Numerous studies have described the importance of nutrition and physical activity in the maintenance of cognitive function( Reference Smith and Blumenthal 1 , Reference Berchicci, Lucci and Di Russo 2 ). Folate and other B vitamins, especially vitamin B12, play important roles in the modulation of gene expression and the synthesis of DNA, as determinants of homocysteine detoxification and neurotransmitter synthesis( Reference Morris 3 ). Deficiencies in folate and vitamin B12 reduce S-adenosylmethionine and increase plasma homocysteine; these effects contribute to cognitive decline through the oxidation of the functional and structural proteins of neurons and the endothelium( Reference Quadri, Fragiacomo and Pezzati 4 ) and the inhibition of methylation-dependent reactions, including neurotransmitter synthesis( Reference Mischoulon and Raab 5 ).
Folic acid supplementation has a positive effect on the cognitive function of older adults who have deficient serum folate (SF) levels( Reference Morris, Evans and Bienias 6 , Reference Durga, van Boxtel and Schouten 7 ). However, studies on seniors in America, where folic acid fortification of foods is mandatory, show that high SF levels combined with deficient vitamin B12 levels might be associated with impaired cognitive function. This association may be a result of the limit that low vitamin B12 levels impose on the rate of methionine synthase activity under conditions of folic acid fortification( Reference Rosenberg and Selhub 8 – Reference Morris, Selhub and Jacques 11 ).
Chile has implemented mandatory folic acid (2·2 mg/kg) fortification of wheat flour since 2000. Studies conducted during the era of food fortification in Chile have demonstrated increased SF levels in the elderly and have revealed that about approximately 50 % of individuals present with supraphysiological levels (>20 μg/l)( Reference Sánchez, Albala and Hertrampf 12 , Reference Castillo-Lancellotti, Margozzini and Valdivia 13 ). Given this background, the goal of the present study was to analyse and compare the relationship between SF, vitamin B12 and cognitive function in older Chilean adults using data from the 2009–2010 National Health Survey (NHS 2009–10)( 14 ).
Materials and methods
Participants
The present study evaluated 1051 older adults (>65 years of age) who participated in the NHS 2009–10( 14 ), which used a national, probabilistic, stratified, multistage sample of 5412 people older than 15 years of age. The sample was representative at the national, urban, rural and regional levels. Each adult was randomly selected from within a household utilising the Kish method. For each individual, an expansion factor was considered which corresponded to the inverse of the probability of selection of that individual and allowed the results of the sample selection to incorporate the unequal selection probability for each respondent given the sample design and demographic post-stratification according to January 2010 census projections. Older adults (>65 years) were particularly over-represented in the sample to obtain statistically significant results that were comparable to the other age groups. Of these respondents, 851 had recorded values for SF and 824 had recorded values for vitamin B12. Outliers, corresponding to values exceeding three times the interquartile range from the median (>62·5 mg/l for SF and >1022 pmol/ml for vitamin B12), were excluded from the mean calculations (twenty-two cases for vitamin B12 and four for SF).
The Research Ethics Committee of the School of Medicine (Pontifical Catholic University of Chile) approved the NHS 2009–10 study protocol. All participants provided signed informed consent.
Biochemical measurements
The samples for measuring SF and vitamin B12 were obtained only from fasting individuals by a trained registered nurse. The collection was standardised according to the NHS 2009–10 manual( 14 ). The country’s laboratories centrifuged and aliquoted the biological samples received according to the protocol and stored them frozen or chilled according to the NHS standards. All samples were transported at −4°C to regional hospitals. The serum samples were frozen at −20°C and sent to the Central Laboratory of the Pontifical Catholic University of Chile for centralised analysis, complying with internal quality controls, within a maximum period of 6 weeks( 15 ). Of all the samples, 91 % were processed within 4 h of venepuncture. SF (µg/l) and vitamin B12 (pg/ml) levels were determined by competitive immunoassay using direct chemiluminescence (Siemens ADVIA Centaur®, Atlanta, GA, USA).
Assessment of cognitive function
A trained nurse assessed cognitive function in the older adults’ homes. First, the Modified Mini Mental State Examination (MMMSE) instrument, which assesses orientation, attention, recent memory and language, was administered to the seniors enrolled in the study. The MMMSE comprises six questions with a maximum total score of 19 points( Reference Folstein, Folstein and McHugh 16 , Reference Chatfield, Matthews and Brayne 17 ) and a cut-off of ≤13 points to identify cognitive impairment( Reference Quiroga, Albala and Klaasen 18 , Reference Icaza and Albala 19 ). Validation studies in Chile have shown that applying the Pfeffer Functional Activities Questionnaire (PFAQ) after assessment with the MMMSE improves the sensitivity and specificity in the identification of impaired cognitive function; therefore, this test was applied when an individual had an MMMSE score ≤13 points( Reference Quiroga, Albala and Klaasen 18 ). The PFAQ includes eleven questions answered by a family member or caregiver to further assess the functional status and degree of daily dependence of the older adult. A score ≥6 points indicates impairment in the performance of daily activities( Reference Pfeffer, Kurosaki and Chance 20 ).
Control variables
The following control variables were considered: age; gender; educational level (EDUL; where basic represents ≤8 years of schooling, medium represents between 8 and 12 years, and higher represents ≥12 years of schooling); area of residence (urban or rural) as defined by the Chilean National Institute of Statistics( 21 ); and the presence of diabetes mellitus (DM) and hypertension.
For SF and vitamin B12 analyses, only participants for whom blood samples were taken after at least 8 h of fasting were included in the DM investigation. Blood glucose was analysed as a continuous variable and extreme values (>300 mg/dl) were excluded. Participants with fasting blood glucose level ≥126 mg/dl or a reported physician diagnosis of DM were considered diabetic( 14 , 22 ). Three blood pressure measurements were performed during a single morning visit while fasting and after resting for 5 min, at 2 min intervals. Each measurement was timed and conducted by a validated automated device (Omron HEM 742®, Matsusaka, Japan)( Reference Coleman, Freeman and Steel 23 ). A high blood pressure cut-off of ≥140/90 mmHg, consistent with the Joint National Committee( Reference Chobanian, Bakris and Black 24 ) guidelines, was utilised. Normotensive individuals who reported drug treatment in the questionnaire were also considered.
Statistical analysis
Student’s t test was used to compare older adults with and without impaired cognitive function employing the MMMSE and the MMMSE plus PFAQ for the following continuous variables: age and SF and vitamin B12 levels. A χ 2 test examined the association of cognitive impairment with gender, EDUL, area of residency, DM and hypertension.
Detailed analysis of the association between the risk of impaired cognitive function in older adults and SF level, vitamin B12 level and the control variables was performed using multiple logistic regressions. Squared SF level (SF2) was also included because both very low and very high levels might be risk factors( Reference Morris, Jacques and Rosenberg 10 ). Interactions between SF and vitamin B12 levels and the control variables (age, sex, area of residence, EDUL, hypertension and DM), as well as the interaction between SF level and vitamin B12 level, were also examined.
OR were calculated for each 5-year increment in age, every 1 µg/l unit change in SF and every 10 pg/ml change in vitamin B12. The following are the reference categories for the categorical variables: for gender, women; for EDUL, higher (≥12 years of studies); for area of residency, the urban region; and for DM and hypertension, the absence of these diseases.
However, quadratic variables and interactions in logistic regression models are not interpretable as OR; therefore, these were omitted because changes in the risk of cognitive impairment occurred as the levels of these variables changed. Notably, changes in the levels of these variables also affect the quadratic and interaction terms. The risk calculations for these variables incorporate the estimates of these variables and their quadratic and/or interaction terms, but these do not correspond to a single value because they change for each value of that variable. As it is not possible to summarise the OR of these variables in a single value, graphs have been drawn describing the OR changes in terms of these variables.
The varying probabilities of presenting with cognitive impairment according to the screening tests (MMMSE and MMMSE plus PFAQ) are presented as a function of folate levels for participants with different characteristics. Two figures present the change in the risk of cognitive impairment by SF level (because SF and SF2 are not independent variables). The probability of cognitive impairment was calculated from the logistic regression coefficients.
Finally, a two-dimensional figure with shaded areas representing different OR presents the impairment of cognitive function according to the MMSE plus PFAQ. This figure identifies the SF and vitamin B12 levels for which a one-unit change in SF (1 µg/l) produces a significant change in the risk of impaired cognitive function, as well as the values for which the risk is not significant. To establish statistical significance for each combination of SF and vitamin B12, the OR was calculated with a 95 % CI, estimates of the variance and covariance of the coefficients. This analysis was performed utilising the R software environment for Windows, version 3·0·1. All other analyses were conducted utilising the complex samples module of SPSS Statistics for Windows, version 17·0. The level of significance was defined as P<0·05.
Results
The characteristics of individuals with normal and impaired cognitive function according to the MMMSE score are displayed in Table 1. The participants with cognitive impairment (n 180) were significantly older and had higher vitamin B12 levels compared with normal participants (n 862). Participants with basic EDUL exhibited a greater prevalence of cognitive impairment (25·9 %) than those with medium or higher EDUL (7·5 and 3·3 %, respectively). Those living in rural areas displayed a higher prevalence of cognitive impairment compared with those living in urban areas (32·7 % v. 14·5 %). Moreover, the prevalence of cognitive impairment was higher among hypertensive individuals (19·5 %; Table 1).
Table 1 General characteristics considering impaired cognitive function according to the Modified Mini Mental Status Examination (MMMSE) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey

SF, serum folate; EDUL, educational level.
Table 2 shows the characteristics of older adults with normal and impaired cognitive function evaluated using the MMMSE plus PFAQ. Using the MMMSE (score ≤13 points) plus PFAQ (score ≥6 points), 906 individuals presented with normal cognitive function and fifty-seven showed impaired cognitive function. When the results using both cognitive tests were compared with those in Table 1 (only MMMSE), differences by age and serum vitamin B12 level were not statistically significant because the estimates for participants with cognitive deficits produced much larger standard errors. Those with impaired cognitive function evaluated by the MMMSE plus PFAQ had SF levels below those found in individuals with impaired cognitive function evaluated with the MMMSE only. A significant difference in the mean levels of SF of normal individuals and those with impaired cognitive function when both tests are applied was observed. As observed with the MMMSE, when both evaluation criteria were utilised, a higher prevalence of cognitive impairment was observed in participants with basic EDUL (10·2 % v. 2·3 % at the medium level and 0·8 % at the higher level). Table 2 demonstrates that cognitive impairment was higher among individuals in rural areas than in urban areas; however, the difference was not statistically significant. The difference between genders was more pronounced when evaluated using both tests than with the MMMSE alone; however, these results were not statistically significant. A similar pattern was observed with hypertension.
Table 2 General characteristics considering impaired cognitive function according to the Modified Mini Mental Status Examination (MMMSE) plus Pfeffer Functional Activity Questionnaire (PFAQ) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey
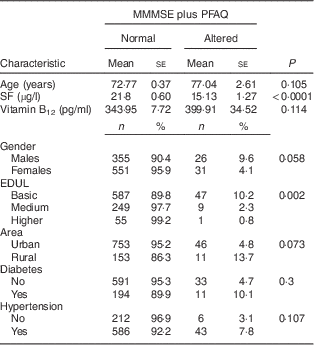
SF, serum folate; EDUL, educational level.
Table 3 presents multivariate logistic models of cognitive impairment assessed with the MMMSE and the MMMSE plus PFAQ. In the first model (MMMSE), a significant increase in the risk of impaired cognitive function was identified in individuals with basic EDUL (OR=5·00; 95 % CI 1·17, 21·35), as well as in those with hypertension (OR=2·96; 95 % CI 1·29, 6·79), DM (OR=2·49; 95 % CI 1·09, 5·71), older age (OR=1·07; 95 % CI 1·01, 1·12) and higher levels of vitamin B12 (OR=1·002; 95 % CI 1·000, 1·003). Both SF and SF2 were significant, but their OR were omitted, as justified above, because the square of SF in the logistic regression changed the risk expressed by the OR at different folate levels of each unit change in SF (1 µg/l). Increases in SF levels below 19·02 µg/l produced significant decreases in the risk of impaired cognitive function (confidence intervals were entirely below 1·00). However, for values above this threshold, changes in SF levels did not significantly affect the risk of cognitive impairment in terms of MMMSE. For example, a change in the SF level of one unit, from 4 to 5 µg/l, decreased the risk of impaired cognitive function by approximately 12 %; an increase in SF from 10 to 11 µg/l decreased the risk by approximately 9 %. Conversely, an increase in SF from 40 to 41 µg/l increased the risk by 7 %.
Table 3 Multivariate analysis using logistic regression for impaired cognitive function according to the Modified Mini Mental Status Examination (MMMSE) and Modified Mini Mental Examination plus Pfeffer Functional Activity Questionnaire (PFAQ) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey
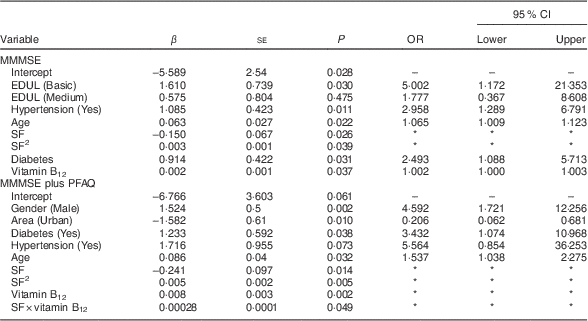
EDUL, educational level; SF, serum folate.
*OR have been omitted since they depend on the values of at least one of the identified variables.
Figure 1 displays the probability of developing cognitive impairment as a function of SF level in individuals with different profiles, which allows for interpretation of the change in risk of cognitive impairment produced by a change in SF. A U-shaped curve was observed, whereby the lowest risk was observed for SF level of approximately 25 µg/l and an increased risk was observed for extreme SF values. The lowest risk profile corresponds to a 65-year-old with higher EDUL, no hypertension or diabetes and normal vitamin B12 values, whereas the highest risk corresponds to an 85-year-old with basic EDUL, diabetes, hypertension and a vitamin B12 serum level of 600 pg/ml.
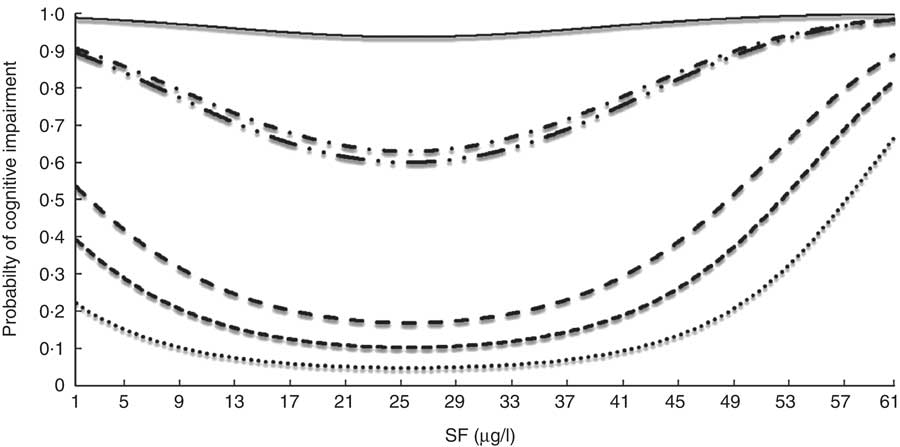
Fig. 1 Probability of cognitive impairment (Modified Mini Mental Status Examination) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey according to serum folate (SF) for individuals with different profiles: ![]() , HEL, no HT, no DM, vit. B12=190 pg/ml, age=65 years;
, HEL, no HT, no DM, vit. B12=190 pg/ml, age=65 years; ![]() .., MEL, HT, DM, vit. B12=600 pg/ml, age=65 years;
.., MEL, HT, DM, vit. B12=600 pg/ml, age=65 years; ![]() , MEL, no HT, no DM, vit. B12=600 pg/ml, age=65 years;
, MEL, no HT, no DM, vit. B12=600 pg/ml, age=65 years; ![]() , HEL, no HT, no DM, vit. B12=600 pg/ml, age=65 years;
, HEL, no HT, no DM, vit. B12=600 pg/ml, age=65 years; ![]() , LEL, HT, DM, vit. B12=600 pg/ml, age=85 years;
, LEL, HT, DM, vit. B12=600 pg/ml, age=85 years; ![]() , MEL, HT, DM, vit. B12=347·9 pg/ml, age=75 years. HEL, high educational level; MEL, medium educational level; LEL, low educational level; HT, hypertension; no HT, no hypertension; DM, diabetes mellitus; no DM, no diabetes mellitus; vit. B12, vitamin B12
, MEL, HT, DM, vit. B12=347·9 pg/ml, age=75 years. HEL, high educational level; MEL, medium educational level; LEL, low educational level; HT, hypertension; no HT, no hypertension; DM, diabetes mellitus; no DM, no diabetes mellitus; vit. B12, vitamin B12
The second model presented in Table 3 (MMMSE plus PFAQ) indicates that individuals living in urban areas were at significantly lower risk for cognitive impairment compared with those living in rural areas (OR=0·21; 95 % CI 0·06, 0·68). The highest risk was observed in men (OR=4·59; 95 %, CI 1·72, 12·26), in those who were older (OR=1·54; 95 % CI 1·04, 2·28) and in diabetics (OR=3·43; 95 % CI 1·07, 10·97). Furthermore, hypertensive patients tended to experience an increased risk but this was not statistically significant (OR=5·56; 95 % CI 0·85, 36·25); however, its inclusion was important to improve the goodness of fit. The OR for folate, SF2 and vitamin B12 were omitted because they were dependent on the values of at least one of the two variables (as explained above).
Figure 2 displays the probabilities of developing cognitive impairment according to the MMMSE plus PFAQ criteria as a function of SF level for participants with different characteristics. As shown in Fig. 2, the probability of impaired cognitive function according to both cognitive tests is described by a U-shaped curve with the lowest risk occurring at SF levels of approximately 29·9 µg/l for some profiles and 41·7 g/l for other profiles. The greatest risk corresponds to a hypertensive, diabetic male who is 85 years old, lives in a rural area and has a vitamin B12 serum level of 600 pg/ml; and the lowest risk corresponds to a non-hypertensive, non-diabetic woman who is 65 years old, lives in an urban area and has a vitamin B12 level of 190 pg/ml. In this model, an increase in cognitive impairment was associated with an increased level of vitamin B12 for SF levels below 20·62 µg/l, with no significant changes beyond this level.
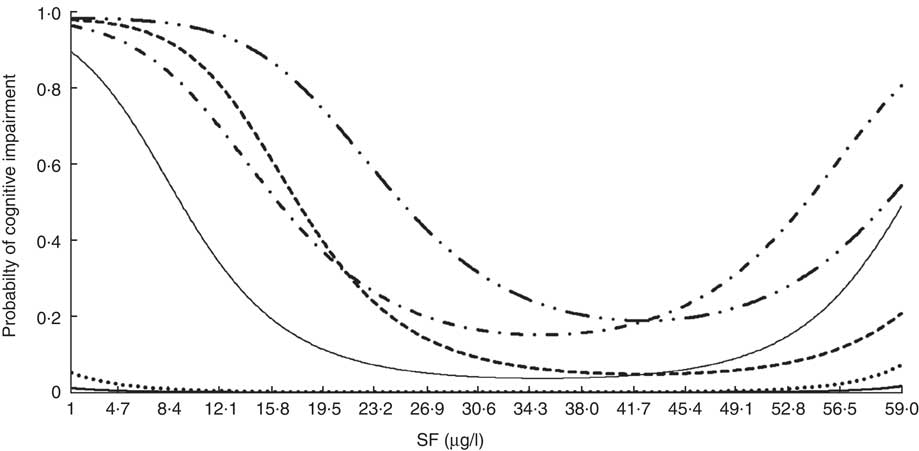
Fig. 2 Probability of cognitive impairment (Modified Mini Mental Status Examination plus Pfeffer Functional Activity Questionnaire) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey according to serum folate (SF) for individuals with different profiles: ![]() , women, urban, no HT, no DM, vit. B12=190 pg/ml, age=65 years;
, women, urban, no HT, no DM, vit. B12=190 pg/ml, age=65 years; ![]() , men, urban, no HT, no DM, vit. B12=190 pg/ml, age=65 years;
, men, urban, no HT, no DM, vit. B12=190 pg/ml, age=65 years; ![]() , women, rural, HT, DM, vit. B12=347·9 pg/ml, age=75 years;
, women, rural, HT, DM, vit. B12=347·9 pg/ml, age=75 years; ![]() , men, rural, HT, DM, vit. B12=347·9 pg/ml, age=75 years;
, men, rural, HT, DM, vit. B12=347·9 pg/ml, age=75 years; ![]() , women, rural, HT, DM, vit. B12=600 pg/ml, age=85 years;
, women, rural, HT, DM, vit. B12=600 pg/ml, age=85 years; ![]() , men, rural, HT, DM, vit. B12=600 pg/ml, age=85 years. Urban, urban residence; rural, rural residence; HT, hypertension; no HT, no hypertension; DM, diabetes mellitus; no DM, no diabetes mellitus; vit. B12, vitamin B12
, men, rural, HT, DM, vit. B12=600 pg/ml, age=85 years. Urban, urban residence; rural, rural residence; HT, hypertension; no HT, no hypertension; DM, diabetes mellitus; no DM, no diabetes mellitus; vit. B12, vitamin B12
Figure 3 displays the differences in the risk of impaired cognitive function according to the MMSE plus PFAQ that are produced by a one-unit increase in SF (1 µg/l) for each combination of SF and vitamin B12. Each grey area represents a different OR level, and we highlight several lines of constant OR. The area with different shades of grey on the left of Fig. 3 displays the OR variation for each unit increase in SF when SF levels are low. For example, the first line on the left shows that a one-unit increase in SF decreased the risk for the impaired cognitive function by 30 % (OR=0·7). This level of protection varies depending on the level of vitamin B12. We observed that the protection against cognitive impairment increased as the vitamin B12 level increased, and vice versa. The area on the right of Fig. 3 shows the risk at high levels of SF (>37·5 µg/l). In this model, a one-unit increase in SF raised the risk for impaired cognitive function, specifically in the context of low vitamin B12 levels. On the other hand, the risk decreased only until vitamin B12 rose to approximately 490 pg/l.
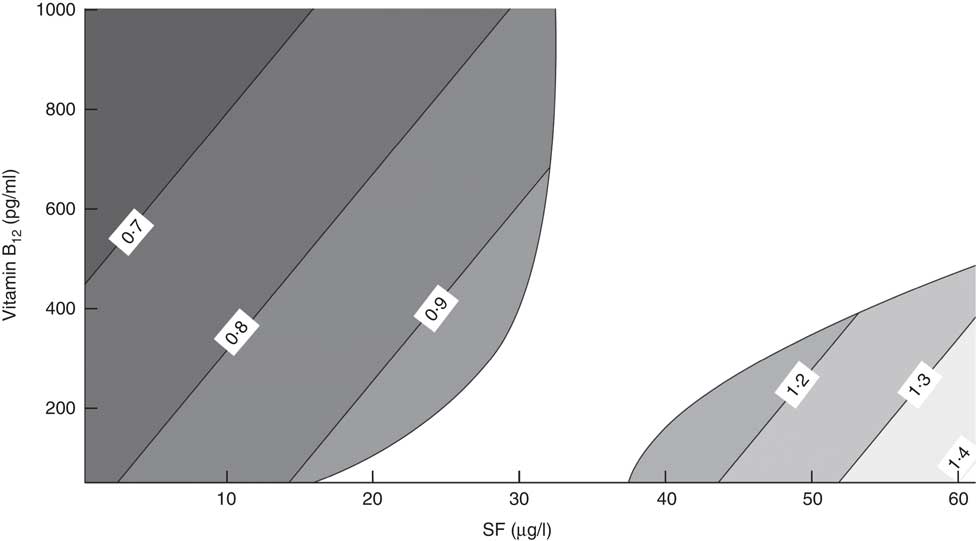
Fig. 3 Variation of OR of impaired cognitive function (Modified Mini Mental Status Examination plus Pfeffer Functional Activity Questionnaire) among older Chilean adults (>65 years) who participated in the 2009–2010 National Health Survey upon increasing serum folate (SF) by 1 µg/l according to different combinations of SF and vitamin B12 levels
As shown in the central white region of Fig. 3, a one-unit change of SF (1 µg/l) and vitamin B12 did not significantly change the risk of cognitive impairment when SF was between 32·7 and 37·5 µg/l. However, when SF was between 15·6 and 32·7 µg/l, increased SF levels had no effect on the risk. Finally, in the same range of SF, the risk of cognitive impairment decreased as the level of vitamin B12 increased.
Discussion
Folate plays an important role in growth, differentiation, development, brain repair, cognition, mood and ageing( Reference Rosenberg 25 ). In the present study, the assessment of cognitive function was conducted using both the MMMSE and PFAQ tests. The MMMSE is a modified screening tool used to evaluate cognitive deficits. A total score ≤13 points indicates an impairment of cognitive function and has a higher sensitivity and specificity (over 90 %) than the extended version of the Folstein Mini Mental State Examination (MMSE)( Reference Folstein, Folstein and McHugh 16 ). The administration of the MMMSE followed by the PFAQ has been validated in Chile and limits bias, particularly when individuals are illiterate or possess limited literacy, thereby increasing the detection of mild cognitive impairment and reducing false positives( Reference Icaza and Albala 19 ). These assessments are simple and can be applied in primary health care with low cost( Reference Quiroga, Albala and Klaasen 18 – Reference Pfeffer, Kurosaki and Chance 20 ).
A significant percentage of the sample was observed to have impaired cognitive function when evaluated only by the MMMSE; however, the number of older adults with cognitive impairment decreased when both tests were applied. This finding is consistent with the increased sensitivity described in validation studies, which has allowed for the use of the MMMSE and PFAQ in similar population studies( 15 , Reference Icaza and Albala 19 ).
Unlike other studies that observed higher rates of cognitive impairment in women( Reference Guerra, Alvarado and Zunzunegui 26 , Reference Zunzunegui, Nunez and Durban 27 ), neither the MMMSE nor the combination of the two screening measures showed a significant difference in cognitive decline by gender in the present study. The superior schooling of Chilean women, as well as their prolonged interactions with family members, might stimulate cognitive function( Reference Helmer 28 , 29 ). As in other studies, a lower risk of cognitive impairment was observed in those with higher levels of education( Reference Rodríguez-Sánchez, Mora-Simón and Patino-Alonso 30 – Reference Moraes, Pinto and Lopes 32 ), which might be explained by greater cognitive reserves from superior cortical and neuronal synaptic capacities or improved compensation for deficiencies arising from other brain regions( Reference Tucker and Stern 33 , Reference Fernández-Ballesteros, Botella and Zamarrón 34 ).
Although the difference in SF levels was statistically significant when comparing our participants with normal and impaired cognitive function (MMMSE plus PFAQ), it should be noted that in both cases SF levels were within the normal range, reaching supraphysiological levels (>20 μg/l)( Reference Dary 35 ) in the group with normal cognitive function. These higher levels might have resulted from the universal folic acid fortification of wheat flour in Chile, the high levels of consumption of farinaceous products or the increased consumption of vitamin supplements among some older adults( Reference Castillo, Tur and Uauy 36 ).
Folate metabolism is closely linked to other vitamins, especially vitamin B12 Reference Morris, Jacques and Rosenberg (37 ). Both folate and vitamin B12 function as cofactors that are dependent on methionine synthase. Severe and prolonged deficiency is associated with decreased synthesis of methionine and a corresponding increase of homocysteine. There is evidence that high levels of homocysteine induce damage in the central nervous system and influence cognitive impairment, dementia and Alzheimer’s disease( Reference Feng, Ng and Chuah 38 – Reference McCaddon, Regland and Hudson 41 ). Different mechanisms have been postulated to explain this cognitive impairment, including failed glutathione metabolism and increased oxidative stress, which lead to greater neuronal injury and hinder the reduction of vitamins to their metabolically active forms( Reference Ramos, Allen and Mungas 42 ).
In the current study, the multivariate analysis utilising only the MMMSE suggested that older adults with lower educational levels, diabetes and hypertension exhibit a significantly increased risk of impaired cognitive function. As shown in other studies, diabetes and hypertension contribute to cognitive deterioration through atherosclerosis, microvascular disease and glucose toxicity( Reference Rodríguez-Sánchez, Mora-Simón and Patino-Alonso 30 , Reference Gavrila, Antunez and Tormo 31 ). Moreover, when cognitive impairment was evaluated using both diagnostic tests, older adults, males, diabetics and those living in rural areas were at the greatest risk. These results are consistent with studies conducted in urban areas that observed greater cognitive function to be associated with superior life conditions and environmental stimuli( Reference Moraes, Pinto and Lopes 32 ).
Deficits in folate are associated with the failure of DNA methylation, which might increase the synthesis of the β-amyloid peptide, a determinant of Alzheimer’s disease( Reference Irizarry, Gurol and Raju 43 ). Low folate and vitamin B12 intakes are associated with failed synthesis, transcription and integrity of DNA due to the lower production of purines, especially thymidine. This would also decrease DNA methylation, which is important not only for gene expression but also for epigenetic mechanisms that contribute to demyelination and the exchange of monoamines in depression( Reference Berchicci, Lucci and Di Russo 2 ).
Previous research described the beneficial effects of folate supplementation under conditions of cognitive impairment( Reference Quadri, Fragiacomo and Pezzati 4 , Reference Durga, van Boxtel and Schouten 7 ). Moreover, the studies that developed post-fortification of foods in the USA suggested that the greater risk associated with high SF levels (>24 µg/l) was also associated with a deficit in vitamin B12 (<200 pg/ml); however, these results have not been described in countries where fortification is not mandatory( Reference Clarke, Sherliker and Hin 44 ). In the present study, a significant increase in the risk of cognitive impairment was observed at low and high SF levels. The risk of impaired cognitive function varied according to the profile of each individual but was higher among the elderly living in rural areas and among those with diabetes and hypertension. These observations suggest the importance of other variables when evaluating the impact of folic acid fortification.
Due to the important metabolic interactions between folate and vitamin B12, the current study evaluated the effects on cognitive function (MMMSE plus PFAQ) of changing SF levels by one unit (1 µg/l) at different levels of both vitamins( Reference Green 45 ). As shown in other studies( Reference Selhub, Morris and Jacques 9 , Reference Morris, Jacques and Rosenberg 10 , Reference Morris, Selhub and Jacques 11 , Reference Morris, Jacques and Rosenberg 37 , Reference Ramos, Allen and Mungas 42 ), we observed that increased folate values in individuals with low SF levels decreased the risk of impaired cognitive function specifically associated with increased vitamin B12 levels. However, when individuals had higher SF levels, increased folate levels increased the risk, especially in patients with the lowest vitamin B12 levels. Unlike other studies, we observed a range of values (SF between 32·7 and 37·5 µg/l) in which the risk of cognitive impairment remained constant, regardless of changes in folate and vitamin B12 levels.
Studies conducted in the USA that described an increased risk of cognitive impairment associated with high SF levels found that this corresponded to 80 % unmetabolised folic acid( Reference Selhub, Morris and Jacques 9 , Reference Boilson, Staines and Kelleher 46 ). The impaired cognitive function was specifically associated with lower vitamin B12 levels, as we described in the present study. It was hypothesised that the increased risk of high SF levels could be explained by the presence of free folic acid( Reference Selhub, Morris and Jacques 9 , Reference Sánchez, Albala and Hertrampf 12 ), which acts as an electron acceptor (oxidant) and results in the irreversible oxidation of intracellular vitamin B12, thereby magnifying the deficiency. This pattern is similar to that observed upon exposure to nitric oxide, which results in the failure of the methionine synthetase enzyme. The reduced availability of vitamin B12 as a cofactor for mitochondrial reactions magnifies the deficiency of vitamin B12 and the levels of homocysteine and methylmalonic acid, as well as their respective haematological and neurological consequences( Reference Selhub, Morris and Jacques 9 , Reference Boilson, Staines and Kelleher 46 ). Considering this background and noting that a significant percentage of Chilean older adults present with supraphysiological SF levels, it appears necessary to assess the proportion that corresponds to free folic acid in future studies.
One limitation of the current study is its cross-sectional design, which did not allow us to identify causal relationships between the studied variables and impaired cognitive function. In addition, other variables such as physical activity and dietary factors, including the consumption of antioxidants that might have important effects on cognitive function, have not been incorporated in the analysis, prohibiting conclusions from being drawn regarding these variables. Another restriction of the study is the exclusive use of serum vitamin B12 levels as an indicator of the vitamin’s nutritional status. The incorporation of other indicators such as methylmalonic acid or transcobalamin would help to better identify vitamin B12 deficiencies. Moreover, the elevated SF and vitamin B12 levels observed in some participants with greater cognitive impairment could be due to extra supplementation given the cognitive defects these individuals display. This possible reverse causation should be investigated in future studies.
Conclusion
In conclusion, the consequences of high SF levels, as well as of other functional results observed in the Chilean elderly require further evaluation in future studies aimed at defining the appropriate levels of food fortification to maximise the benefits such as decreasing neural tube defects while minimising the potential adverse effects on cognitive function in older people. Our data also suggest the need to monitor serum folic acid levels achieved by universal fortification in older people to prevent the adverse effects of excess folic acid as well as a potential need for vitamin B12 supplementation in this age group.
Acknowledgements
Acknowledgements: The authors acknowledge the Public Health School of Pontificia Universidad Católica de Chile for its support in this investigation. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: C.C-L. participated in the design and analysis, and wrote and reviewed the manuscript. G.V. and P.M. reviewed the manuscript and contributed in the discussion. O.P. participated in the statistical analysis and interpreted and reviewed the results in all stages of the investigation. R.U. and J.A.T. participated in the design and interpreted the results. J.R. reviewed all of the manuscript, including the present version. All authors read and approved the final revised draft for publication. Ethics of human subject participation: Ethical approval was not required.











