Adequate dietary intake during pregnancy plays an important role in women’s health, in perinatal outcomes, and in fetal growth and development( Reference Wu, Bazer and Cudd 1 , Reference Meinila, Koivusalo and Valkama 2 ). The quality of a woman’s diet during pregnancy( Reference Meinila, Koivusalo and Valkama 2 – Reference Grieger and Clifton 4 ) and excessive( Reference Poston, Harthoorn and Beek 5 ) or restricted intake( Reference Purandare 6 ) are known risk factors and may impact maternal health in addition to childhood and adolescent health( Reference Poon, Yeung and Boghossian 7 ). Dietary restrictions are associated with nutritional deficiencies and low birth weight( Reference Grieger and Clifton 4 , Reference Purandare 6 ), whereas excessive gestational weight gain seems to be associated with fetal macrosomia( Reference Poston, Harthoorn and Beek 5 , Reference Mamun, Callaway and O’Callaghan 8 ). These conditions are considered risk factors for the development of obesity and chronic diseases later in adult life.
Dietary intake during pregnancy is traditionally assessed by examining the energy and macronutrient intakes or the intakes of certain food groups or micronutrients( Reference Wu, Bazer and Cudd 1 – Reference Lee, Talegawkar and Merialdi 3 ), which may then be associated with inadequate nutritional status and imbalanced maternal diet. Since the early 1990s, principal component analysis has been used to identify dietary patterns during pregnancy( Reference Castro, Souza and Vilela 9 – Reference Northstone, Emmett and Rogers 11 ). This method allows for diet to be described as a whole, and it can be used to assess the role of social and economic factors in the likelihood of adherence to a certain dietary pattern. Thus, principal component analysis seems to be a relatively good and comprehensive method for exploring food intake during pregnancy. The use of dietary patterns may also help capture some of the complexities of diet, which are often lost in nutrient-based analyses. Similarly, principal component analysis provides additional information for exploring the relationship between nutrition and health( Reference Wirfält, Drake and Wallström 12 – Reference Hu 14 ).
There are still gaps regarding the impact of various socio-economic and demographic factors on adequate dietary intake during pregnancy( Reference Castro, Souza and Vilela 9 , Reference Sommer, Sletner and Jenum 15 ). In Brazil, a study of 421 pregnant women, conducted by Castro et al.( Reference Castro, Souza and Vilela 9 ), indicated that schooling and family income were positively associated with a ‘healthy’ dietary pattern. In a multi-ethnic population study of pregnant women conducted in Norway it was observed that non-European women were more likely to adhere to an unhealthy dietary pattern compared with European women( Reference Sommer, Sletner and Jenum 15 ). Additionally, a positive association between education and a ‘health-conscious’ diet was verified in a British population-based study with 12 053 pregnant women( Reference Northstone, Emmett and Rogers 11 ). Also, age, regional habits, lower parity and marital status have been suggested to be associated with dietary quality( Reference Arkkola, Uusitalo and Kronberg-Kippilä 10 , Reference Hoffmann, Camey and Olinto 16 , Reference Ricardo and Claro 17 ).
The identification of whether certain sociodemographic factors are associated with adherence to specific dietary patterns may help shape the actions of health professionals according to the social and economic profile of their patient population. Particularly, in a developing country such as Brazil, it was shown that low income may affect food and dietary patterns( Reference Castro, Souza and Vilela 9 , Reference Hoffmann, Camey and Olinto 16 , Reference Ricardo and Claro 17 ). These relationships emphasise the recent evidence that suggests these possible associations should be further explored. Therefore, the aim of the present study was to identify gestational dietary patterns and verify whether they are associated with sociodemographic factors in low-income Brazilian women who are pregnant.
Materials and methods
Design and study population
A cross-sectional study was conducted of women who gave birth between February 2009 and February 2011 in the maternity ward of the Municipal Hospital Leonel de Moura Brizola, located in Mesquita, Rio de Janeiro, Brazil.
The maternity ward sees women with low-risk term pregnancies. Women aged 18–45 years, without twin pregnancy or pre-existing chronic diseases (diabetes mellitus, cancer and renal disease) were eligible to participate in the study. Of the 338 women who were invited to participate in the study, four (1·2 %) refused to answer the questionnaire and seven (2·1 %) were excluded from the analysis because their energy intake exceeded 25 104 kJ (6000 kcal)( Reference Oken, Kleinman and Olsen 18 ). A dietary intake of less than 2510 kJ (600 kcal) was an exclusion criterion, but none of the participants were excluded based on this criterion. Thus, the study included data on 327 (96·7 %) participants.
Sociodemographic data were collected during the immediate postpartum period (first week after birth) through a structured questionnaire and included the following variables: schooling (years of schooling), parity (number of parturitions), marital status (married/stable partnership or single/other), age (years), self-reported skin colour (white or black/brown) and monthly per capita family income ($US). The sociodemographic variables were used as predictor variables of the dietary patterns.
Early pregnancy weight was measured until 13 weeks of gestation and was obtained from the women’s prenatal cards. When the early pregnancy weight was not recorded on the prenatal cards, it was self-reported by the women. Height was measured while the women were barefoot by using a portable stadiometer that was calibrated to 0·1 cm (Alturexata, Belo Horizonte, Brazil). The anthropometric measures were assessed following the protocol established by Lohman et al.( Reference Lohman, Roche and Martorell 19 ).
Dietary patterns
All of the women answered a validated semi-quantitative FFQ( Reference Sichieri and Everhart 20 ); the time frame included the last six months of pregnancy to avoid distortions in consumption by nausea and dislike of the early stages of pregnancy. The FFQ options were converted into grams of daily intake. The daily intakes of nutrients and energy were estimated using a program developed by Sichieri( Reference Sichieri 21 ) in the statistical software package SAS version 9·1, and were obtained by multiplying the previous portion of each food( Reference Pinheiro 22 ) by its respective daily frequency. The Brazilian Food Composition Table( 23 ) was used as the nutritional composition database for the conversion of foods into nutrients and energy. The US Department of Agriculture Food Composition Table( 24 ) was used in the absence of certain food items from the Brazilian table.
The eighty-one items on the FFQ considering nutritional composition were grouped into twenty-eight food groups, including the following foods and beverages: (i) green leafy vegetables (lettuce, kale and cabbage); (ii) legumes and other vegetables (okra, chayote, cucumber, onion, pumpkin, zucchini, carrot, pea pod, beet, cauliflower, red/green/yellow pepper and tomato); (iii) fruits (orange, banana, papaya, apple, melon, pineapple, mango and grape); (iv) rice; (v) beans; (vi) wheat; (vii) breads; (viii) potatoes and cassava; (ix) pasta and Italian foods; (x) milk; (xi) dairy products (yoghurt, cheese and cream cheese); (xii) eggs; (xiii) fish; (xiv) sardines; (xv) chicken; (xvi) red meat; (xvii) fatty and processed meats (steak, oxtail, pork, sausages and giblets); (xviii) sugar; (xix) cakes and cookies/crackers; (xx) butter and margarine; (xxi) other fats (bacon and mayonnaise); (xxii) snacks (pizza, savoury snacks, French fries, popcorn and patties); (xxiii) soda; (xxiv) candy; (xxv) herbal mate and tea; (xxvi) fruit juice; (xxvii) beer and wine; and (xxviii) coffee. Some of the individual food and beverage items were kept separately due to their higher frequency of intake among the pregnant women and their cultural relevance (e.g. rice and beans). Complex dishes such as lasagne, pasta and gnocchi were included in the pasta and Italian foods group. The questionnaire included a specific Brazilian mixed dish called churrasco (steak) that comprises more than one type of meat; it was included in the fatty and processed meats group.
Dietary patterns were identified using factor analysis for principal component analysis. A Kaiser–Meyer–Olkin statistic >0·60 was applied to test for method adequacy. The following criteria were used to identify the number of patterns (factors): (i) eigenvalues >1·50 and (ii) the inflection point of the eigenvalues from the Cattell scree test (screeplot)( Reference Wirfält, Drake and Wallström 12 , Reference Kant 13 , Reference Newby and Tucker 25 ). After orthogonal rotation of the varimax matrix, we used a factor loading cut-off of 0·20 to limit the inter-correlation between the dietary variables that were obtained. Cronbach’s α was used to measure the internal consistency of a set of items that was identified as a group. Subsequently, the factors were named according to the dietary composition of the predominant food groups.
Data analysis
After extraction of the factors, unvariate linear regression was performed to evaluate the association between the sociodemographic variables and the identified dietary patterns. The sociodemographic variables that had P<0·20 in the univariate regression were included in the multivariate linear regression model. Energy intake (kcal) and pre-pregnancy BMI (early pregnancy weight (kg)/height2 (m2)) were used as adjustment variables. Aside from skin colour, all of the other predictor variables (schooling, monthly per capita family income, parity, age and marital status) were associated with at least one of the factors retained (P<0·05) in the univariate linear regression model. All estimates were calculated using SAS version 9·1.
Ethical aspects
The study and the informed consent form were approved by the Research Ethics Committee of the Institute of Social Medicine at the State University of Rio de Janeiro (protocol number CAAE 0022.0.259.000–09). The proposed project was in accordance with the ethical principles recommended by the Brazilian National Health Council.
Results
The mean baseline characteristics of the 327 postpartum women were as follows: 24·8 (sd 5·5) years of age; 2·2 (sd 1·4) parturitions; monthly per capita family income of $US 338 (sd 211); pre-pregnancy BMI of 23·6 (sd 4·6) kg/m2; and total daily energy intake of 12 108 (sd 4523) kJ (2894 (sd 1081) kcal). More than 80 % of the pregnant women reported that their skin colour was black or brown, and 73·3 % of the women were classified as married or having a stable partner (Table 1).
Table 1 Baseline characteristics of the 327 postpartum women. Rio de Janeiro, Brazil, 2011
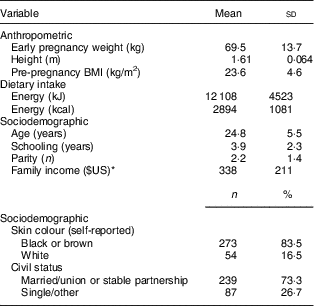
* Monthly per capita family income.
Three dietary patterns were identified: ‘healthy’, ‘mixed’ and ‘traditional’. The Kaiser–Meyer–Olkin statistic was 0·65, and the eigenvalues were 3·37, 2·05 and 1·67, respectively. The cumulative variance was 25·3 %. The ‘healthy’ pattern explained 12·0 % of the total variance and comprised green leafy vegetables, legumes and other vegetables, milk, dairy products, fruits, red meat, chicken, herbal mate and tea, and fruit juice. The second pattern was the ‘mixed’ pattern; it explained 7·3 % of the variance and comprised eggs, pasta and Italian foods, other fats, cakes and cookies/crackers, butter and margarine, fatty and processed meats, snacks, soda, sardines and candy. The third pattern was the ‘traditional’ pattern; it explained 6·0 % of the variance and comprised rice, beans, wheat, potatoes and cassava, breads, sugar and coffee. The standardised Cronbach’s α coefficient was 0·65, 0·59 and 0·55, respectively, for the ‘healthy’, ‘mixed’ and ‘traditional’ patterns. Prior communality was estimated, and the low and high communality was 0·44 for beer and wine and 0·82 for fruits, respectively (Table 2).
Table 2 Factor loadings of rotated factor matrix and initial communalities for major pregnancy dietary patterns for 327 women at postpartum. Rio de Janeiro, Brazil, 2011

* Factor loadings ≥0·20 were included in each dietary pattern.
The older women (β=0·021; 95 % CI −0·001, 0·042; P=0·058) and the women with a higher monthly per capita family income (β=0·0006; 95 % CI 0·0001, 0·001; P=0·011) tended to adhere more to the ‘healthy’ dietary pattern, whereas the women with lower parity (β=−0·097; 95 % CI 0·184, −0·009; P=0·030) tended to adhere less to the ‘healthy’ dietary pattern. The women with more parturitions (β=0·098; 95 % CI 0·021, 0·175; P=0·012) were more likely to adhere to the ‘traditional’ dietary pattern. Although the association was not statistically significant, maternal age was negatively associated with the ‘mixed’ (β=−0·017; 95 % CI −0·037, 0·003; P=0·075) and the ‘traditional’ (β=−0·018; 95 % CI −0·037, 0·001; P=0·061) dietary patterns (Table 3).
Table 3 Unadjusted and adjustedFootnote * regression coefficients for the ‘healthy’, ‘mixed’ and ‘traditional’ dietary patterns according to sociodemographic variables for 327 women at postpartum. Rio de Janeiro, Brazil, 2011

* The models were adjusted for total energy intake and pre-gestational BMI.
† Monthly per capita family income.
‡ Married/union or stable partnership or single/other.
Discussion
In the present study, three dietary patterns were identified among low-income Brazilian pregnant women, and they were labelled as ‘healthy’, ‘mixed’ and ‘traditional’. The women with a higher monthly per capita family income were more likely to adhere to the ‘healthy’ pattern, whereas the women with higher parity were less likely to adhere to the ‘healthy’ pattern. In contrast, the women with higher parity were more likely to adhere to the ‘traditional’ pattern, whereas the older women showed a borderline negative association with this pattern. With the exception of a borderline association with maternal age, the sociodemographic factors examined were not associated with the ‘mixed’ dietary pattern.
Some studies suggest that sociodemographic factors influence adherence to dietary patterns( Reference Castro, Souza and Vilela 9 ) and are related to differential access according to economic status and ethnicity( Reference Sommer, Sletner and Jenum 15 ). In our study, a positive association was observed between family income and the ‘healthy’ pattern. This finding is in agreement with other national studies that verified that women with the lowest education and income have been associated with the intake of traditional Brazilian food items( Reference Hoffmann, Camey and Olinto 16 ) and with a low consumption of healthy foods( Reference Ricardo and Claro 17 ). Brazil is a developing country and the frequency of low-income families is still quite common. In this regard, the present finding is particularly important and adds new evidence to the current literature as it shows that income is strongly associated with dietary choices. In a study among British pregnant women, the ‘health-conscious’ pattern (comprising salad, fruit, rice, pasta, oat- and bran-based breakfast cereals, fish, pulses, fruit juices and non-white bread) showed a negative association with financial difficulties and increasing parity( Reference Northstone, Emmett and Rogers 11 ); this is similar to our findings.
Our study found an inverse association between parity and the ‘healthy’ pattern. According to Goñi et al.( Reference Goñi, Cuervo and Santiago 26 ), a diet comprising fruits, dairy products and nuts during pregnancy was more common among nulliparous than multiparous women. This finding was based on a cross-sectional survey of 5087 pregnant women in Spain. Additionally, the authors found a higher intake of sausages, bread and meat among the multiparous pregnant women. Ferrer et al.( Reference Ferrer, García-Esteban and Mendez 27 ) showed that parity was positively associated with the ‘caloric’ pattern characterised by a high intake of energy-dense foods and with the low intake of fruits and vegetables. Other studies are in accordance with our results( Reference Northstone, Emmett and Rogers 11 , Reference Cucó, Fernández-Ballart and Sala 28 ), where it was observed that increasing parity is associated with high adherence to unhealthy dietary patterns during pregnancy. This finding raises the hypothesis that health consciousness may occur more frequently among nulliparous women. In general, higher-income and older individuals tend to choose healthier foods( Reference Laaksonen, Prattala and Helasoja 29 , Reference Lenz, Olinto and Dias-da-Costa 30 ).
Parity and maternal age were positively and negatively associated with the ‘traditional’ pattern that mainly comprised rice and beans. Rice and beans are some of the most frequently consumed food items in Brazil( Reference Souza A de, Pereira and Yokoo 31 ) and are considered to be appropriate when consumed in moderation( 32 ). However, this pattern also comprised higher energy-dense foods such as potatoes and cassava, bread and sugar. As noted above, the scientific literature reveals that higher number of parturitions and younger age have been associated with a greater adherence to unhealthy patterns( Reference Northstone, Emmett and Rogers 11 , Reference Hoffmann, Camey and Olinto 16 , Reference Ferrer, García-Esteban and Mendez 27 , Reference Cucó, Fernández-Ballart and Sala 28 ). In our study, older women were more likely to adhere to the ‘healthy’ dietary pattern.
We did not observe associations between the sociodemographic factors and the ‘mixed’ pattern. This pattern was labelled ‘mixed’ because it comprised food groups with different nutritional compositions, such as eggs, bacon and mayonnaise, cakes and biscuits, and sardines. The majority of these food groups comprised more energy-dense foods. Some studies that have assessed sociodemographic factors and dietary patterns during pregnancy observed that factors such as smoking during pregnancy, living without a partner, higher parity, lower education and maternal age were positively associated with dietary patterns that are similar to the ‘mixed’ pattern in the present study( Reference Arkkola, Uusitalo and Kronberg-Kippilä 10 , Reference Northstone, Emmett and Rogers 11 , Reference Ferrer, García-Esteban and Mendez 27 , Reference Cucó, Fernández-Ballart and Sala 28 ). Castro et al.( Reference Castro, Sichieri and Brito 33 ) showed that high adherence to the ‘mixed’ dietary pattern could decrease postpartum body weight loss. This may contribute to the maintenance of maternal overweight or an increase in the prevalence of obesity during the reproductive period.
No association was observed between skin colour and dietary pattern. This may be explained by the fact that more than 80 % of the sample comprised black and brown women. Some studies on black–white dietary differences have shown that black populations tend to report lower intakes of vegetables, vitamin C, Ca and K( Reference Kant, Graubard and Kumanyika 34 ). Lacerda et al.( Reference Lacerda, Kac and Cunha 35 ) found that compared with white women, black women tended to have higher energy and carbohydrate intakes during pregnancy and higher postpartum energy intake. Boggs et al.( Reference Boggs, Palmer and Spiegelman 36 ) identified two dietary patterns among the 41 351 women enrolled in the Black Women’s Healthy Study. At baseline, a lower score on the ‘vegetables/fruit pattern’ was associated with younger women and a higher score on the ‘high meat/fried foods pattern’ was associated with less educated women. Another possible explanation for the lack of association in our study may be that skin colour is a proxy for income.
Some limitations should be considered when interpreting our results. First, the cross-sectional design of the study does not allow for establishing causal relationships. Also, the FFQ previously validated for adults( Reference Sichieri and Everhart 20 ) was not developed for pregnancy; however, epidemiological studies have shown that FFQ can be used to measure nutritional dietary intake during pregnancy with acceptable validity and reproducibility( Reference Poon, Yeung and Boghossian 7 , Reference Arkkola, Uusitalo and Kronberg-Kippilä 10 ), including in Brazil( Reference Barbieri, Nishimura and Crivellenti 37 ). Finally, the small sample size and the low-income profile of the sample must be considered when generalising the results.
Nevertheless, the study provides results that highlight the need to develop new understanding of dietary patterns during pregnancy. Moreover, the findings of the present study can be adopted in primary-care settings to provide new strategies for maternal dietary counselling during pregnancy and for women’s health.
Conclusion
In summary, our study showed that sociodemographic factors may determine dietary patterns during pregnancy. Among other factors, income and parity, respectively, positively and negatively influenced food intake. Higher family income was associated with the ‘healthy’ pattern, whereas greater parity was associated with the ‘traditional’ pattern. These factors should be considered to provide pregnant women more appropriate and effective prenatal care.
Acknowledgements
Financial support: The project was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Edital FAPERJ/SESDEC/MS/CNPq/no. 18/2009). The funder had no role in the design, analysis or writing of this paper. Conflict of interest: None. Authorship: M.B.T.d.C. designed the study, analysed and interpreted the data, and drafted the paper. A.A.F.V., A.S.D.O., M.C. and R.A.G.S. interpreted the data and drafted the paper. G.K. and R.S. designed the study and drafted the paper. All authors reviewed and approved the final version submitted for publication.








