiner and Braun2005) showed that cut-mark patterns could be distinct in different carcass sizes. The premise is that to maintain the appropriateness of the comparability of experiments, cut-mark patterns obtained from specific carcass sizes should not be applied to interpret cut marks in different carcass sizes. In the Zinj case, the use of complete carcasses for experimentation might also be more adequate than partial carcasses. Only Capaldo (Reference Capaldo1995) uses this premise correctly. The other researchers either use one variable alone correctly, or both variables are inadequate. While Pobiner (Reference Pobiner2007) used complete carcasses, she never conducted a study of cut marks.
Assumption 5. Sample size and composition (see discussion in Domínguez-Rodrigo, Reference Domínguez-Rodrigo2003) are crucial for correct inference. Sample sizes in all the compared sets of experiments are highly variable, from large samples, like those obtained by Capaldo (Reference Capaldo1995, Reference Capaldo1998) or Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a, Reference Domínguez-Rodrigo1997b) for butchery of fleshed carcasses, to samples composed of multiple experiments of single elements or a pair of bones per carcass (Selvaggio, Reference Selvaggio1994), which do not reproduce the assumption that carcasses were accumulated at Zinj in a more complete state (whether hunted or scavenged). The interpretive model developed by Pobiner (Reference Pobiner2007) for flesh availability on large carcasses at lion kills is derived from a total of nine carcasses in contrast with Domínguez-Rodrigo's (Reference Domínguez-Rodrigo1997c, 1999) sample of twenty-nine individuals where flesh distribution was documented and almost twenty carcasses from lion kills where secondary access was experimentally modeled. If an arbitrary threshold of a minimum of ten carcasses4 per tested hypothesis (comprising at least complete limbs in each experiment) is used as a premise, some researchers’ samples would be left out (Figure 2.1).
Assumption 6. To interpret the validity of cut marks to infer different butchery behaviors and primary or secondary access to carcasses by hominids, the observation of the anatomical distribution of flesh (whether bulk or scraps) in carnivore kills is not enough; experimental butchery is also necessary. Within Gifford-Gonzalez's (1991) conceptual scheme of hierarchical order of inference, the documentation of flesh at carnivore kills would be situated in the ecological sphere. From there to the final obtainment of cut-mark patterns (traces), one should be able to document how actors, with the aid of effectors, produce specific traces. Pobiner (Reference Pobiner2007) did not conduct any of these experiments, and her interpretation of the use of cut marks rests on the assumption that one can skip the experimental process linking ecology and traces by indirect assumption. This is conceptually flawed.
Assumption 7. Butchery at Zinj was carried out with stone tools. The primary access hypothesis would assume that if hominids were hunters, they had regular access to carcasses and they might have been efficient and knowledgeable butchers. The alternative hypothesis, that hominids were scavengers that had only sporadic access to carcass remains, would imply that they might not have been expert butchers. The premise in the former hypothesis is that experimental butchery must be carried out by expert butchers, because butchery implies a learning process that is reflected in the decreasing number of cut marks imparted on bones according to experience; novice butchers leave more cut marks on bones than do expert butchers (Domínguez-Rodrigo, Reference Domínguez-Rodrigo1997c). The premise in the latter hypothesis that stone tools would be used to remove every single scrap of flesh and will not be focused on flesh bulk removal alone, which is most habitual in common butchery practices. For both hypotheses, a second premise is that the use of stone tools, preferably of the same raw material type as is archaeologically documented, is an experimental requirement.
Most researchers use these variables (tool type and butcher type) differently. Capaldo's and Lupo and O’Connell's butchery samples were made with metal knives, whereas Selvaggio's and Domínguez-Rodrigo's implied the use of stone tools of the same kind as found at Zinj. Selvaggio did not consider the experience of the butcher an important factor, however, and probably obtained higher frequencies (especially in certain bones) of cut marks than if an expert butcher model were used.
Assumption 8. The assumption of the type of carcass processing carried out at Zinj depends on the hypothesis to be tested. In the primary access hypothesis, processing assumes three butchery behaviors: skinning, disarticulation, and defleshing. From an optimal foraging point of view, the secondary access hypothesis assumes that the most efficient behavior is the removal of the flesh scraps at the kill. Disarticulation is the most time-costly butchering activity and also produces the highest degree of tool wear. Disarticulation of fairly defleshed carcasses, like those that one would obtain at carnivore kills, is unnecessary. In both cases, it is assumed that demarrowing followed. The premise is that in each of these hypotheses, no other type of processing activity should be experimentally reproduced.
Of the comparative sample of experiments, Domínguez-Rodrigo did not reproduce skinning and disarticulation. Capaldo's introduced an activity (periosteum removal) that is unnecessary for the butchery of most elements. This apparently irrelevant activity can actually produce a high frequency of tool marks on bones, biasing the assumed butchery behavior described above. Lupo and O’Connell's butchered carcass samples obtained from Hadza also include another processing activity not assumed for the Zinj hominids: grease extraction by bone boiling. Whereas this might not directly affect the resulting cut-mark frequencies, it indirectly affects them by limiting the type of bone fragmentation introduced by postravaging hyenas, thus, conditioning the resulting frequencies. Hyenas have been suggested to play a secondary role in bone modification and fragmentation at FLK Zinj (Bunn and Kroll, Reference Bunn and Kroll1986; Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Egeland and Barba2007). Bone fragmentation ultimately determines bone surface modification frequencies. The hyena was one of the agents, other than hominids, that probably played a role in bone breakage at Zinj. Experiments suggest that hyenas are interested in modifying bones in human-accumulated bone assemblages when grease is available and preferably while it is fresh (Marean et al., Reference Marean, Spencer, Blumenschine and Capaldo1992; Capaldo, Reference Capaldo1995; Pickering et al., Reference Pickering, Marean and Domínguez-Rodrigo2003; Marean et al., Reference Marean, Domínguez-Rodrigo and Pickering2004). By deterring hyenas from early access to bones or by removing grease from bones during boiling, hyena postravaging is modified and therefore the degree of bone fragmentation is also modified, affecting the resulting bone surface modification frequencies.
Assumption 9. The species of the animals used for butchery experiments also could determine both the amount of flesh available for secondary access and the resulting cut-mark pattern from their processing. For instance, equids have stronger muscular attachments to bones (as reflected in the stronger muscular/ligament insertions on certain bones (e.g., caudal tibia and femur) than bovids, and consumption of their bones by carnivores tends to leave more flesh on abandonment (personal observation5). Likewise, human bulk defleshing of equids also produces more scraps of flesh than in bovids. As a result, equids tend to appear more highly cut marked than bovid remains. An example is provided by Lupo and O’Connell (Reference Lupo and O’Connell2002) with various assemblages created by Hadza. In these bone sets, zebras tend to appear cut marked at rates more than one-third higher than bovids. Most of the processed animals at FLK Zinj were bovids. The assumption is therefore that experimental butchery should preferably be carried out on the same kind of carcasses for the sake of comparability. The premise is that experiments should be made by using bovids to test both hypotheses, and that experiments based on either butchery of equids or observation of flesh distribution in carnivore kills composed only of equids are not heuristically valid.
All but one of the experimental samples used for this comparative approach accepted the premise. Pobiner's (Reference Pobiner2007) sample of lion kills in the wild is primarily composed of zebras (eight zebras and one eland). The eland appears more defleshed than several zebras, and midshafts from upper limb bones appear virtually defleshed (see figure 2.10 in Pobiner Reference Pobiner2007: 56). The resulting pattern documented in the equid sample is not adequate to infer flesh availability in bovids scavenged from lion kills.
Pobiner (Reference Pobiner2008) is critical with this point and requested some citations showing that cut-mark frequencies are different in equids versus bovids. She dismisses Lupo and O’Connell's (2002) data because she argues it depends on the data set. Domínguez-Rodrigo (2008) used all the data from equids butchered by Hadza that Lupo and O’Connell report, whereas she selects only the base-camp subsample because it is convenient for her arguments, not because they support any justified selection criteria. By doing that she renders the analogical sample smaller (which already is fairly reduced) and does not justify why selecting a smaller subsample is better than using the complete sample. Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997c) showed that experimental zebra remains frequently were more highly cut marked than bovid remains. A much larger body of data on cut-marked bones from bovids and equids can be obtained from the archaeological record. In extensive fossil samples of cut-marked bones, it can be seen that in assemblages where equids and bovid remains occur together, equids are substantially more highly cut marked than bovids (Voormolen, Reference Voormolen2008; Yravedra, Reference Yravedra2005; see also Yravedra [Reference Yravedra2001] for a summary of data and references from the Iberian Upper Paleolithic showing the same trend).
Pobiner (Reference Pobiner2008: 471) argues that “that the difference between flesh abundance on these two carcasses is due not to species, but to the number of lions feeding on them.” To make such a statement, Pobiner (Reference Pobiner2008) should have contrasted both hypotheses, and whereas the number of lions feeding on a carcass obviously determined the amount of available flesh after consumption, the hypothesis that the prey taxa also determines the amount of available scraps remains untested by her study and cannot be rejected. Further experimental studies should be conducted by taking into account the taxonomic factor, but the reported differences suggest the influence of such variables as indicated earlier.
Assumption 10. This is one of the most important assumptions. Flesh is differentially distributed across the anatomy of an animal. The assumption is that a methodological approach that does not consider the type of element and the actual location of cut marks on these elements would not accurately reflect human butchery behaviors and the dynamics of these vis-à-vis the available scraps of flesh from carnivore kills, which are differentially distributed across the anatomy of carcasses. See an extensive methodological critique in Domínguez-Rodrigo (Reference Domínguez-Rodrigo2002). The premise is that to reflect butchery behaviors accurately, cut marks have to be tallied according to element and bone section, as described in Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a).
In the experimental comparative sample, Capaldo and Selvaggio are the only ones not to consider this assumption. They use a general method of tallying marks according to bone portion, irrespective of element type and the actual location of marks. They are also the only ones who have experimentally advocated equifinality in the use of cut marks as a result of their method, which lacked resolution in differentiating butchery behaviors.
Discussion
On the use of analogy
Some of the previous assumptions have failed to establish a link between their theoretical premises and the way in which they have been experimentally replicated (Figure 2.1). Their validity as analogies is therefore questionable. This, as published in Domínguez-Rodrigo (2008), has encountered some criticism (e.g., Pobiner, Reference Pobiner2008) that relates to the core of the present debate: how analogy is built and how interpretations are epistemologically justified.
Paleoanthropological thinking is necessarily analogical. Some (Aronson et al., Reference Aronson, Harre and Cornell Way1995; Bunge, Reference Bunge2006) would argue that all scientific reasoning is based on analogical modeling. Modern scientific analogical modeling differentiates between “descriptive models” and “explanatory models” in the relations of constituent models of hypotheses and theories (Aronson et al., Reference Aronson, Harre and Cornell Way1995).6 A school of thought in scientific realism argues that theories are structured around model systems (Giere, Reference Giere, Churchland and Hooker1985) articulated in the form of type-hierarchy frameworks (Aronson et al., Reference Aronson, Harre and Cornell Way1995). This school differentiates among positive, neutral, and negative analogies, although nonqualitatively.7 A correct use of analogical reasoning should combine these types. A mere formal analogy (e.g., female chimps use tools more frequently than male chimps do, and therefore early hominid females also preferentially used tools) uses the analog incompletely and incorrectly.8 How could we test that female Australopithecus would have the similar tendency as female chimpanzees? Furthermore, very frequently the use of formal analogies is made without knowing their contextual or universal character (e.g., are female chimpanzees more habitual tool users everywhere?).9 This dichotomy (formal and relational analogies) has been also referred to as the difference between “trivial” and “nontrivial” analogies (Harré, Reference Harré1986; Aronson et al., Reference Aronson, Harre and Cornell Way1995). Analogical reasoning in scientific interpretation is not based on the use of trivial formal analogies but on the elaboration of testable models. These are created through a dialectic use of groups of nontrivial analogies linked together.
Although archeological research has thrived under the use of analogical reasoning, little has been done conceptually to expand the use of analogy and to make it epistemologically supported. Analogy is at the core of several natural scientific disciplines. The field of theory of general systems has produced clearly defined concepts that scientific realists use to claim (1) that not all the analogies are equally valid, and (2) that to differentiate between valid and invalid analogies, an heuristic devise needs to be applied, which (3) can also be used to discriminate among the validity of scientific analogies that initially could be equally well structured and reliable.
One of the most widespread concept in the use of analogical reasoning for general and dynamic systems stems from Bunge's (1981) definition. Bunge criticized that most analogical reasoning was either undefined or too narrowly defined under isomorphic (and sometimes homomorphic) applications of the concept. He developed a qualitative concept of analogy embedded within the concept that most analogical reasoning in science occurs in dynamic systemic structures. These systems depend on the tight interaction of three components: composition, structure, and environment. Composition refers to the collection of components in any of two given systems. Structure refers to the relationship of those components within each system. Environment impacts the structure by determining how the system components interact. This third element is of utmost importance because it shows that when comparing two systems (as analogical reasoning does), even if both systems have similar composition, their structure could be different because of the environmental differences of each of them.
From this point of view, two systems are substantially analogous when they share the same components, structurally (or formally) analogous when they share similar structures, and environmentally analogous when their contexts are similar.
To emphasize that not all analogical reasoning is equally valid, Bunge (Reference Bunge1981) stressed that there were different (heuristic and epistemic) degrees of analogy. The degree of similarity between system A and system B could be proportional to their similarity in composition (degree of substantial analogy), structure (degree of structural analogy), and environment (degree of environmental analogy). The most important criterion in using degrees of analogy lies in the combination of the three types of intertwined parts of analogical reasoning, which is what Bunge (Reference Bunge1981) identified as the degree of total analogy defined as the average of the degree of substantial, structural, and environmental analogies shared between two systems. Bunge expressed this definition in the following formula:
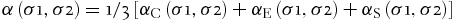
where α is the degree of analogy, σ is for each system, C is for substantial analogy, E is for environmental analogy, and S is for structural analogy.
Bunge (Reference Bunge1981) thus produced a final definition of analogy according to the result in the application of this logical formula in which he described two systems as analogous if their degree of total analogy was greater than 0, weakly analogous if their degree of total analogy was close to 0, and strongly analogous if their degree of total analogy was close to 1.
Bunge used this to show that not all analogies were equal in their heuristic power. Unless archaeologists (and especially taphonomists) assimilate this, they will continue to make epistemically blind interpretations produced as the result of matching prehistoric data with modern analogical frameworks, which could be conceptually inappropriate.
Pobiner's (Reference Pobiner2008) recent response to the critical description stated earlier on why several experimental programs fail to reproduce hominid butchery behavior ignores all these epistemological references (especially those that relate substantial and structural analogy to their environmental contexts) and raises several points that can be used to differentiate trivial from nontrivial referential analogues as we have seen above. She denies the relevance of the anthropogenic impact of the context where she conducted her research and justifies it by arguing that many of the settings where Domínguez-Rodrigo (1997a, Reference Domínguez-Rodrigo1997b, Reference Domínguez-Rodrigo1997c) conducted his experiments were also anthropogenically modified owing to the presence of pastoralists and the existence of poaching. This is inaccurate. The bulk of Domínguez-Rodrigo's research was conducted either in Maasai Mara or in the Olchorro le Musiara area of the reserve, where no pastoralists lived while he was conducting his research, nor was any poaching documented there for that period. Poaching and problems with humans became an issue only in the past decade and only in peripheral areas outside the region where Domínguez-Rodrigo conducted his studies, owing to the increase of the population surrounding the reserve (Norton-Griffiths, Reference Norton-Griffiths, Sinclair and Arcese1995; Norton-Griffiths et al., Reference Norton-Griffiths, Said, Serneels, Kaelo, Coughenour, Lamprey, Thompson, Reid, Sinclair, Packer, Mduma and Fryxell2008). Despite this, the figures of game currently poached there are far below those reported for Serengeti (Campbell and Hoffer, Reference Campbell, Hoffer, Sinclair and Arcese1995). Furthermore, poaching targets specific herbivore taxa, not carnivores, as is the case in the ranch where Pobiner conducted her research (Campbell and Hoffer, Reference Campbell, Hoffer, Sinclair and Arcese1995). Therefore, the carnivore trophic dynamics of Maasai Mara and Olchorro le Musiara regions when Domínguez-Rodrigo was conducting his study remained similar to those of the Serengeti, thus explaining the similar results obtained in flesh availability at lion kills by independent studies (Blumenschine, Reference Blumenschine1986; Domínguez-Rodrigo, Reference Domínguez-Rodrigo1997a, 1997b, Reference Domínguez-Rodrigo1997c). The only place where Domínguez-Rodrigo (Reference Domínguez-Rodrigo1996) conducted some independent research in a human-impacted environment was in Galana and Kulalu, near Tsavo, and lions abandoned their cattle prey in state similar to that described by Pobiner, because humans chased them. This is an informative coincidence. It was never argued that Serengeti and Maasai Mara were pristine ecosystems but that they are the closest we have (with other protected reserves and national parks) to natural trophic dynamics in savannas prior to the arrival of food producers. These ecosystems are fairly different in terms of mammalian trophic dynamics from private properties used by humans as hunting grounds, where certain carnivore taxa are reduced to the limit of survival. The former remain the closest proxy for Plio-Pleistocene savannas. The latter are something different, and their application to the past remains epistemologically unjustified.
Pobiner questions the assertion that felids and hyenids were abundant in Olduvai Bed I times, which is crucial to select the adequate modern proxy for interpreting trophic dynamics in the Olduvai paleolandscape and modeling the resulting availability of scavengeable resources. Obviously neither predator populations nor herbivore biomass can be determined for the past; however, Domínguez-Rodrigo justified it because their remains are relatively abundant for this period compared with other Plio-Pleistocene sites and because in all the sites that have been taphonomically analyzed, their intervention in the formation of faunal assemblages has been documented (Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Egeland and Barba2007). As a matter of fact they have been argued to be responsible for all but two of the Olduvai Bed I and Bed II sites (Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Egeland and Barba2007). This interplay of felids and hyenids is documented in several modern national parks and reserves but lacking in the reserve where Pobiner conducted her studies. It is therefore logical to claim that the former can be potential proxies and the latter ought to be excluded.
Pobiner argues that three ecological circumstances could produce similar meat surpluses to those documented in her research: droughts, mass drownings, and scavenging from saber-toothed felids. There is a problem with this: mass drownings are a very marginal occurrence in modern savannas and affect only specific herbivore taxa. They do not occur in reduncini and antilopini, nor in alcelaphini adapted to edaphic grasslands (e.g., topi in modern savannas or its extinct counterpart Parmularius), which form the bulk of the FLK Zinj herbivores. None of the bulk of the taxa exploited at FLK Zinj have been documented in mass drownings. Ongoing work at Olduvai Bed I has uncovered various sources of water during the formation of the Bed I sites (Domínguez-Rodrigo et al., Reference Domínguez-Rodrigo, Bunn, Mabulla, Ashley, Díez-Martín, Barboni, Prendergast, Yravedra, Barba, Sanchez, Baquedano and Pickering2010). This presents an excellent testing case for the drought and mass drowning hypotheses. Both processes produce concentrations of carcasses near the remaining water sources (mass drowning in lake environments [Capaldo and Peters, Reference Capaldo and Peters1995] and carcasses accumulating during droughts in river beds and ponds [Haynes, Reference Haynes1991]). The number of carcasses that accumulated near these water sources during the formation of FLK Zinj is extremely small (much smaller than at water sources in modern savannas in the absence of extreme climatic conditions), showing that neither phenomenon is the source of the carcasses butchered by hominids during FLK Zinj times.
Regarding the other possibility of scavenging large amounts of flesh from saber-toothed felids, it should be stressed that Pobiner references Marean (Reference Marean1989) as support, selectively ignoring the later work by Marean and Ehrhardt (Reference Marean and Ehrhardt1995) on a Homotherium den, which showed that saber-toothed felids defleshed carcasses more thoroughly than previously thought based on tooth morphology alone. Furthermore, saber-toothed felids were also subjected to the competition created by thousands of other carnivores in Plio-Pleistocene savannas. This brings into question: (1) whether flesh availability as documented by Pobiner in an almost competition-free environment could be applied to these felids, and (2) whether these felids could have afforded to be such inefficient flesh eaters given the competitive environment to which they were adapted. What Pobiner has epistemologically modeled is not resource availability at sabertooth kills, but strictly resource availability at lion kills in a savanna context lacking competition and with altered carcass consumption habits by lions because of human presence and humans chasing them. The epistemological bridge between this analogue and its application to the Plio-Pleistocene past is missing. Despite this, Pobiner's (2008) claim that saber-toothed felid kills could be a good source of scavengeable meat remains untested and with decreased heuristics after Marean and Ehrhardt's (1995) work. Recently, the hypothesis of a scavenging niche made viable by sabertooths because they may have lacked the morphology necessary to use all parts of carcasses fully, leaving an open niche in the form of high-quality scavengable remains available for hominins, has received a further blow. Quantifications of occlusal radii-of-curvature (ROC) of carnivore premolars and the study of the correlation of this morphology with carcass-processing behavior
do not support the hypothesis that sabertooth felids were more hypercarnivorous than modern felids (but the opposite). Thus, this study shows no evidence that members of the paleo-carnivore guild were capable of producing higher quality scavengable carcasses than are modern carnivorans, and based on these analyses of fossil carnivorans, it does not appear that high-quality scavengable remains were more available in the Plio-Pleistocene than there are today” (emphasis added). (Hartstone-Rose and Wahl, Reference Hartstone-Rose and Wahl2008: 630)
Pobiner's belief in the “scavenging from sabertooth kill” hypothesis, in absence of empirical support and contradicted by currently available evidence, requires another leap of faith.
Pobiner argues that “the amount of flesh abandoned on lion kills is highly variable, e.g. bulk or flesh scraps on 18 per cent of lion-eaten larger prey carcasses (Domínguez-Rodrigo Reference Domínguez-Rodrigo1997a, Reference Domínguez-Rodrigo1999) vs. 56 per cent (Tappen Reference Tappen, Stanford and Bunn2001 – unknown predators on larger adult ungulates) vs. 70 per cent (Blumenschine Reference Blumenschine1986) vs. 95 per cent (Pobiner, Reference Pobiner2007), and dependent on a series of ecological variables.” I argue that this is incorrect. Blumenschine (Reference Blumenschine1986) does not report 70% of bulk flesh surviving lion consumption of carcasses (otherwise he could not claim that flesh and viscerae are not available for a secondary scavenger). Pobiner is using data from the short periods of prey abundance, which do not reflect what happens in the Serengeti the rest of the year (see also Schaller [Reference Schaller1972]). Blumenschine's (Reference Blumenschine1986: table 4.8) data for bulk flesh from medium-sized adult carcasses after abandonment by lions is less than twelve, more similar to what Domínguez-Rodrigo (Reference Domínguez-Rodrigo1999) reports in Maasai Mara. Likewise, Tappen's (Reference Tappen, Stanford and Bunn2001) data should be taken with caution. She documents a list of carcasses found in virtually two states: complete or defleshed. The description of the defleshed carcasses is similar to those reported by Blumenschine and Domínguez-Rodrigo. There is no variation in lion feeding behavior if lions were responsible for consuming them. The complete carcasses present an important problem: they cannot be shown to be the result of lion kills. Most of them were found without any definitive indicators that lions had hunted them. They could easily be natural deaths. Once again there is a sample derived through an uncontrolled procedure. If lions have not been witnessed to kill the carcasses, the interpretation that lions abandoned fully fleshed carcasses cannot be fully supported. By lumping the complete carcasses with the defleshed carnivore-eaten carcasses, Pobiner is artificially creating a bulk estimate that is epistemologically unsupported. If we consider the carcasses that carnivores (probably lions) ate, their description does not support the 56% bulk flesh survival inferred by Pobiner and is very similar to what has been reported for Serengeti and Maasai Mara. Furthermore, Tappen never published any quantification of resource availability that would have allowed any quantifiable estimates of surviving bulk flesh. Pobiner's (Reference Pobiner2008) method of deriving it from mere general descriptions is thus flawed.
In sum, no heavily fleshed carcass survives lion consumption in the Serengeti or Maasai Mara on a regular basis but instead on exceptional occasions. Simpson (Reference Simpson, Hecht and Steere1970) argued that our analogs should be constructed based on regularities and not exceptionalities. From what is the most commonly documented pattern, therefore, secondary access to flesh in small and medium-sized carcasses abandoned by lions in modern savannas (not modified by humans) remains a highly marginal scenario. This is ultimately reflected on the frequencies and anatomical location of cut marks when these carcasses are butchered with stone tools.
Conclusion
I have argued here that a systemic evolutionary taphonomic approach (as outlined by Fernández-López, Reference Fernández-López2006), considering taphonomic entities as endowed with properties subjected to change according to their structure, behavior, and environment, also shows that the selection of criteria to be replicated in experiments ultimately depends on what has been called taphonomic redundancy. Taphonomic redundancy, a crucial element in the way that we construct analogies, is the capacity of taphonomic elements to repeat the same message. Taphonomic redundancy, as well as replication, allows the estimation of the “repeatedness” of taphonomic groups under particular environmental conditions, on the basis of their actual properties (Fernández-López, Reference Fernández-López2006). This was also argued as essential by Simpson (Reference Simpson, Hecht and Steere1970) in his concept of configuration and regularity. Unlike universal immanent properties, configurational properties are based on regularities in the variables that structure them. There are two types of configurational processes: (1) those that are highly variable and therefore difficult to predict; and (2) those that are highly regular and therefore predictable. Obviously, only the latter can be reliably applied to past dynamics.
The way we construct analogies determines to which extent their configurational properties respond to highly variable or highly regular properties. Pobiner's reliance on exceptional occurrences (e.g., “as lions sometimes abandon kills of larger animals with large amounts of flesh, hominins scavenging from social felid kills could have access to well-fleshed carcasses” [Pobiner Reference Pobiner2008: 476]) produces analogies that cannot be applied to the past: they lack taphonomic redundancy, and therefore they produce ambiguity. They are not highly regular and thus are unpredictable and by extension nonapplicable, because they are not solidly tied to environmental conditions observable in the absence of human impact on carnivore trophic/competition dynamics. Furthermore, their application to interpreting the past is linked to inferential scenarios based on untestable hypotheses (e.g., scavenging large amounts of flesh from saber-toothed felids was feasible). There is an important missing link between past and present, which is the correspondence between the premises used for testing hypotheses and the way in which testing is implemented.
The range of interpretations about the use of cut marks to infer human butchery behaviors, derived from the experimental sets compared in the present work, is not a reflection of the variability of these behaviors and their ecology but is a methodological artifact of the diversity of assumptions made in experimental design and their corresponding experimental premises. Researchers have reacted differently to what is supposed to be tested and the way in which testing was conducted. Some researchers claim ambiguity in the use of cut marks not because they can prove it, but because of their methods of documenting cut marks (Assumption 10), or because they disregarded the determinant interrelated inferential categories of ecology behavior and skipped the hierarchy of inferential categories. Others failed to document the utility of cut marks to reconstruct butchering behaviors because their selection of premises to articulate their hypotheses and the corresponding variables used during experimentation were different from those that should have been inferred and used to interpret the targeted fossil assemblage. Figure 2.1 shows how far each researcher is from the experimental matrix created by the articulation of assumptions and their corresponding premises. The more distant the experimental sets are from the matrix, the less heuristically appropriate they are to interpret cut marks from the fossil assemblage. Some of the studies casting doubt on the utility of cut marks (e.g., Pobiner, Reference Pobiner2007) mistake the degree of comparability of the data sets used, disregarding ecology, behavioral variability, and confiding in untested assumptions to the point of not even replicating butchery when testing the secondary access hypothesis.10
Only one out of the researchers whose work has been compared claims that cut marks can be used successfully to differentiate between primary access to fleshed carcasses or secondary access (Domínguez-Rodrigo, Reference Domínguez-Rodrigo1997a). Challengers to this claim could support their position either by proving that the set of assumption-premises used by that researcher is equivocal or by using the same experimental premises to document a greater variety of results than that reported in the referential framework provided by his or her research. Instead of that, by selecting a different experimental path, they set themselves up to test-prove something similar but essentially different.
This brings us to reconsider the use of analogy and the importance of combining its substantial, structural, and contextual-environmental properties (Bunge, Reference Bunge1981). Given the variability of criteria when one is designing experiments and using analogies, an outline containing the set of inferences and premises guiding hypothesis modeling seems necessary. This will help researchers understand when new results from experiments provide new compelling evidence to challenge established ideas, or when they simply represent the testing of a completely different set of premises and assumptions, even if the hypotheses appear to be the same.
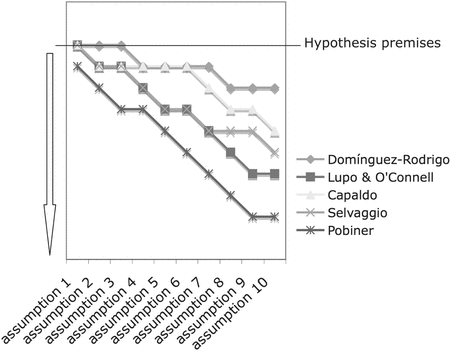
Figure 2.1. Experimental matrix showing the conceptual assumptions and premises of hypothesis testing as described in the text (top horizontal line) and the deviations from these by various researchers. Each experimental premise for each researcher that does not take any given outlined assumption into consideration is reflected in a step down from the horizontal line of the matrix. The lower the experimental research appears (direction of arrow) compared with the top horizontal line, the more conceptually distant the experiment is from the hypothesis premises, and the more inappropriate it is for comparison with the specific case of the behavioral meaning of cut marks at FLK Zinj.
References
1 Taphonomic redundancy has been defined as the capacity of taphonomic elements to repeat the same message. Taphonomic redundancy, as well as replication, allows the estimation of the “repeatedness” of taphonomic groups under particular environmental conditions, on the basis of their actual properties (Fernández-López, Reference Fernández-López2006).
2 Gould was rewriting Occam's Razor: One should not increase, beyond what is necessary, the number of entities required to explain something. Even fourteenth-century scholars can remind experimental archaeologists of the need to keep it simple.
3 This refers to human-modified ecosystems (like that used by Pobiner for her experiments) and savannas not impacted by humans in their trophic dynamics (like those used by the other researchers referenced in the previous paragraph).
4 Experiments with a smaller number of carcasses usually yield large variation ranges, which make hypothesis testing more difficult.
5 Several zebras were used by Domínguez-Rodrigo (Reference Domínguez-Rodrigo1997a, Reference Domínguez-Rodrigo1997b, Reference Domínguez-Rodrigo1997c, Reference Domínguez-Rodrigo1999) in his observation of availability of flesh in lion kills and in his experimental replication of the scavenging hypothesis.
6 Similarly, in theoretical archaeology, and applied to a smaller inferential scale, one has differentiated between “formal” analogies and “relational” analogies, the former being a mere transcription of an observed analog to the past, the latter being a constructed inference built on analogical reasoning (Gifford, Reference Gifford and Schiffer1981; Gifford-Gonzalez, Reference Gifford-Gonzalez1991).
7 These are defined as follows: “If A is a theoretical model for some real system B, then the positive analogy is those properties or respects in which A and B are similar. The negative analogy consists of those respects in which A and B are different, and the neutral analogy consists of those properties or respects which either have no corresponding map to the other or which have not yet been explored” (Aronson et al., Reference Aronson, Harre and Cornell Way1995, p. 91).
8 Incompletely because it assumes that there is a perfect match between the analog (only positive analogy) and the model that needs to be elaborated to explain the reality of a past behavior. Formal analogies are also frequently used incorrectly because it is assumed that analogy and model are the same concept, whereas the latter is frequently composed of sets of analogies with attached testable hypotheses to overcome two facts: single observed analogies cannot represent the totality of a past behavior, and the analogy per se does not provide any bridging apparatus to test the adequacy of its application to the past.
9 For a nonsupporting view see Carvalho et al. (Reference Carvalho, Cunha, Sousa and Matsuzawa2008).
10 Pobiner (Reference Pobiner2007) assumes that the distribution of flesh in lion kills that she observed is enough to discredit the utility of cut marks when applied to fossil assemblages, without proceeding to remove flesh through butchery and compare it to a null-hypothesis experimental scenario.






