The human life course is marked by a number of important events and transitions. Some of these are biologically based, involving notable changes to the human body, such as teething, sexual maturation, and menopause. However, many transitions are simultaneously, or exclusively, marked by cultural and ritual events, such as weddings, feasts, or other social gatherings to celebrate and advertise such transitions. While the biological transitions are relatively standardized in how they are expressed, including the timing of their onset and completion, especially within the sexes, there is tremendous cultural variation in how societies practice and observe life history transitions (Crittenden and Meehan Reference Crittenden, Meehan, Meehan and Crittenden2016).
Food and diet play an important part in the expression of these different human life history stages and transitions. For example, one of the most important and stressful transitions is the weaning process, when a young individual transitions from breast milk to solid foods (Hill and Hurtado Reference Hill and Hurtado1996; Quinlan Reference Quinlan2007; Trivers Reference Trivers1974). This is a transition that all individuals experience, typically at an early age (between a few months and a few years; though in some recent settings formula is substituted for breast milk entirely). Other stages in life may also be expressed by dietary shifts. For example, in the United States today, early adolescent years are often expressed by increased consumption of sugar-rich and highly processed foods (Ogden et al. Reference Ogden, Kit, Carroll and Park2011; Wang et al. Reference Wang, Bleich and Gortmaker2008).
Because many life history stages are expressed culturally, bioarchaeological methods provide the best means to reconstruct them in ancient societies. In particular, stable isotope analyses are well suited to reconstructing these processes when life history transitions involve dietary or residential changes. Because human tissues, including bones and teeth, are synthesized from ingested foods, they record signatures of the paleodiet of an individual. Furthermore, teeth grow in sequential layers over windows of time. Isolating tissue from different layers, then, facilitates diachronic reconstruction of diet for an individual during the growth and development period. In other words, by analyzing different tissues that form at different points in a person's life, we can track paleodietary patterns over a number of potential life history stages. These techniques allow us to reconstruct something of an isotopic biography, or an “isobiography,” for individuals from the past.
In this article, we analyze stable isotopes of carbon and nitrogen in 100 dentinal collagen samples extracted from first (n = 5) and third (n = 11) molars. A total of 16 individuals (one tooth per person) was sampled from five archaeological sites on the island of Tutuila in American Samoa (see Figure 1). Teeth were cut in serial sample fashion to obtain a time-transgressive estimate of diet for that person during the window of time the dentinal collagen formed (see Eerkens et al. Reference Eerkens, Berget and Bartelink2011). We then examine life history patterns in diet through the distribution of stable isotope values across serial sections.
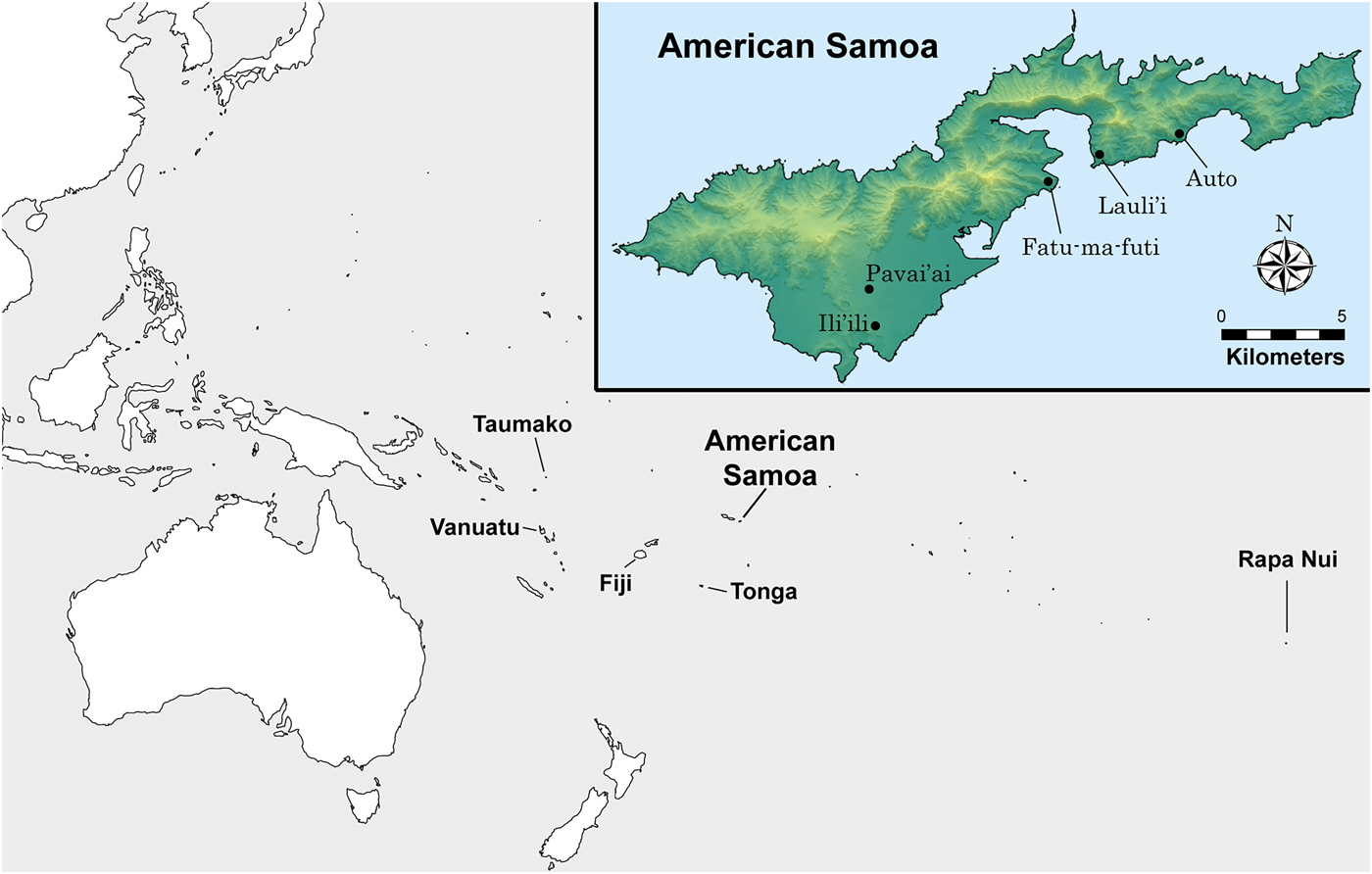
Figure 1. Map showing the location of Tutuila Island, American Samoa (inset), and sites included in this study.
A Samoan Dietary Model
Before examining ancient remains, we first establish a pattern based on ethnographic sources of dietary life history in American Samoa, focusing on information available from the late nineteenth through mid-twentieth century. Precise dietary life history data are not available for individuals, as diet was typically of secondary concern in anthropological studies on the islands, especially in earlier studies. Instead, we rely on aggregate or more general information recorded by ethnographers that relates to subsistence practices of villages and/or households. In this regard, we examine two types of information, household social organization and foraging behaviors. Although we use the past tense to connote an ethnographic past, people continue many of these behaviors today.
American Samoans were dependent on the ocean (e.g., fish, shellfish, crustaceans, sea turtles), inland agricultural fields (e.g., banana, taro, coconut, breadfruit, yams), and domesticated animals (e.g., pig, dog, chicken) for the majority of their basic subsistence. Villages were made up of a number of household economic units (5–40), often spread over large areas. While most villages in ethnohistoric times were near the coast, archaeological data indicate that inland areas were more heavily occupied in precontact times (Addison et al. Reference Addison, Tago, Toloa and Pearthree2006; Davidson Reference Davidson1969; Eckert and Welch Reference Eckert and Welch2013; Quintus and Cochrane Reference Quintus and Cochrane2017; Rieth and Addison Reference Rieth, Addison, Addison and Sand2008). Households within villages varied in size from nuclear families, to larger extended families, to multifamily houses, but each was presided over by a chief or matai (Ember Reference Ember1962; Tcherkézoff Reference Tcherkézoff2000). In general, Samoan society was ranked, both households within a village and individuals within a household (Krämer Reference Krämer and Verhaaren1994; Mead Reference Mead1928).
As in all traditional human societies, dietary life history on American Samoa began with infants consuming breast milk. Typically within the first few months after birth, small amounts of solid or liquid food, often premasticated by the mother, were introduced to the infant (Mead Reference Mead1928:21). This point marks the beginning of the weaning process, a slow transition to solid food. The precise length of time that infants consumed breast milk is not well established in ethnographic studies in American Samoa, especially prior to the twentieth century, when breastfeeding behavior was not an important topic in anthropology. More detailed studies in the 1960s through 1980s indicate that weaning was usually complete between one and two years (Bindon Reference Bindon1984, Reference Bindon1986; Gardner Reference Gardner1965:29; Nardi Reference Nardi1984). However, Nardi (Reference Nardi1984) suggests that greater involvement in cash economies in the middle of the twentieth century in the Pacific placed a greater labor burden on women and may have promoted earlier weaning. Indeed, Malcolm notes that in earlier times women used to “breastfeed the infants until they were about two years old or even older” (Reference Malcolm1954:19), and Mead (Reference Mead1928:22) stated that mothers nursed their babies until they were two to three years old. Based on these observations, we expect a transition from breast milk to solid foods between two and three years of age for most individuals in precontact times. There are no suggestions of a difference in the age of weaning between boys and girls from these sources; thus, this dietary transition appears to have happened around the same time for all individuals.
Ethnographic studies of social organization in American Samoa suggest additional age-linked transitions in diet as individuals moved from adolescence into adulthood. Within households, extended families ate evening meals together, but with a particular order in which people took food from the day's harvest. Gardner (Reference Gardner1965:14–16) observed that male heads of house typically ate first, followed by other adults and only then children. Likewise, Mead (Reference Mead1928:18) suggested a similar ordering, with adult males eating first, followed by women and children and finally boys (presumably adolescent boys). This partitioning in the timing of eating often resulted in many of the most desirable foods being consumed first, with lower-status foods left for children, especially younger boys and girls. These dietary differences, especially in protein intake, have been recorded in more recent studies in American Samoa as well (e.g., Bindon Reference Bindon1984, Reference Bindon1986). As a result, there was significant variation in the composition of diet by age and sex within households, especially during evening meals.
Of course, not all foods were consumed during evening meals. “Snacking” while foraging is commonly observed by anthropologists during field studies and can contribute significant numbers of calories in the diets of individuals (Ladio and Lozada Reference Ladio and Lozada2001:368; Lupo Reference Lupo2006:39; Stearman Reference Stearman1991:253). Thus, an evaluation of foraging behavior is also warranted, especially because many researchers observed that foraging efforts were also partitioned by age and sex in American Samoa.
In a detailed survey on American Samoa in 1975, Hill (Reference Hill1978) documented significant age-grading in participation in subsistence activities involving marine environments. Overall, he found that males were engaged in these activities far more often than females (more than 1,100 observations over a six-month period). Of the children under age 15 years, 70% of those harvesting food from marine environments were boys; of adolescents (age 15–20 years), 75% were male; and of adults (age 21–60 years), 69% were male. The only age category where females outnumbered males in these activities was among elderly adults over the age of 60 years, where just 37% were male. The specific types of activities undertaken by the sexes also varied and changed with age categories. Females predominantly engaged in “gleaning,” defined as the gathering of sessile animals, including echinoderms, mollusks, and crustaceans. Males engaged more often in fishing, either by diving (spearfishing), hook-and-line, or using boats and nets. Young boys under age 15 years tended to engage more in gleaning (i.e., typically “female” activities) but transitioned to more fishing, or male activities, with age. Mead (Reference Mead1928) made similar observations on sex and age-linked foraging behaviors, though far less detailed and nonquantitative in nature.
Hill's (Reference Hill1978) survey also showed that the likelihood of participation in marine-focused subsistence activities was initially low for boys and girls, then increased with age through early adult years, after which it decreased again. The magnitude of increase, however, was less for females than for males, and the timing of the decrease in adulthood was earlier for males. In other words, adolescent boys and young men rapidly increased investment in fishing activities early in life but after reaching early adult years shifted most of their labor to tending gardens and raising domesticated animals (Bindon Reference Bindon1988; Holmes Reference Holmes1974). By contrast, a higher percentage of adult women continued to engage in fishing long after adolescence. Although these adult women contributed to garden production by weeding and harvesting, the success of a garden was said to depend mainly on male labor input (Bindon Reference Bindon1988:63). Thus, many of the high-status foods, especially pigs, were said to derive from the labors of adult males.
If snacking was important during foraging efforts, we would expect boys and girls to synchronously increase their marine food intake after early childhood. Fully adult males should decrease their marine intake as they shifted their labor efforts to tending gardens and domesticated animals. Likewise, the snacking diets of females should be focused more on lower-trophic-level sessile invertebrates, rather than on higher-trophic-level reef fish, well into adult years.
In sum, if precontact populations followed a similar dietary life history as described in ethnographic times, then we expect males and females to start life with identical diets (breast milk) and continue with similar diets during childhood, where the majority of solid foods were provisioned within the household. In adolescence, we expect a divergence by sex, where male diets should be focused more on marine fish and female diets more on marine invertebrates. Finally, as adults, males should shift toward a more terrestrial-focused diet, while females may shift slightly in this direction but maintain a greater marine focus than males.
Materials
The human remains in this study derive from various archaeological excavations undertaken by the American Samoa Power Authority (ASPA) in the villages of Fatu-ma-futi, Lauli'i, Ili'ili, Auto, and Pavai'ai (Bartelink and Johnson Reference Bartelink and Johnson2014), although a few burials from Fatu-ma-futi village were originally excavated through a cultural resource management firm in 2004 during a road improvement project (Kailihiwa et al. Reference Kailihiwa, Beck and Cleghorn2005). The majority of human remains were located as inadvertent discoveries during ASPA archaeological monitoring for various construction projects, including the installment of sewer lines and a septic tank. For each village, the ASPA principal investigator received permission for bioarchaeological analyses of human remains by landowners in consultation with village leadership. Table 1 lists the individuals included in this study, the tooth sampled, the number of dentin samples from that tooth, and demographic information for the individual. Stable isotope analysis on bone collagen from some of the same individuals we sampled was previously reported by Valentin and colleagues (Reference Valentin, Herrscher, Petchey and Addison2011). We resampled these individuals to extract bone and tooth collagen for stable isotope analysis to ensure that the tooth samples came from the same individual as the bone collagen and that data are directly comparable.
Table 1. Individuals Included in This Study and Associated Demographic Information.

Note: AMS = accelerator mass spectrometry. Unless otherwise noted, AMS samples submitted by P. R. Johnson and Q. Winterhoff (American Samoa Power Authority) to Waikato Radiocarbon Dating Laboratory.
a Waikato Radiocarbon Dating Laboratory dates reported in Valentin et al. (Reference Valentin, Herrscher, Petchey and Addison2011).
b Beta Analytic dates reported in Kailihiwa et al. (Reference Kailihiwa, Beck and Cleghorn2005).
The majority of the samples (9 of 16 individuals) come from the site of Fatu-ma-futi, a coastal habitation site with significant accumulations of shellfish and fish (Addison et al. Reference Addison, Walter and Morrison2008; Morrison and Addison Reference Morrison and Addison2008, Reference Morrison and Addison2009). Additional samples come from the coastal sites Lauli'i (n = 2) and Auto (n = 1). The remaining four samples are from slightly inland sites; one is Pavai'ai (n = 3), about 3 km from the coast, and the other is Ili'ili (n = 1), about 2 km from the coast (Addison et al. Reference Addison, Tago, Toloa and Pearthree2006). While the earliest known evidence of human occupation of Tutuila derives from site components currently dated to around 2500 BP (Addison and Asaua Reference Addison and Asaua2006; Addison et al. Reference Addison, Tago, Toloa and Pearthree2006; Cochrane et al. Reference Cochrane, Rieth and Dickinson2013; Rieth and Hunt Reference Rieth and Hunt2008; Rieth et al. Reference Rieth, Morrison and Addison2008), all individuals in the study date to between 1200 and 100 cal BP, as demonstrated by direct radiocarbon dates on human bone collagen (Table 1). The distribution of the radiocarbon dates in our sample does suggest some degree of clustering, with an older group of individuals dating between 1070 and 950 BP (n = 7) and a younger group between 530 and 120 BP (n = 9).
Methods
Human teeth grow in thin incremental layers, accruing dentinal tissue from the dentino-enamel junction apically over time (Hillson Reference Hillson1996, Reference Hillson2014). Each increment forms over a number of weeks to months as tissues are initially deposited and subsequently mineralized. Once deposited, these tissues do not remodel, recording isotope ratios within the tooth increments.
The ontogenetic age at which a tooth forms varies significantly by tooth type and position. In this study, we examine permanent first molars and third molars. First molars begin forming around the time of birth until about age 9.5, when the apical root closes. Third molars begin forming between 7 and 10 years of age and continue growing through age 18 to 25 years, until the root apices close (Hillson Reference Hillson1996, Reference Hillson2014).
Within a tooth it is possible to further isolate tissues that grew over different windows of time by sectioning the tooth along the axis of growth and measuring stable isotope composition within each section (e.g., Beaumont et al. Reference Beaumont, Gledhill, Lee-Thorp and Montgomery2012; Beaumont et al. Reference Beaumont, Montgomery, Buckberry and Jay2015; Burt Reference Burt2015; Eerkens and Bartelink Reference Eerkens and Bartelink2013; Eerkens et al. Reference Eerkens, Berget and Bartelink2011; Fuller et al. Reference Fuller, Richards and Mays2003). By analyzing serial samples, it is possible to reconstruct isotopic changes in diet across successive windows of time for a particular individual. Using first and third molars, we can trace paleodiet across the first and second decade of life, respectively.
Collagen extraction followed a modified Longin (Reference Longin1971) method. Tooth serial sampling follows procedures established by Eerkens and colleagues (Eerkens et al. Reference Eerkens, Berget and Bartelink2011; Eerkens et al. Reference Eerkens, Sullivan and Greenwald2016). Briefly, a tooth was cleaned with a small brush of any adhering soil or other exogenous material, sonicated in deionized water (dH2O), and cut in half longitudinally (i.e., crown to root) with a slow-speed diamond-coated saw. All cementum and enamel was removed, and the pulp chamber was reamed out from one half of the tooth using a handheld drill to remove secondary dentin. This tooth half was demineralized in a solution of 0.5M HCl in a refrigerator set at 5 °C. HCl was changed every other day until the sample was completely demineralized (generally one–two weeks).
The tooth was then rinsed with dH2O and sliced into parallel, horizontally oriented serial sections, beginning at the apical root tip and working up toward the crown. These cuts are parallel to growth layers within the crown but cut across diagonal growth layers in the root (see Eerkens et al. Reference Eerkens, Berget and Bartelink2011). Because layers accumulate in a cone-like manner within the root, we were unable to manually cut cones out of the demineralized root but had to cut horizontally across growth planes. As a result, adjacent serial samples in the root include some material from the same layers of growth (i.e., adjacent sections do not represent mutually exclusive temporal windows). This will cause fluctuations of stable isotope data to be somewhat smoothed within increments of the root sections. We estimate that every other sample within the root has less than 10% of the same (synchronous) material, by volume. The number of serial sections produced varied slightly by tooth depending on the degree of occlusal wear, as well as the size, length, and structure of the tooth.
Following demineralization, any secondary dentin that was not reamed out during drilling was also removed manually (typically it separates from the primary dentin). Following sectioning, each serial sample was placed in a separate vial and immersed in 0.125M NaOH for 24 hours to remove humic acids. The sample was rinsed with dH2O to remove any residual NaOH and placed in slightly acidic pH3 water in an oven set to 70 °C to solubilize collagen. Solubilized collagen was then freeze-dried to remove all remaining water, isolating the collagen fraction.
Approximately 1.0 mg of collagen was weighed out from each serial section for stable isotope analysis. In some cases, there was not enough collagen from a serial sample, and one or two adjacent sections were combined to achieve a total of 1 mg. Carbon (13C/12C) and nitrogen (15N/14N) isotope ratios for each serial sample were measured by continuous-flow mass spectrometry (PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer) at the Stable Isotope Facility, University of California, Davis. Carbon isotope ratios are reported using the delta notation (δ13C) and expressed in parts per thousand (permil, ‰) relative to the Vienna Pee Dee Belemnite standard (arbitrarily set at 0‰). Similarly, nitrogen isotope ratios are reported using the delta notation (δ15N) and expressed against N2 in modern atmospheric air (also arbitrarily set to 0‰). Repeated analyses of standards with known isotopic composition show that internal instrument precision is less than 0.1‰ for δ13C and less than 0.2‰ for δ15N.
In paleodietary studies, carbon isotopes provide an estimate of the consumption of C3 versus C4 plants and marine foods (Cerling et al. Reference Cerling, Ehleringer and Harris1998; Ehleringer et al. Reference Ehleringer, Sage, Flanagan and Pearcy1991; Farquhar et al. Reference Farquhar, Ehleringer and Hubick1989). In American Samoa, δ13C primarily provides an estimate of the importance of marine versus terrestrial foods (Schoeninger et al. Reference Schoeninger, DeNiro and Tauber1983). C4 plants, such as sugarcane (Saccharum officinarum), may have been available prehistorically, although conclusive evidence is lacking for American Samoa. Furthermore, sea grapes (Caulerpa racemosa), a type of macro-algae, follow a C4 pathway (Casu et al. Reference Casu, Ceccherelli, Sechi, Rumolo and Sara2009) and were likely consumed in prehistoric American Samoa. Despite the potential availability of these C4 resources, they are poor sources of dietary protein and thus are expected to be underrepresented in collagen isotope signatures (Leach et al. Reference Leach, Quinn and Lyon1996). Nitrogen isotopes reflect the general trophic level of consumed foods. Nitrogen fractionates during the synthesis of biological tissues, favoring the retention of the heavier 15N. δ15N increases by about 3‰–6‰ with each trophic level (Minagawa and Wada Reference Minagawa and Wada1984; O'Connell et al. Reference O'Connell, Kneale, Tasevska and Kuhnle2012; Schoeninger Reference Schoeninger1985). In most island terrestrial systems, there are three trophic levels—plants, herbivores, and carnivores. In aquatic environments there are more trophic levels, resulting in greater enrichment of 15N at the top of the food chain (Minagawa and Wada Reference Minagawa and Wada1984; Schoeninger Reference Schoeninger1985). The latter include large fish, predatory birds, and aquatic mammals.
In humans, collagen is synthesized mainly from consumed protein (Ambrose and Norr Reference Ambrose, Norr, Lambert and Grupe1993; Kellner and Schoeninger Reference Kellner and Schoeninger2007; Tieszen and Fagre Reference Tieszen, Fagre, Lambert and Grupe1993). A recent study estimates that 72% of the carbon in collagen comes from dietary protein, with the remaining 28% derived from carbohydrates and lipids (Fernandes et al. Reference Fernandes, Nadeau and Grootes2012). Combined, δ13C and δ15N of human collagen can be used to discriminate foraging patterns, especially for dietary protein, in different environments. In general, foraging in coastal environments leads to elevated δ13C values. Because nitrogen isotopes fractionate with each trophic level and marine environments tend to have more trophic levels, δ15N values also tend to be elevated in these settings. By contrast, foraging in terrestrial environments leads to lower δ13C and δ15N isotope values, and brackish water and some reef environments lead to intermediate, but still distinctive, values (Eerkens et al. Reference Eerkens, Mackie and Bartelink2013).
A comparison of published bone collagen stable isotope values among different Pacific Island groups from Fiji, Solomon Islands, Vanuatu, Rapa Nui, and Tonga shows distinctive ranges of δ13C and δ15N values for many island populations. Figure 2 plots δ13C and δ15N for six different populations (data from Commendador et al. Reference Commendador, Dudgeon, Finney, Fuller and Esh2013; Kinaston et al. Reference Kinaston, Buckley and Gray2013; Kinaston et al. Reference Kinaston, Bedford, Richards, Hawkins, Gray, Jaouen, Valentin and Buckley2014; Stantis et al. Reference Stantis, Buckley, Kinaston, Nunn, Jaouen and Richards2016; Valentin et al. Reference Valentin, Buckley, Herrscher, Kinaston, Bedford, Spriggs, Hawkins and Neal2010; Valentin et al. Reference Valentin, Herrscher, Petchey and Addison2011). Inhabitants of Tutuila, American Samoa, have lower δ13C and δ15N values compared with other island groups (Figure 2). This is consistent with the general interpretation that American Samoan diets were focused more on lower-trophic-level terrestrial foods, especially C3 plants, rather than on marine resources. Such foods included common C3 plant domesticates (bananas, taro, coconuts, breadfruit, yams), as well as domesticated animals (pig, dog, and chicken) and some wild animals (frugivorous bats) that feed on local terrestrial plants. The smaller marine component of the diet would have included reef and pelagic fish, shellfish, crustaceans, and sea turtle.
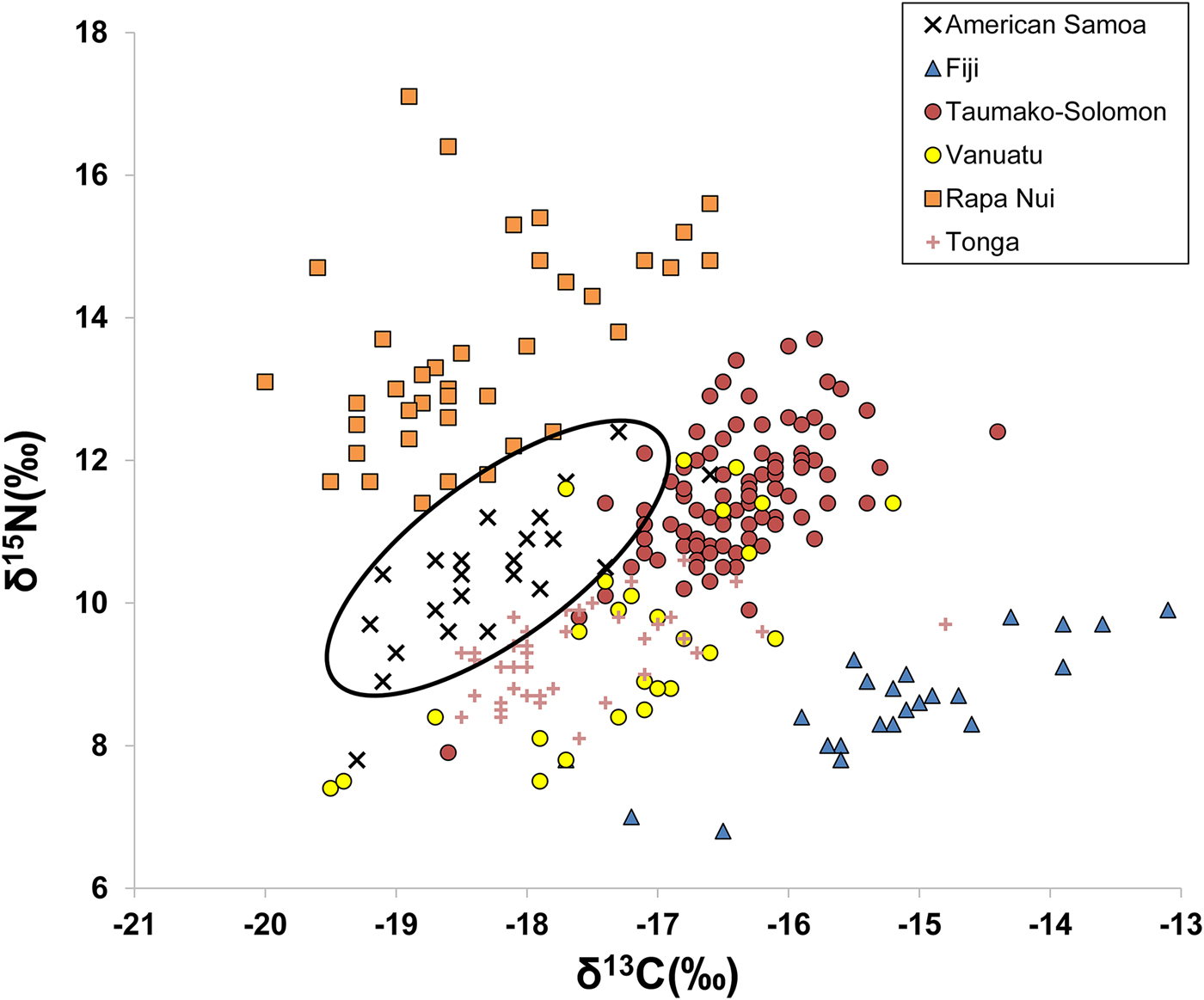
Figure 2. δ13C and δ15N from bulk bone collagen reported in six previous Pacific Island studies (Commendador et al. Reference Commendador, Dudgeon, Finney, Fuller and Esh2013; Kinaston et al. Reference Kinaston, Buckley and Gray2013; Kinaston et al. Reference Kinaston, Bedford, Richards, Hawkins, Gray, Jaouen, Valentin and Buckley2014; Stantis et al. Reference Stantis, Buckley, Kinaston, Nunn, Jaouen and Richards2016; Valentin et al. Reference Valentin, Buckley, Herrscher, Kinaston, Bedford, Spriggs, Hawkins and Neal2010; Valentin et al. Reference Valentin, Herrscher, Petchey and Addison2011), with ellipse highlighting the distinctive American Samoa signature.
One additional potential source of isotopic variation is artificial manipulation of soils by humans, for example, through fertilizing agricultural fields with exogenous materials. Thus, Jarman and colleagues (Reference Jarman, Larsen, Hunt, Lipo, Solsvik, Wallsgrove, Ka'apu-Lyons, Close and Pop2017) suggest that the higher δ15N but lower δ13C values observed among humans from Rapa Nui are due to the addition of guano, household waste, and/or lithic mulch to gardens. Lacking other sources of information, it is difficult to account for these practices on a site-by-site basis. In this study, we assume that fertilizing practices were similar between different sites and time periods, at least on Tutuila. As a result, all individuals on the island should have experienced similar baseline δ15N and δ13C, and variation in these values in collagen should reflect differences in diet, not differences in field fertilization. We acknowledge this shortcoming and suggest that future research examine whether fertilization practices varied over space or changed over time on the island (see also Quintus and Cochrane Reference Quintus and Cochrane2017).
Relevant to the discussion below, in an analysis of archaeological burials from American Samoa, Valentin and colleagues (Reference Valentin, Herrscher, Petchey and Addison2011) report that the mean bulk δ13C in the bone collagen of adult females is −18.2 ± 0.5‰ (one standard deviation; n = 8), while bulk δ15N values have a mean of 10.7 ± 0.6‰ (one standard deviation). Males are similar, with a mean δ13C of −18.6 ± 0.6‰ (one standard deviation; n = 8) and a mean δ15N of 9.6 ± 0.9 (one standard deviation). Furthermore, although the number of radiocarbon-dated burials was small (n = 12), the Valentin study suggested a slight change in stable isotope values over time. Individuals dating around 1000 BP seem to show slightly higher δ13C compared with those dating younger than 600 BP, suggesting a shift from a more marine-focused to a more terrestrial-focused diet over time.
For the current study, we have expanded the number of burials sampled from Tutuila. In many cases, we resampled the same individuals reported in Valentin and colleagues (Reference Valentin, Herrscher, Petchey and Addison2011) in order to ensure that tooth samples were directly associated with bone (and radiocarbon dates). Because many of these burials were commingled, we were able to identify and sample additional individuals not included in the Valentin study. We were also able to sample several additional burials housed at the American Samoa Power Authority, as well as four burials that were on loan through Texas A&M University from an earlier excavation project at Fatu-ma-futi. To ensure data comparability, we use our complete analyzed dataset from human bone collagen to compare with our dentin serial samples.
Results
Table 2 provides all the stable isotope values for the 100 serial samples. No obvious dietary differences are apparent between the two slightly inland sites (Ili'ili and Pavai'ai) and the coastal sites (Lauli'i, Auto, and Fatu-ma-futi). For example, the average values in third molars of the two inland females for δ13C (−17.3‰) and δ15N (12.4‰) are similar to those for three females from coastal villages (δ13C = −17.6‰; δ15N = 12.0‰). Likewise, one inland male averages −17.4‰ and 11.4‰ for δ13C and δ15N, respectively, while four coastal males average −18.1‰ and 11.3‰ for δ13C and δ15N.
Table 2. δ13C, δ15N, and C/N for All Serial Samples in the Study, along with Median Estimated Age for Each Section.

In addition, there do not appear to be significant differences in isotopic values by archaeological age. If we group the burials by those dating older than 900 BP versus those younger than 600 BP, similar to the temporal division suggested by Valentin and colleagues (Reference Valentin, Herrscher, Petchey and Addison2011), we do not see significant isotopic changes across time. Thus, δ13C and δ15N values in third molars for females dating older than 900 BP are −17.6‰ and 12.0‰, respectively, while females younger than 600 BP show average values of −17.3‰ and 12.4‰. Likewise, males dating older than 1000 BP show average values of −18.2‰ and 11.5‰, while males dating younger than 600 BP show average values of −18.0‰ and 11.3‰ for δ13C and δ15N, respectively. Note that these results do not necessarily indicate a lack of dietary change through time in Tutuila. The results only suggest that any such effects on collagen stable isotope signatures are minor when compared with other sources of variation. However, the dietary shift reported by Valentin and colleagues (Reference Valentin, Herrscher, Petchey and Addison2011) toward the increased use of terrestrial resources through time is not supported by our expanded dataset.
In short, the minor differences by site and archaeological age explain only a small percentage of the total isotopic variation in our dataset. Instead, the major sources of variation appear to be by sex and tooth type. Because they represent different periods in life, we separate reporting of results by tooth type below.
First Molars
The five permanent first molars provide estimates on infant and early childhood dietary patterns (Figure 3). Two of the individuals from Fatu-ma-futi (B5 and B6) died at approximately two years of age. These teeth were incomplete at the time of death and only include the earliest-forming part of the crown, and the serial samples represent less than two years of diet. Both individuals display elevated δ15N values, 3.3‰ and 4.5‰ higher than the mean value (10.6‰) of bone collagen among adult females from American Samoa. Such a signature is consistent with one full trophic level above adult values, as would be expected for an infant who was receiving all of his or her protein through breastfeeding. δ15N values do not decrease significantly throughout the serial sections, suggesting that both toddlers were still receiving most or all of their protein from breast milk at the time of death.

Figure 3. δ15N in permanent first molar serial sections for five individuals from American Samoa, plotted by estimated median age of each section.
The other three individuals had more complete first molars, with dentin extending into the roots, facilitating the reconstruction of longer dietary life histories. Still, one individual from Fatu-ma-futi (Feature 2) and one from Pavai'ai (B1B) died between six and eight years of age, before the apical root closed, and our serial samples extend out to only age 5.5 and 6.0 years, respectively. The third individual, from Auto (B2A), had a complete first molar and had lived into adulthood. The teeth from these three individuals also show elevated δ15N values in the coronal dentin but much lower values within the lower sections of the crown and into the root. This is consistent with a weaning process. From these results, we conclude that the three individuals were completely weaned before ages 4.0, 4.4, and 1.4 years, respectively (mean = 3.3 years).
By contrast, intra-M1 and interindividual variation is less pronounced for δ13C. The mean intratooth range (maximum-minimum) for δ13C is just 0.6‰, compared with 1.3‰ for δ15N. Likewise, the standard deviation of intratooth averages, a measure of interindividual variation, is 0.5‰ for δ13C, compared with 1.4‰ for δ15N. The lower variation in δ13C suggests a fairly steady and consistent mixture of terrestrial and marine-derived protein for all children in the sample. Relative to adult bone collagen values with an average of −18.3‰, δ13C is elevated in M1 teeth, averaging −16.9‰. This suggests that children consumed higher amounts of marine-derived protein compared with adults in the sample.
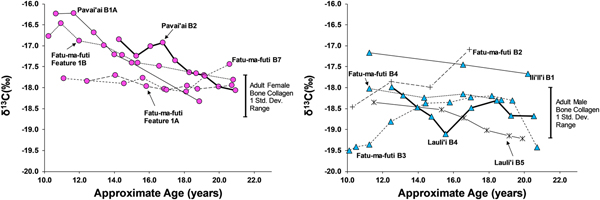
Third Molars
Eleven third molars provide additional evidence on dietary patterns during the second decade of life on American Samoa. As shown in Figures 4 and 5, there is a clear difference between males and females during this window of life, especially for δ13C values, which are elevated for females, particularly between 10 and 15 years of age. While male δ13C values are relatively stable during the second decade of life, δ13C values decrease notably with age for females, only approaching the male average around 20 years of age. This suggests that the dietary pattern observed in first molars, where children seem to consume greater amounts of marine foods, changes for males around age 10 but continues for females throughout much of the second decade of life. Given the overall sex-linked dietary patterns evident in the data, the two individuals that are indeterminate for sex (Burial 5 from Lauli'i and Burial 2 from Fatu-ma-futi; plotted with males in Figures 4 and 5) have a more male-like dietary pattern.
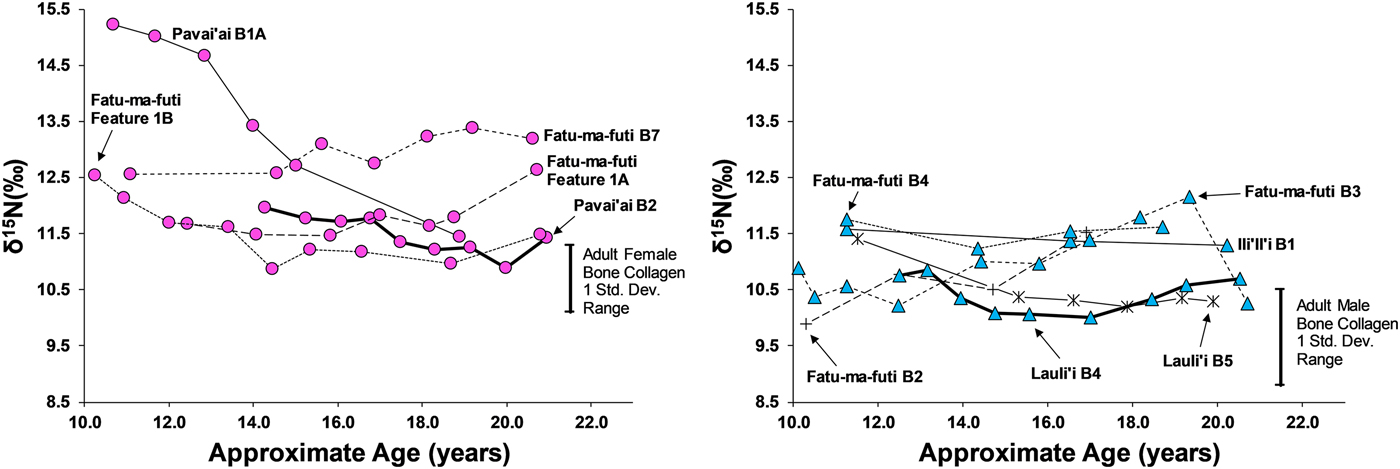
Figure 4. δ15N in serial sections of third molars from American Samoa for females (left) and males and suspected males (right), with one standard deviation range for adult male and female bone collagen.
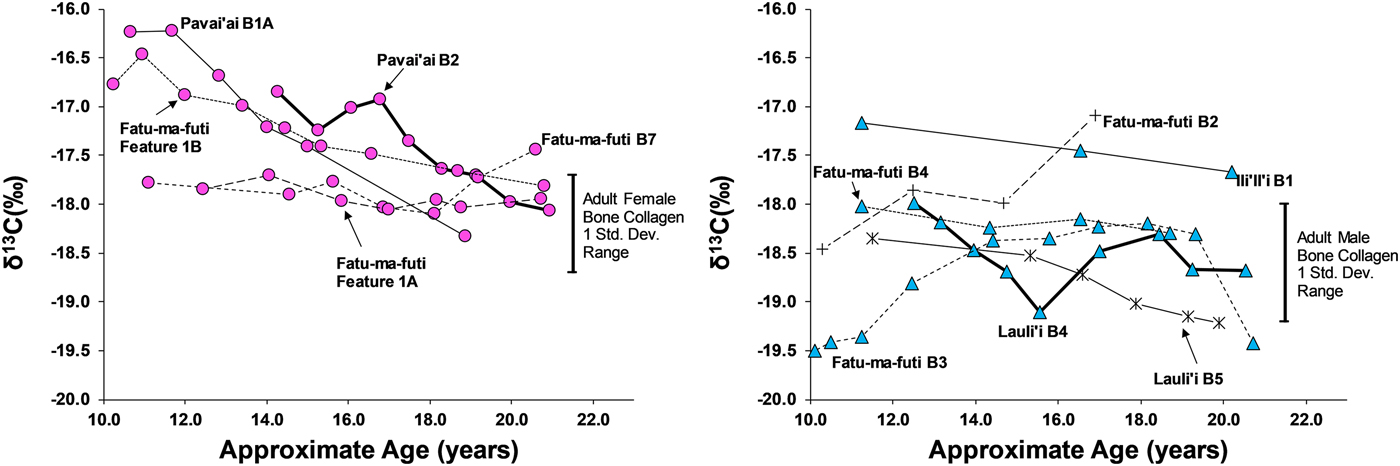
Figure 5. δ13C in serial sections of third molars from American Samoa for females (left) and males (right), with one standard deviation range for adult male and female bone collagen.
Discussion
Results from the present study did not identify a significant dietary change between 1200 and 100 cal BP, suggesting that isotopically similar food resources were consumed through time. Contrary to previous isotope data on a smaller set of samples suggesting that human paleodiets became more terrestrially focused over time in Tutuila (Valentin et al. Reference Valentin, Herrscher, Petchey and Addison2011), our expanded isotope sample suggests dietary stability between 1200 and 100 BP. Some zooarchaeological studies from the Samoan archipelago have also failed to identify significant changes in faunal assemblages, implying that human populations did not have a large negative impact on the relative abundance or diversity of species (Morrison and Addison Reference Morrison and Addison2008, Reference Morrison and Addison2009; Nagaoka Reference Nagaoka, Kirch and Hunt1993). This was true of both fish and invertebrate species (shellfish), which showed few changes in relative abundance and species diversity through time. However, Morrison and Addison (Reference Morrison and Addison2009) found evidence for a significant decline in the trophic level of fish species at Fatu-ma-futi between 300 and 100 BP, which could reflect human-induced harvest pressure or could have been caused by environmental changes. A fruitful line of research for the future would be to further test this proposed diachronic dietary change (or lack thereof) by examining isotopes in samples from additional sites on the island.
We were able to estimate a maximum age for the termination of breast milk for three individuals on Tutuila, American Samoa. Based on a drop in δ15N values from the coronal to root sections, we estimate the weaning age at 1.4, 4.0, and 4.4 years for these three individuals. Although the sample size is small, these figures are somewhat later than the average weaning age of one–two years reported in studies in the mid-twentieth century (Bindon Reference Bindon1984, Reference Bindon1986; Gardner Reference Gardner1965:29; Nardi Reference Nardi1984). Instead, the reconstructed ages from archaeological samples are more in line with historical memory on the island, where weaning was typically after age two years (Malcolm Reference Malcolm1954; Mead Reference Mead1928). This suggests some discordance between breastfeeding behaviors in the last century with those earlier in time.
We did not sample enough first molars of individuals of known sex to determine whether the diets of young boys and girls less than 10 years of age were similar (as we had expected based on the ethnographic model). However, results from the study do suggest significant differences in the diets of early adolescent males and females. Thus, by age 10 years, there is a significant isotopic difference, where females are notably higher in δ13C and δ15N, suggesting a much greater role of marine foods in the diet of these juvenile girls. Further, the data show that the male-female difference in diet decreased throughout the second decade of life, but there was still a notable gap, even by age 20 years, where young women were still consuming greater amounts of marine food than men.
This archaeological pattern contrasts somewhat with historic and ethnographic accounts. Based on foraging behaviors and accounts of eating order within households, we had expected maturing boys (ca. ages 10–15) to be consuming greater amounts of marine food, similar to girls of that age. Instead, by age 10 most boys had transitioned to a diet lower in δ13C and δ15N, close to that of fully adult males, suggesting that they were already eating higher amounts of terrestrial food. Although there is interindividual variation in the diets of adolescent-aged males, this pattern was relatively stable through age 20 years (i.e., no noticeable shift with age for males between ages 10 and 20 years). If fishing and gleaning were an important part of the foraging behaviors of these maturing males, they do not seem to have been consuming a significant amount of the by-products of their labor.
Females, on the other hand, did show higher δ13C around age 10 years, as expected from the ethnographic model. However, throughout their second decade of life these maturing females decrease in δ13C and δ15N, suggesting a slow increase in terrestrial foods. By age 20 years, females are close to the bone collagen values of fully adult females for δ13C but still notably higher for δ15N. Based on the ethnographic model, where adult females continue to invest heavily in gleaning, we had expected adult females at age 20 years to remain above their male age cohort in δ13C.
Together, the archaeological patterns suggest either that precontact activity patterns were different from ethnographic ones (i.e., boys and adult women did much less fishing in the past) or that harvested food and labor investment was discordant from actual diet and, hence, that there was widespread food sharing. There is much historical and ethnographic support for the latter. Detailed studies show significant redistribution of food within extended families or villages in the region (Bell Reference Bell1931; Holmes Reference Holmes1957:312). This result also suggests that snacking explains very little of the age-sex variation in stable isotope results.
At the same time, we did document significant differences in the diets of young adolescent girls versus boys. This general pattern is shown in Figure 6. Thus, while life on Tutuila began and ended with similar dietary compositions, breast milk early in life and a terrestrial focus later in life, in between there was a marked difference in the role of marine versus terrestrial foods. Around age 10 this difference seems to have been greatest. If there was an age-sex bias in the order of food consumption within the household, and if marine foods were of lower status, this suggests that adolescent girls likely ate last and were of lowest status within the household. With age, the status gap decreased between males and females. Yet, even by 20 years of age, when many of the women were presumably starting their own families, there still existed a slight difference in diet by sex between individuals from the same age cohort.

Figure 6. Generalized ontogenetic dietary profile for American Samoan males and females.
Conclusions
We document a particular dietary life history for ancient American Samoa society wherein individuals transition from breast milk to greater incorporation of marine-derived foods and to consumption of more terrestrial-derived foods. This dietary life history seems to be accelerated for males relative to females. This finding contrasts slightly with ethnographically described labor life histories for individuals. Those studies suggest increased investment in fishing on the part of adolescent and young adult males and a lifelong focus on the gathering of sessile marine foods by females. Instead, the stable isotope data show less consumption of marine foods among adolescent and young adult males and fully adult females. In other words, assuming there is a direct correlation between diet and labor, the precontact diet does not seem to follow precisely the historic descriptions of sex- and age-based labor practices.
This result could represent, in part, a bias on the part of the ethnographic studies we consulted, which were, largely by design, focused on fishing and the exploitation of marine environments, rather than gardening and animal husbandry. On the other hand, the result may also reflect a key dynamic of precontact Samoan social organization, namely, the pooling and redistribution of foods within households. We expect discordance between labor efforts and diet when individuals specialize in exploiting particular environments for foods but give up and redistribute the fruits of their labor within sharing or exchange networks. In other words, the diet of individuals does not correlate directly with their daily subsistence activities when there is significant food sharing. As discussed above, ethnographic descriptions of day-to-day meals on American Samoa support such a redistributive system, with different foods tied to rank and feeding order within a household, rather than day-to-day subsistence activities.
Along these lines, because social status within the household was typically marked by greater access to certain foods (e.g., especially terrestrial-based foods such as pig), and these higher-status terrestrial foods contrast isotopically with lower-status marine foods, serial samples in teeth may provide insight into the ages at which males and/or females transitioned in their social rank within households. In particular, shifts from less negative (i.e., higher) to more negative (i.e., lower) δ13C values on Tutuila may signal a change in social status and an earlier eating order position within the household. Likewise, differences between males and females in the ontogenetic timing of such shifts may reflect sex-biased access to social status and may be a means to reconstruct patriarchal or matriarchal social organizations in ancient societies.
Given the multitude of factors that play into the isotopic composition of diet (i.e., residence shifts, changing environment, etc.), it will always be important to contextualize serial samples within sites and to particular individuals. That is, we do not believe that serial samples, by themselves, will directly indicate social status or changes therein. Rather, combined with other contextual information from burials, such data might help delineate life history transitions for particular individuals.
In summary, rather than them being static, we record significant intra-individual variation in the isotopic composition of paleodiets in permanent first and third molars from American Samoa. Analysis shows that age and sex explain a large proportion of this isotopic variation. We interpret these findings within the context of life history processes, especially weaning and social status, that affected access to different foods for individuals. While the overall life history trajectory is similar, from breastfeeding, to a diet higher in marine foods, to a diet focused on terrestrial foods, the male life history appears to be accelerated relative to that of females. This finding may indicate earlier access to higher social status rungs for males.
Acknowledgments
We thank Dr. Pat Barker for discussions regarding ethnographic dietary patterns in American Samoa and his insights regarding status and eating order. We also thank Alex Greenwald for assistance in preparing teeth samples for analysis; Joy Matthew of the University of California, Davis, Stable Isotope Facility for help in analyzing collagen samples; and the American Samoa Power Authority for its support of this research. We thank Edgar Huerta for providing the Spanish abstract. Finally, we thank the peer reviewers and editor for their suggestions, which have greatly improved this article.
Data Availability Statement
All original data for this study are presented in Tables 1–2 within the body of the article.