INTRODUCTION
Rabies is a fatal disease of antiquity with the highest case-fatality rate of all infectious diseases, accounting for over 55 000 deaths annually [Reference Hemachudha, Laothamatas and Rupprecht1]. One person dies from rabies every 10–15 min and around 300 are exposed [Reference Rupprecht, Hanlon and Hemachudha2, 3]. According to the global burden of disease report revised in 2013, rabies accounted for the loss of 1462 disability-adjusted life years in 2010 compared to 3234 in 1990 [Reference Murray4]. Even though this may suggest a decrease in the rabies burden worldwide, rabies continues to be one of the most important viral zoonoses given its widespread distribution, public health concerns, veterinary implications, and economic consequences [Reference Rupprecht, Hanlon and Hemachudha2]. Each year, more than 10 million people, many of whom are unvaccinated, endure protracted anxiety after exposure to animals with suspected rabies [Reference Meslin and Stohr5]. Despite this anxiety, rabies ignorance is a major public health problem that requires educating both the public and health professionals [Reference Hemachudha, Laothamatas and Rupprecht1, Reference Rupprecht, Hanlon and Hemachudha2, Reference Dodet6].
Rabies is an acute, progressive, viral encephalitis with poorly understood pathogenesis and a predictable fatal outcome [Reference Jackson7]. Rabies infection is eminently preventable despite the absence of any treatment for the disease. Controlling the infection in dogs is the best measure of prevention, yet this is still not achieved in many developing countries, contributing to the higher incidence of rabies [Reference Knobel8, Reference Wilde9]. A major contributor to the absence of canine rabies control in these countries is the lack of competent measures taken by relevant authorities. Other measures for rabies prevention include rabies post-exposure prophylaxis (PEP) with rabies vaccine and immunoglobulin. Most humans die of rabies because of inappropriate, delayed or lack of PEP administration [Reference Senior10]. According to a World Health Organization (WHO) report, post-exposure rabies prophylaxis prevents about 272 000 deaths each year in Asia and Africa [11].
Worldwide, rabies is spread by various warm-blooded mammalian species, such as red foxes in North America, Europe and Eurasia, skunks in North America, raccoons in USA, mongooses in the Caribbean Islands and South Africa, jackals in Africa, and vampire bats in Southern and Central America [Reference Chomel12]. Dogs are the main source of human infection in the Middle East, while cats constitute the second most important source. Wild animals, the most important vector in the developed world, are less important in Middle Eastern countries. Infection of cattle, sheep, goats, camels, and donkeys, although rare, is also potentially possible. Around 300 human cases of rabies are reported annually in the Middle East region [Reference Aylan13, Reference Seimenis14]. Certain countries of the region are facing increasing problems due to wildlife rabies, such as Saudi Arabia, Oman, Yemen, Israel, Iran, and Turkey [Reference Seimenis14].
Animal rabies is known to be endemic in countries bordering Lebanon, mainly Syria and Israel [15, 16]. Between 2004 and 2007, the biological and molecular characterization of the rabies isolates by David et al. showed stray dogs to be the main animal reservoir in Northern Israel [Reference David17]. Fifty-seven percent of confirmed rabies isolates between 2001 and 2007 were in dogs compared to 43% confirmed isolates in other wild and domestic animals [Reference David17].
Human rabies is a reportable disease in Lebanon. Few reports were published about the burden of rabies in the country and the public attitude towards it [Reference Akkoca and AA18–Reference Saad and Ghosn20]. The last major report about the burden of rabies in Lebanon was published in 2000. A total of eight cases of human rabies were reported to the Lebanese Ministry of Public Health (LMOPH) between 1991 and 1999 together with an annual average of 184 animal bites to humans between 1991 and 1996. Dogs were the only vector of human disease in that report and the most commonly reported cause of animal bites to humans [Reference Bizri19].
In this study, we aim to revisit the status of rabies infection and exposure in Lebanon as reported to health authorities and compare it to studies from other countries. We also evaluate available rabies surveillance, control and preventative measures.
METHODS
Rabies record collection
Records from the American University of Beirut Medical Center (AUBMC), the major medical center in Lebanon, together with records from the LMOPH were reviewed between 2001 and 2012. Records before 2001 were excluded as they were reviewed by our team in a previous study [Reference Bizri19].
Documented cases of rabies were reviewed for their setting of occurrence, demographics, source of infection, and mode of management.
Collection of animal bite data
Data about animal bites to humans occurring between 2001 and 2012 along with data on PEP and vaccine administration to individuals exposed to bites were also reviewed from the records of the LMOPH Epidemiological Surveillance Unit in terms of age, location and offending animal. The number of vaccines per animal bite was calculated as an approximate measure of rabies PEP adequacy as the majority of animals reported during that period were not followed, necessitating four doses of vaccine per bite. Data on the number of incomplete PEP is not available.
Statistical analysis
To assess the change in reported rabies exposure, collected data for the period 2001–2012 was compared to that reported between 1991 and 1999. The means of annual animal bites to humans reported in each period was compared using unpaired t tests.
RESULTS
In total, eight cases of human rabies were reported to LMOPH (Table 1), of which one case only was admitted to AUBMC. Out of the eight cases, seven occurred in the North and Bekaa provinces that are predominantly farming areas close to the Syrian border. Only one case was reported from Beirut, the capital of Lebanon. The vector of infection was invariably a dog bite. The eight cases were distributed in different age groups including children, young adults and the elderly. All the reported cases had an unfortunate fatal outcome. The outcome was assessed based on the clinical picture without pathological confirmation. Presentations were classical including agitation, aggression, drooling, excessive sweating, fever and hallucinations.
Table 1. Reported human rabies cases in Lebanon between 2001 and 2012
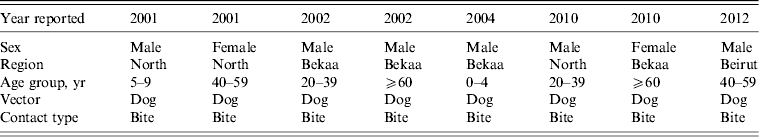
Modified from the Lebanese Ministry of Public Health website (www.moph.gov.lb).
A total of 5280 animal bites to humans were reported to LMOPH between 2001 and 2012 with an annual average of 431 bites per year. Table 2 shows the number of people exposed to animal bites and the total number of vaccine doses provided by LMOPH. On average, 3·15 vaccine doses were delivered per animal bite during the study period. The offending animals included domestic and stray dogs as well as cats, rats, monkeys, donkeys, and foxes. Dogs, however, were by far the major offending animal responsible for around 91% of all bites with stray dogs constituting 55% of all bites. The highest incidence of animal bites was in older children and adults. Yet, the incidence in those aged <15 years was marked, with an average of 134 bites per year. With respect to region, the highest incidence of exposure to bites was in the North and Mount Lebanon despite animals being unprovoked (Fig. 1). Table 3 illustrates the distribution of individuals exposed to bites by age and region.

Fig. 1. (colour online) Epidemiological map showing the distribution of rabies exposure and cases across the different provinces of Lebanon.
Table 2. Total number of animal bites to humans and number of exposed people
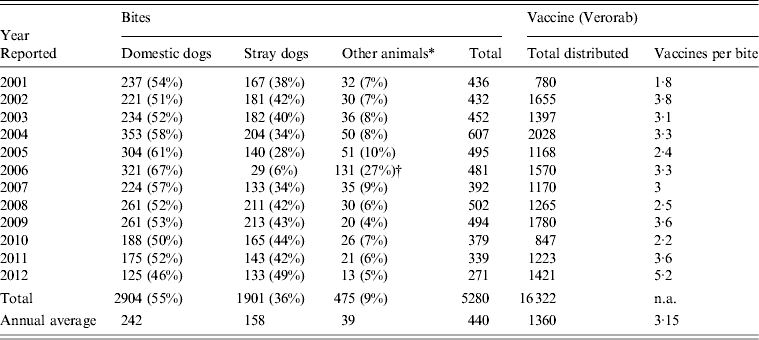
Modified from the Lebanese Ministry of Public Health website (www.moph.gov.lb).
n.a., Not applicable.
* Cats, wild animals, bats, rodents, and others.
† Peaked levels of other animals in 2006 relative to previous years mainly included wild animals. One possible explanation is the war state in Lebanon where families were homeless or living in camps in rural areas and more exposed to unusual bites.
Table 3. Distribution of animal bites to humans by location and age
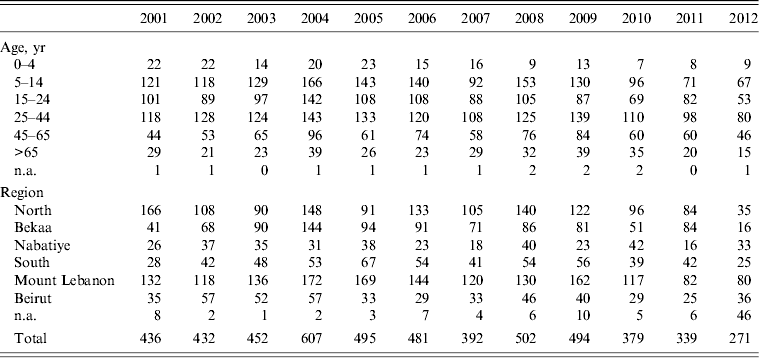
Modified from the Lebanese Ministry of Public Health website (www.moph.gov.lb).
n.a., Not assigned.
The comparison of annual animal bites in Lebanon during 2001–2012 to that during the 1990s showed a significantly higher number of reported animal bites in the 2001–2012 period (431 bites per year vs. 184, P < 0·0001).
DISCUSSION
The annual average of animal bites to humans reported to LMOPH between 2001 and 2012 was 440 bites per year, ranging between 271 and 607 bites. This may not reflect the actual number since animal bites are mainly reported when the victim consults a physician who, in turn, suspects a possible rabies risk. The surveillance system of LMOPH is a passive system where physicians in different districts of Lebanon are required to report rabies cases to district authorities who in turn are responsible for reporting to LMOPH as well as the follow-up of suspected animals if possible. Hospitals are also required to report rabies cases to LMOPH directly. However, some cases may go unnoticed by LMOPH if families did not consult a physician or the physician failed to report the exposure, which is quite possible in rural areas where the disease is more prevalent. Active educational campaigns are the best way to enhance the power of surveillance and allow a better evaluation of the rabies burden.
The annual average of vaccine doses distributed during this period was 1360 doses per year with a ratio of 3·15 doses per animal bite. This ratio still lags behind the Centers for Disease Control and Prevention (CDC) recommendation of four doses per bite [21]. The reason for this could be either that there was an incomplete follow-up of victims or that the animal was identified as non-rabid and no further measures were needed. Rabies vaccine is delivered free of charge by LMOPH healthcare centres present in different provinces of Lebanon. In total, eight cases of rabies were reported to LMOPH and were diagnosed on a clinical basis without pathological identification. Vectors in all cases were dogs indicating that human rabies in Lebanon is still secondary to canine rabies as it was in the previous decade [Reference Bizri19].
Annually, an average of 0·6 cases of rabies is reported to LMOPH. As with many reportable diseases in Lebanon and worldwide, reported numbers may be an underestimation especially in rural areas with poor awareness and limited access to medical care [Reference Saad and Ghosn20]. The observed annual occurrence in this study is very close to that reported in a previous study by our group between 1990 and 1999 [Reference Bizri19] (0·8 cases per year). This may not necessarily reflect stability in disease burden in the country, promoting concerns that this state of balance between near elimination and sporadic emergence of cases may lead to second reservoir species such as foxes or other animals as was the case in Turkey [Reference Johnson22]. Such worries seem possible given the presence of similar reservoirs in foxes as reported in Israel, an adjacent country to Lebanon [Reference David17]. This is often referred to as rabies ‘host switching’ and was found to be responsible for the appearance of the virus variants in several wildlife animals such as foxes and skunks originating from dogs and bats in the USA [Reference Velasco-Villa23, Reference Leslie24]. This emphasizes the need to monitor the circulation of rabies virus variants in different wild animals for the initiation of adequate preventative measures.
Despite this, Lebanon is considered a country with low incidence of rabies compared to other developing countries, especially in Africa and South East Asia [Reference Horton25–Reference Munang'andu27]. One explanation is the limited exposure to rabies reservoirs in Lebanon compared to those countries. Even though the global burden of rabies is on the decrease [Reference Murray4], the disease continues to be reported in several regions in Europe and the USA [Reference Johnson28, Reference Vercauteren29]. However, many of the reported cases of rabid animals in developed countries, e.g. France, Germany, Italy, Canada and the USA, are believed to be secondary to imported rabid animals [Reference Johnson28, Reference Blanton30]. In addition, imported human rabies immigrants and travellers are another concern in those developed countries as reported recently by Carrara et al. [Reference Carrara31].
In our report, the majority of cases were in adults (six cases) while two cases occurred in children aged <15 years of age (Table 1). A possible explanation may be the fact that children are less likely to go outside and be exposed to animals than adults [Reference Senauer32]. This can be inferred from the distribution of animal bites across age groups where the highest numbers occurred in the adult group (Table 3). Although, the occurrence of two cases of this fatal and low-prevalence condition is still alarming. A similar picture can be deduced from our previous report published in Lebanon [Reference Bizri19]. On the contrary, studies in high-prevalence areas, especially Africa, indicate that the majority of rabies cases were in children. More than half of the deaths from rabies in Africa involve children raising serious concerns about childhood rabies prevention and control in these areas [Reference Liu and Ertl33].
With regard to geographical distribution, all but one of the reported cases was from the North and Bekaa provinces (Fig. 1, Table 1). The remaining case was from Beirut. The outcome of all the reported cases was fatal, secondary to the lack of available treatment for this disease, emphasizing the crucial role of rabies prevention.
The socioeconomic conditions in a country and the residential area (rural vs. urban) besides age and occupation are influential factors on the epidemiological features of animal bites [Reference Eslamifar34]. In the present study, animal bites were most frequently reported in the 20–60 years age group. Similar to our results, the study of Gautret et al. on the epidemiology of dog-related injuries in Marseille, France revealed a mean age of 32 years for affected people [Reference Gautret35]. This was not, however, the case in Kerman Province, Iran, where animal bites were more common in younger age groups (10–19 years) [Reference Eslamifar34]. Although less than adults, the incidence of animal bites in children is uncomfortably high with an average of 134 bites per year (31% of average annual bites). It was suggested that children may trigger an attack by a dog through provocative playful actions. Even behaviours not regarded as provocative may be interpreted by a dog as an invasion of territory and may incite an attack. This is important in view of the way children behave around dogs especially if dogs live in the same household or community [36]. Children who have been bitten or scratched by suspect rabid dogs may not tell their parents or guardians, especially if they have been instructed not to approach animals that are not their own pets [Reference Bhanganada37, Reference Cleaveland38]. Dzikwi et al. studied the effect of educating Nigerian children about rabies on rabies exposure. The study revealed that properly educating children about rabies can result in these children avoiding dogs [Reference Dzikwi, Ibrahim and Umoh39].
The annual animal bite counts in Lebanon reported in this study was much higher than that reported in the previous decade (431 vs. 184 bites per year; P = 0·0001) [Reference Bizri19]. This may seem to reflect a deterioration of animal control strategies in the country resulting in an increased exposure to rabies through animal bites. However, it can also imply advancement in the reporting and surveillance system at LMOPH.
Dog bites constitute 91% of all bites to humans in Lebanon; therefore, dog control, especially stray dogs, is a major avenue for prevention. Stray dogs are those that are not under the direct control of an owner either because they have no owner or because the owner has not restricted them from roaming freely. Domestic dogs are those that are under direct control and restraint by their owners. Other animals, including wild animals, account for 8% of all bites. This continuous exposure necessitates stronger measures for the control canine rabies in Lebanon as a means of controlling the disease. Control of canine rabies starts with action by the veterinary authorities, i.e. taking responsibility for monitoring and limiting the dog population. Possible measures to be taken include registering domestic dogs and educating owners, capturing stray dogs, controlling dog reproduction, vaccination of non-rabid dogs, humane measures of rabid dog euthanasia, and observation of suspected animals for neurological symptoms or laboratory diagnosis. Several massive canine rabies vaccination programmes were successful in establishing canine rabies immunity in many countries [Reference Franka40]. In the systemic review of Davlin & Vonville of the canine rabies vaccination programmes in the developing world, the majority of the campaigns were capable of accomplishing the WHO-recommended vaccination coverage of ⩾70% [Reference Davlin and Vonville41]. Despite that, countries like China, India and Pakistan where the highest burden of rabies exists did not have any studies reporting such campaigns. Control of canine reproduction is also of importance given the fact that the majority of stray dogs in the developing world are young and short-lived, posing a major limitation to efficiency of dog vaccination campaigns [Reference Davlin and Vonville41].
In Lebanon, attempts to control canine rabies are still inadequate. Canine vaccination is limited to pet dogs in the absence of any governmental vaccination campaigns. In addition, stray dogs to which highest exposure is recorded are not captured, neither are suspected animals followed up after biting humans. Studies pertaining to rabies cases in animals are lacking, and there are no studies that have reported confirmed cases of animal rabies in Lebanon during the study period. However, despite the ability of the Lebanese surveillance system to record cases of human rabies and animal exposure, interventional measures for long-term rabies control are still lagging. Such measures require a competent authority as well as the help of non-governmental organizations to have a good surveillance and interventional system that can control the rabid canine population and its possible wildlife interactions. Such measures should be applied at a national level in order to ensure long-term reduction of rabies reservoirs by limiting rabies infection in dogs and its transmission to humans as well as other animals. Consequences of the lack of interventional measures as well as the absence of follow-up of offending animals also include the inappropriate administration of PEP and rabies immunoglobulin resulting in shortage of supplies at different locations. This problem is shared in many countries of the developing world where canine rabies is the major contributor to human rabies cases [Reference Franka40].
The low level of reported bites in animals other than dogs may be due to poor awareness of the potential risk of rabies transmission through these animals. Despite the fact that dogs are known as the most common source of rabies transmission to humans throughout the world, especially in Asia, Latin America, and Africa [Reference David17], bites by other animals should also be addressed in control programmes and awareness campaigns.
A closer look at the distribution of animal bites revealed the highest cluster was in Mount Lebanon where no cases of rabies were reported. However, seven out of eight cases were from remote rural areas in the country. These findings cannot be attributed to greater numbers of wildlife in that area as the distribution of wild vs. domestic bites was comparable in both areas (data not shown).
One possibility is the movement of infected stray dogs into Northern Lebanon across the borders resulting in a spillover, especially from Syria which has a higher incidence than Lebanon with 24 reported fatal cases between 1997 and 2002 [Reference Seimenis, Morelli and Mantovani42]. Spillover from neighbouring countries like Syria, Lebanon and Turkey was reported to contribute to an outbreak of rabies in Northern Israel in 2009. This was based on biological and molecular characterization of the rabies strains isolated that revealed the stray dogs as the main animal reservoir [Reference David17]. This highlights the need for regional cooperation to ensure the best rabies control measures in neighbouring countries. However, this can be complicated in regions of political and economic instability with shifting governmental priorities [Reference Rupprecht43]. For instance, Hatch et al. reported increased rabies and a need for massive rabies vaccination during the civil war in Sierra Leone [Reference Hatch, Sneddon and Jalloh44]. The unstable political situation in the region, mainly in countries bordering Lebanon, adds further challenges to efforts aiming at controlling wildlife and urban rabies infection.
Another reason for increased incidence of rabies in the North and Bekaa provinces is the lower adherence to PEP by affected patients in rural areas compared to Mount Lebanon. This may be due to the lack of awareness about the disease and the need for PEP, as well as difficulty in accessing centres where PEP is provided.
Death from human rabies results from the lack of awareness and the untimely administration of PEP along with the failure to follow WHO recommendations on wound washing and vaccination [Reference Hemachudha45, Reference Wilde46]. Wasay et al. [Reference Wasay47] evaluated the public knowledge regarding predisposing factors, fatality and prevention of tetanus and rabies and the attitudes towards vaccination and PEP in Pakistan. Most of the participants were not aware of the fatality of these diseases and the importance and affordability of vaccination in case of dog bites and minor trauma. In that study, only 11% of 1201 persons bitten by dogs received some kind of vaccine or PEP. In Lebanon, a study was conducted by Saad & Ghosn [Reference Saad and Ghosn20] to assess knowledge, attitudes and practices towards rabies in two major towns in the North and Bekaa provinces (Zagharta and Baalback); 80% of 196 participants reported having no or little information about PEP, despite the fact that the majority of respondents had heard about rabies. This lack of awareness might explain the cluster of rabies cases in these areas.
Despite the low level of awareness towards rabies PEP in rural areas of Lebanon, there is an improvement in the delivery of vaccines after bite exposure at the national level. This is illustrated by an increase in the vaccine/bite ratio from 2·25 doses per bite between 1990 and 1999 to 3·15 doses per bite between 2001 and 2012. This recent ratio is very close to the newest CDC recommendation of delivering four doses/bite [21]. In contrast to Lebanon, where the majority of human victims of suspected animal bites receive the full PEP course, many developing countries still lag behind. Almost 10% of human rabies cases in India received incomplete PEP vaccination [Reference Sudarshan48], and 47% of patients started on PEP in the Ivory Coast failed to complete the course of injections [Reference Tiembre49]. Interestingly, many of the developed countries are facing the problem of PEP overuse and lack of monitoring of PEP administration as reported in the USA by Christian et al. [Reference Christian50].
CONCLUSION
Rabies control remains elusive in both the developing and developed world. The variability in rabies epidemiology in different regions adds to the challenges that face efforts to prevent and eradicate the disease.
The stable incidence of the disease in a country should not be reassuring due to the host-switching ability of the virus and its circulation in different species of wild animals. Dogs remain the most important vector in the developing world compared to wild animals in the developed world. Children are a major high-risk group for acquiring the disease mainly in developing counties. The high incidence of dog bites in the young age group (up to age 15 years) is a cause for concern worldwide. Public awareness is of tremendous importance and disseminating information about all relevant aspects of the disease is essential. Such information should also be included in school curricula and school awareness programmes targeting pediatric age groups.
The ability of animals to cross borders, the reports of imported cases from many countries, and the higher incidence of rabies in border areas such as in Lebanon emphasize the importance of regional cooperation. Cooperative efforts can be impeded by political instability experienced in many regions including the Middle East.
The capacity of national surveillance systems to evolve and cope with the increased risks of exposure is of enormous importance given the future challenges of rabies control. PEP including timely and adequate vaccination and administration of rabies immunoglobulin remains the cornerstone for prevention of the infection and its fatal outcome in the exposed.
Monitoring viral circulation in the various hosts, control of canine rabies in the developing world, regional cooperation, raising public awareness mainly in children, and timely administration of PEP are essential for decreasing the rabies burden worldwide.
DECLARATION OF INTEREST
None.






