Introduction
CHD, the most common birth defect diagnosed in infants, is present in approximately 40,000 live births annually in the United States of America. One-quarter of infants diagnosed have critical CHD and are hospitalised after birth or within the first few months for surgical and medical management. 1,2 Following cardiac surgery, infants with critical CHD are at risk for neurological and motor abnormalities that impact muscle tone, active movements, and motor skill development, and roughly one-third have delayed motor skills that can perpetuate as they age, with the most severe impairments seen in infants and very young children. Reference Stegeman, Sprong and Breur3–Reference Butler, Sadhwani and Rofeberg7 At school entry, compared to children born very preterm, children with critical CHD are one-and-a-half times more likely to have motor delays. Reference Wehrle, Bartal and Adams8 Comparatively, studies have found that children and adolescents with critical CHD tend to have motor scores that are about one standard deviation lower than those of typically developing peers. Reference Bolduc, Dionne, Gagnon, Rennick, Majnemer and Brossard-Racine6
The aetiology of motor skill delays seen in infants with critical CHD is multifactorial. Prenatally, infants with critical CHD often exhibit brain dysmaturation, growth abnormalities, and genetic co-morbidities. Reference Jansen, Blom and Haak9–Reference Thomason, Hect and Waller14 After birth, infants with critical CHD are at an increased risk of neurologic injury and events (e.g., seizure, hypotonia, hypertonia, and stroke) in the peri-operative period, associated with a higher risk of long-term motor delays. Reference Liamlahi and Latal15 Noxious environmental stimuli, painful procedures, sleep deprivation, and invasive lines and tubes impact infants’ brain development and affect their ability to calm, self-regulate, and maintain an optimal behavioural state for motor learning. Reference Cong, Wu and Vittner16 Further, a longer hospital stay predicts poor motor performance in infants independent of other risk factors, and infants with critical CHD who are hospitalised for more than 2 weeks are at increased risk for compounding global neurodevelopmental and motor delays. Reference Subedi, DeBoer and Scharf17–Reference Gaynor, Stopp and Wypij19
With the knowledge of these ongoing motor delays, there is a focus on optimising motor outcomes through early developmental therapies of occupational therapy, physical therapy, and speech-language pathology in critical and acute care settings. This article begins with an overview of typical infant motor development and then aims to describe the contributors to motor skill disruptions and the roles of developmental therapists in addressing those disruptions. Motor assessment and interventions specific to hospitalised infants with critical CHD are recommended.
Typical infant motor development
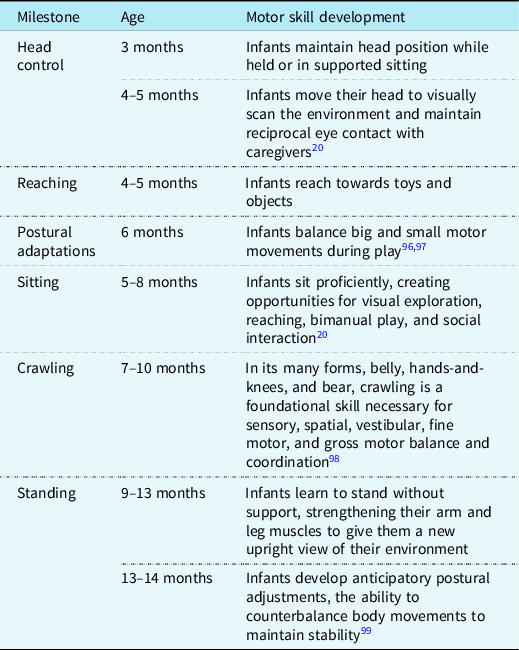
Motor development begins prenatally when a fetus is in utero. Early fetal movements are necessary for proper physical development and vital to exercise muscles, flex joints, stretch skin, and circulate amniotic fluid. Reference Adolph, Robinson and Damon20 By 16 weeks, a full fetal movement repertoire is established, which includes startles, stretches, periodic breathing, and sucking and swallowing movements, and continues throughout gestation. As gestation progresses, at 34 weeks, the fetus can coordinate the suck/swallow/breathe pattern, allowing for non-nutritive sucking and efficient breast or bottle feeding after birth. Reference Jones, Desai and Fogel21 Once born, early infant experiences profoundly affect future motor skill development, and the extrauterine environment exposes infants to a range of physical and sensory input and motor demands, which prepare them for the development of new motor skills. Postural control, the ability of the infant to maintain head and trunk stability while coordinating extremity movements against gravity, is the foundation upon which motor skill development occurs. Infants learn to maintain a stable position while performing more complex motor movements as postural control matures (see Table 1). Reference Adolph, Robinson and Damon20,Reference Dusing22 For infants with critical CHD, these early opportunities for variations in motor movements that shape future motor skills may occur in ICUs. Reference Hadders-Algra23
Table 1. Infant postural control and facilitation of motor development
Motor development interruptions in the critical care setting
During the perinatal and peri-operative period, multiple factors affect typical motor patterns and developmental experiences of infants with critical CHD. Reference Marino, Lipkin and Newburger24
Infant sensory experiences
Intrauterine fetal experiences do not prepare infants for the bright and loud intensive care setting or excessive or inappropriate social interactions. The Synactive Theory of Newborn Behavioral Organization and Development suggests that the infant is in constant interaction with the physical (i.e., temperature, lights, noise, and other sensory stimuli) and social (i.e., family, healthcare professionals, and other caregivers) environment, and development is a result of the balance and regulation between the two. Infants actively strive for the next steps in development and depend on the environment and care to promote their developmental trajectory. Reference Als25 When an infant is repeatedly exposed to ongoing environmental stressors or less than ideal caregiving, there is the potential for changes to brain development and long-term neurodevelopmental consequences. Reference Nist, Harrison and Steward26
Autonomic dysregulation, often measured via heart rate variability, is an imbalance of the autonomic nervous system and a gauge of autonomic nervous system maturity as it responds to sensory stimulation. Reference Cardoso, Silva and Guimarães27 Autonomic dysregulation increases muscle tone, decreases movement tolerance, and impacts an infant’s ability to integrate new motor experiences. Disruption of early newborn development through sensory overstimulation may negatively impact the newborn’s ability to achieve regulation of cyclical processes and integrate sensory experiences, which is crucial to achieving more complex developmental tasks and motor progression. Reference Geva and Feldman28
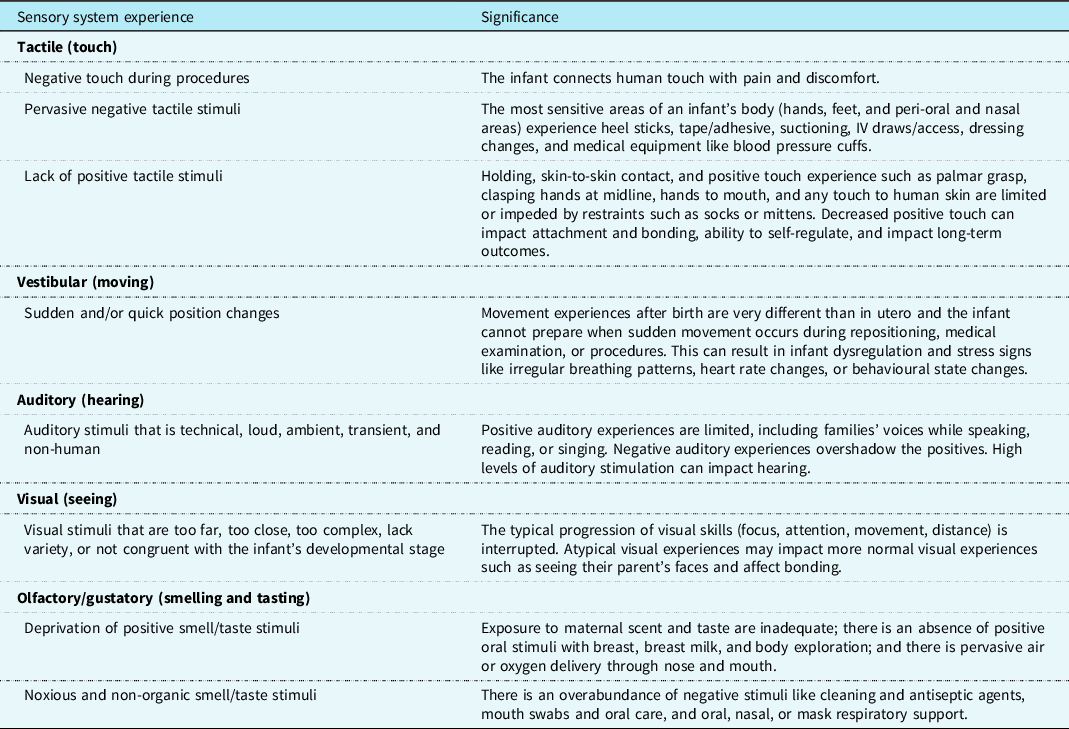
Inadequate or lack of positive sensory experiences also can lead to physiological and psychological stress for the infant. Signals of infant stress may include changes in colour or vital signs (autonomic signs of stress), disorganised patterns of body movements or sudden muscle tone changes between flaccidity and tension (motor signs of stress), or gaze aversion and irritability (state signs of stress). In the intensive care setting, infants can experience unpredictable, intense, atypical, and complex sensory stimuli (see Table 2), which is challenging for the developing sensory system to integrate. Stress can impact the infant’s regulation and motor development.
Table 2. Sensory experiences of the infant with CHD in the critical care setting
Infant movement experiences
In transitional and post-operative periods, the infant’s clinical status, or ability to maintain physiological stability, impacts caregiving such as touch, movement, and therapeutic positioning. Life-sustaining medical interventions also limit activity and movement opportunities, resulting in prolonged supine, asymmetric, or extended postures and deprivation of movement, which is adverse to the typical progression of motor sequences. Reference Sweeney and Gutierrez29 Peri-operative low cardiac output syndrome may result in physiological intolerance to movement. Delayed sternal closure limits position changes, resulting in prolonged periods where the infant is supine. Reference Pye and McDonnell30 Post-operative analgesia and neuromuscular blockage result in active movement limitations. Reference Naguib, Dewhirst, Winch, Simsic, Galantowicz and Tobias31 Often, common practice is to limit movement or stimulation to decrease autonomic instability. However, there is evidence that positive touch and gentle passive range of motion in infants following sternal closure are safe, feasible, and may have positive benefits. Conversely, non-reciprocal interactions, sudden position changes, movement without preparatory touch, and negative touch and sensory stimulation are more likely to result in irregular autonomic patterns and traumatic movement experiences for the infant. Reference Lambert, Pemberton and Trachtenberg32
Lines, tubes, and respiratory support devices are important to maintain physiological stability but also limit typical infant movement patterns within and outside the crib. Restrictive measures, such as placing socks on an infant’s hands or wrapping their arms to their sides, hinder the infant’s ability to self-regulate. By limiting their movements, they are unable to bring their hands to midline and mouth, touch their face, and soothe themselves. This can impede the infant’s ability to self-regulate and manage pain and discomfort in a non-pharmacological way. Further, improper use of positioning devices in practices that trap, weight, or limit normal movement patterns like physiological flexion, symmetry, hands to mouth and face, palmar grasp, and body movements against gravity impedes sensorimotor development, musculoskeletal integrity, and developing postural control.
Congenital heart surgery and critical CHD have many potential adverse complications, including haemodynamic instability, persistent hypoxaemia, infection, arrhythmias, cardiac arrest, seizures, and cardiovascular collapse requiring extracorporeal membrane oxygenation. Each intervention that is necessary and imperative for the infant’s survival also has resulting implications on motor function and the infant’s ability to interact with their surroundings. All complications may lead to further movement limitations, activity restrictions, decreased opportunities for normal movement patterns, and a longer length of stay in the critical care setting resulting in an increased risk for motor delays. Reference DeWitt, Rossano and Bailly33 When the infant lacks feedback from actively moving their muscles against gravity, stretching against boundaries, and coordinating successful movement patterns, there is often a resulting impact on the development of typical motor, cognitive, sensory, and self-regulation skills. Reference Field34
Neurological injury
Infants with critical CHD are subject to potential neurological insult from pre-existing brain anomalies, abnormal perfusion from cardiac physiology, hypoxia, and injury to developing brain structures. Reference Wernovsky and Licht35 During the third trimester, fetuses with some forms of critical CHD exhibit gestationally immature brain volume, weight, and development compared to those without. Reference Limperopoulos, Tworetzky and McElhinney36 Peri-operative brain injury has been identified in approximately 55% of infants with single ventricle critical CHD, primarily affecting white matter, and neurologic complications ranging from 36 to 44% inclusive of other critical CHD defects. Reference Andropoulos, Stayer, Diaz and Ramamoorthy37–Reference Beca, Gunn and Coleman39 Cardiopulmonary bypass, hypothermic circulatory arrest, extracorporeal membrane oxygenation, or other temporary circulatory support pose risks for thrombotic, embolic, or haemorrhagic neurological complications. Reference Svrckova, Meshaka and Holtrup40 Additionally, seizures from neurological injury lead to an increased risk for mortality and neurodevelopmental delays. Reference Naim, Gaynor and Chen41
Injury to developing brain structures in infants with critical CHD is linked to delayed motor performance and milestone achievement. Reference Peyvandi, Chau and Guo38 Some infants with critical CHD display neurological and motor concerns such as hypotonia, hypertonia, abnormal suck, oral motor discoordination, and asymmetrical body movements following cardiac surgery and are at significant risk of fine and gross motor delays. Reference Gorantla, Chan, Shen, Wilkes and Bratton5,Reference Butler, Sadhwani and Rofeberg7,Reference Jones, Desai and Fogel21,Reference Khalil, Suff, Thilaganathan, Hurrell, Cooper and Carvalho42 Additionally, sedatives and neuromuscular blocking agents can compromise motor tone to maintain physiological flexion or move freely, affecting the infant’s ability to calm, move to comfortable positions, and self-regulate.
Musculoskeletal integrity
Typical infant motor skill development depends on maintaining and protecting musculoskeletal structural integrity, symmetry, midline, and physiological flexion. Reference Sweeney and Gutierrez29 The hospitalised infant with critical CHD is subject to many factors that can interrupt this alignment, and musculoskeletal complications develop quickly in infants who experience movement limitations. Hospitalised infants who spend a significant time supine instead of side-lying or prone are particularly at risk. When one joint or muscle group is affected, the entire musculoskeletal complex can become stressed and misaligned.
Musculoskeletal misalignment of shoulders, hips, trunk, and head and neck asymmetry creates a cascading effect by disrupting typical motor patterns and fine, gross, and oral motor milestone attainment, for instance:
-
Shoulder immobility disrupts self-soothing skills like bringing hands to the mouth or clasping hands at midline. Reference Sweeney and Gutierrez29
-
Hip immobility and misalignment of lower extremities result in delayed gross motor transition movements and milestones such as rolling, crawling, standing, and walking. Reference Rajantie, Laurila and Pollari43
-
Extension-dominant or poorly supported physiological flexion positioning can affect oral muscle tone, result in poor proprioceptive awareness of oral structures, and increase fatigue, making it challenging for infants to latch on to a nipple for non-nutritive suck or feeding. Reference Jones, Desai and Fogel21
-
Neck hyperextension and head turn preference can lead to postural or acquired torticollis (neck range of motion limitation caused by asymmetrical positioning over time leading to a muscular imbalance), positional deformational plagiocephaly (changes in the shape and structure of the cranium, often with facial asymmetry), and exaggerated cervical spinal lordosis. Reference Sweeney and Gutierrez29 Torticollis and plagiocephaly may cause visual range of motion deficits, inner ear structural anomalies, primitive reflex interruption, compound fine, gross, and oral motor delays and are linked to brain asymmetries and worse developmental outcomes. Reference Cabrera-Martos, Valenza and Valenza-Demet44-Reference Sargent, Kaplan and Coulter46
Sternal and prone precautions
Haemodynamic instability, neuromuscular blockade, and positioning restrictions due to an open sternum or sternotomy limit movement in infants with critical CHD post-operatively. It is important to recognise the difference between sternal precautions that limit picking an infant up from under their arms or pulling on their arms, and prone precautions that restrict tummy time. Reference Clifton, Cruz, Patel, Cahalin and Moore47 There is high variability in both implementation and duration of sternal and prone precaution practices in paediatric cardiac surgical institutions across the country, and those precautions are largely based on institution-specific preferences versus evidence-based research. Reference Rodman Uher, Nguyen and Monge48
When prone positioning is restricted, it may contribute to motor delays in infants. Reference Hewitt, Kerr, Stanley and Okely49 After cardiac surgery, infants who were permitted more than 15 minutes of prone positioning daily exhibited a significantly greater motor score than those with less. Reference Uzark, Smith and Yu50 Active, awake, and engaged time in the prone position influences and even predicts the onset of walking in infants with critical CHD. Reference Dagenais, Materassi and Desnous51 Prone strengthens the upper extremity, neck, and back muscles and promotes development of the chest and rib cage, improves ability to sit and stand, and facilitates the progression of locomotive activities. Reference Hewitt, Kerr, Stanley and Okely49 Clearly defining the difference between sternal and prone precautions and encouraging evidence-based practices regarding activity limitations post-sternotomy help support earlier mobility and long-term developmental outcomes.
Contextual challenges to normal infant experiences
Parents of hospitalised infants with critical CHD face challenges in supporting their child’s motor development. Reference Mitteregger, Wehrli and Theiler52 The critical care setting creates contextual and temporal obstacles for families to fulfil their parental roles, bond with their infant, provide calming and nurturing comfort, and promote motor development. Reference Mitteregger, Wehrli and Theiler52,Reference Lisanti, Golfenshtein and Medoff-Cooper53 Challenges to touch, holding and providing sensory, motor, and social interactions with their infant may adversely impact motor development. Further, hospitalisation in a critical care environment is stressful for families and may lead to anxiety, depression, post-traumatic stress, and trauma symptoms, which are associated with poorer infant neurodevelopmental outcomes, including motor milestone achievement in infants with critical CHD. Reference Lisanti, Allen, Kelly and Medoff-Cooper54–Reference Wu, Lu and Jacobs56 Families face uncertainty, altered roles, medical complexity, and varied emotions as their infant grows. Reference Mitteregger, Wehrli and Theiler52 It is essential that families have opportunities to care for, bond with, and participate in typical parenting activities with their hospitalised infant to support normalised infant motor movement experiences. Reference Mitteregger, Wehrli and Theiler52–Reference Wu, Lu and Jacobs56
Addressing social determinants of health that affect diversity, equity, and inclusion for under-represented minority families of infants with critical CHD is necessary as they are at a higher risk for worse medical outcomes, increased re-hospitalisations, and are less likely to receive follow-up services, including developmental therapies. Reference Lopez, Baker-Smith and Flores57,Reference Neukomm, Ehrler and Feldmann58 Families face many barriers to their presence at the bedside such as financial concerns (difficulty taking time off of work, transportation, and housing near the hospital), caring for other children and family members at home, and emotional challenges (difficulty seeing child ill, mental health challenges). While there are no published studies investigating the correlation between early motor development and social determinants of health in hospitalised infants with critical CHD, it is crucial to consider the influence of these factors on infant development. Families with limited engagement at the bedside, whatever the barrier may be, likely have fewer opportunities to hold their infant, provide positive social interaction, provide breastfeeding or breast milk, and bond with their infant. These interactions and experiences are critical for promoting early motor development, as well as the overall health and well-being of the infant and family. COVID-19 increased barriers for at-risk families through visitation limits and fewer financial, medical, and psychosocial resources. Reference Cena, Biban and Janos59,Reference Litmanovitz, Silberstein, Butler and Vittner60
Supporting motor development in the hospitalised infant with critical CHD
Holistic motor support for hospitalized infants with critical CHD considers how the infant’s body systems work together, combines neuroprotective measures for physiological and developmental support, is individualized to each infant and family unit, and is understood within the context of the critical and acute care setting. Although healthcare professionals’ responsibilities may vary within and between institutions, a multi-disciplinary approach provides a robust avenue for collaboration and information sharing. Reference Miller, Lisanti and Witte61–Reference Butler, Huyler and Kaza63 All healthcare professionals providing medical and developmental care benefit from knowing infant behavioural patterns, how they impact neurobehavioural functioning, and how motor development evolves as a result. Developmental therapists work with hospitalised infants with critical CHD and with other developmental specialists like medical professionals, psychologists, child life therapists, and music therapists.
Developmental therapies
Developmental therapists are increasingly involved in the care of critically ill children with a focus on early mobility, assessing and regaining functional skills after illness or injury, and dysphagia management that interferes with safe oral intake. Reference Wieczorek, Ascenzi and Kim64,Reference Hanna, Zhao, Shannon and Betters65 Typically, developmental therapists are occupational therapists, physical therapists, and speech-language pathologists as they are specifically trained to provide neurodevelopmental, fine and gross motor, oral motor, and feeding interventions to support hospitalised infants in intensive and acute care units. Reference Ubeda Tikkanen, Nathan and Sleeper66–Reference Ross, Heiny, Conner, Spener and Pineda68 Hospitalised infants often work on recovering lost skills, along with acquiring new skills that form building blocks for future milestones while also healing from invasive interventions. Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler69 This is a habilitative approach and is implemented to support the infant’s developing brain, body, and sensory systems to encourage motor milestone development and limit deficits. Reference Craig and Smith70 Developmental therapists have in-depth knowledge of typical infant developmental milestones and trajectories, recognising that motor development is the result of behavioural patterns that build upon one another to support the infant’s progression to the next stage of development.
Developmental therapists support infant development and provide early holistic interventional therapeutic strategies to mitigate the many motor interruptions noted for children with critical CHD. Reference Ross, Heiny, Conner, Spener and Pineda68,Reference Borges Nery, Snider and Camelo71 All infants with critical CHD would benefit from close assessment of their motor abilities on admission in order to provide appropriate referral to occupational therapy, physical therapy, and/or speech-language therapy. Developmental therapies play a vital role in creating developmental plans and ensuring the continuity of interventions throughout an infant’s hospitalisation. Reference Lisanti, Vittner and Peterson72 The following descriptions aim to promote a shared understanding and encourage referrals by providing information on the areas targeted by each developmental discipline, as well as the corresponding methods used to achieve the desired outcomes:
-
Occupational Therapy: Employs sensory, neuromotor, neurobehavioural, developmental, and social-emotional interventions to enhance infant and family participation in daily activities and co-occupations (holding, skin-to-skin, soothing, and adapted infant care tasks) within the hospital. Reference Carroll, Ludwig and Sturdivant73
-
Physical Therapy: Applies movement science principles to optimise progressive postural and motor skill development in alignment with the infant’’s neurobehavioural and neuromotor systems. Reference Byrne and Garber74-Reference Sweeney, Heriza, Blanchard and Dusing76
-
Speech-Language Pathology: Utilises sensory, neuromotor, neurobehavioural, and oral motor interventions to improve infant communication, speech, feeding, and swallowing skills to promote infant-driven experiences. Reference Craig and Smith70,Reference Barbosa77
Promoting motor skill development requires a family-centred and multi-disciplinary team that considers the infant’s unique sensory, movement, and bonding experiences. This approach integrates concepts of infant mental health, such as building trust with the infant and family, particularly in an environment which is different from what the infant’s brain is expecting and atypical for early experiences. Reference Als78,Reference Als, Lawhon, Duffy, McAnulty, Gibes-Grossman and Blickman79 Developmental therapies use standardised and non-standardised assessments to determine specific areas for interventions to support the acquisition of motor skills; provide developmentally supportive environmental modifications, facilitate essential self-care activities such as oral motor skills for feeding, self-calming, and state regulation; provide hands-on therapeutic interventions to mitigate atypical musculoskeletal changes; and promote typical motor movement patterns and sensory experiences. During a critical period of brain development, developmental therapies promote family bonding and interaction through shared participation in activities that encourage the infant to attain long-term motor milestones and encourage the typical development of functional skills. Reference Barbosa77,Reference Sturdivant80
Motor skill assessment
Standardised neurobehavioural and neuromotor evaluations are recommended for hospitalised infants between 0 and 12 months diagnosed with critical CHD before discharge home. Reference Ware, Butcher and Latal81 Standardised assessments provide objective, accurate, reliable, consistent, and criterion-referenced measurements and are best practice to identify early neurodevelopmental challenges, tone and motor discrepancies, predict future developmental outcomes, and guide discharge referral to early intervention, home health, or outpatient developmental therapies. Reference Noble and Boyd82–Reference Desai, Jones and Fogel84 Several standardised assessments are recommended to assess the skills of hospitalised infants (see Table 3).
Table 3. Standardised assessments of motor skill development for the hospitalised infant with CHD

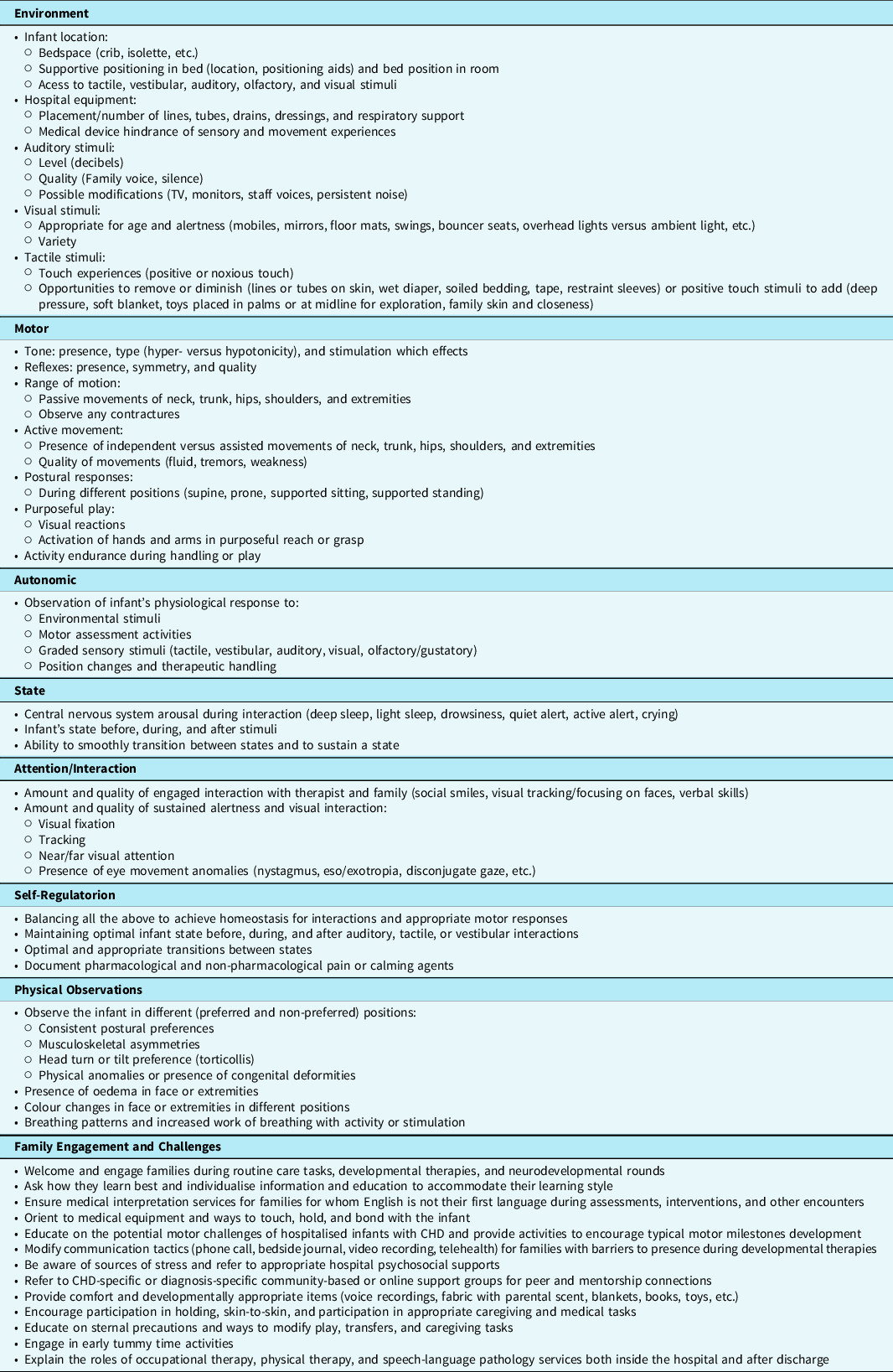
In situations where an infant is sedated, ill, or medically unstable, a standardised assessment is often contraindicated, and a non-standardised assessment following many of the components of individualised developmental care is used to gather information and guide inpatient therapy goals, interventions, and discharge therapy recommendations (see Table 4). Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler69,Reference Als, Lawhon, Duffy, McAnulty, Gibes-Grossman and Blickman79 In addition, early infant motor assessment supports individualised developmental care, family knowledge of the infant’s motor developmental trajectory, and neurodevelopmental follow-up recommendations. Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler69 When families are encouraged to support their infant’s motor development, they become more attuned to their infant’s strengths and weaknesses, specific developmental goals, adjust caregiving tasks to meet those goals, and incorporate customised interventions into their daily routines.
Table 4. Variables to assess/observe in support of motor skill development for the hospitalised infant with CHD
Motor skill interventions
Motor interventions in the critical care setting (see Table 5) are individualised and adapted after careful and repeated observation of the infant’s behavioural cues in response to therapeutic activities and engagement with their family. Interventions continually evolve and change based on factors such as medical acuity of the infant (the level of medical care and attention that a patient requires based on the severity and complexity of their medical condition), surgical timing, infant response, and family comfort. The developmental therapy provided by occupational therapists, physical therapists, and speech-language pathologists is a specialised field that requires competence and adaptability to address many factors, including the medical diagnosis, individualised infant and family dynamics, and the rapidly changing clinical stability of a critically ill infant with CHD in the complex hospital environment. Reference Sturdivant80,Reference Desai, Jones and Fogel84
Table 5. Interventions to support motor skill development for the hospitalised infant with CHD

Interventions to support optimal environment for motor learning
Environmental modification interventions (see Table 5) decrease noxious stimuli and help the infant maintain a calm and optimal behavioural state for motor learning. Providing developmentally supportive stimuli enhances active engagement in motor activities and exploration. For example, light cycling, visual stimulation, and positional devices encourage the infant to interact and connect to the people and objects around them, thus promoting motor development. The appropriateness of all stimulation is determined by reading the infant’s cues and responses. Reference Als and Goldson85
Interventions to support infant self-regulation
Infant neurobehavioural cues guide therapeutic interventions, as learning is hindered when cues are misrepresented and an infant is overwhelmed, stressed, or physiologically unstable. Supporting infant strengths and utilising non-pharmacological calming and regulation activities like developmentally supportive positioning, environmental modifications, positive touch experiences, and family co-occupations create a healing environment for the infant (see Table 5). Reference Cardin86–Reference Altimier and Phillips88 Careful, detailed, and repeated observation of infant behavioural patterns and motor responses are essential for individualised adaptation and planning of care, taking into account strengths and vulnerabilities to tailor interventions for the individual infant. Reference Noble and Boyd82,Reference Als and Goldson85 Utilising behavioural assessments prior to any handling or engagement ensures the infant is well regulated and calm in order to best receive intervention.
Interventions to support optimal infant movement experiences
Interventions for infant motor development are two-fold; graded body movements and weight-bearing activities require support for physiological stability and neurobehavioural readiness. Reference Altimier and Phillips88 Infants first tolerate static touch, progressing to body and extremity movements, then position changes and facilitated targeted motor skills (see Table 5). Reference Hadders-Algra89 It is also important to engage the infant’s sensory systems while engaging in motor movement – paying close attention to what the infant sees, hears, smells, touches, feels, and tastes along with how they respond during therapeutic handling and postural control activities. Reference Hadders-Algra89 The goal is active engagement of the muscles and joints to participate in stretching, reaching, and kicking; visual tracking and head control in prone, supine, and sitting; integration of sensory experiences into motor movements; and safe exploration of new movement patterns. Reference Altimier and Phillips88
Interventions to support the musculoskeletal system
Developmentally supportive positioning interventions facilitate midline development and symmetrical support of the musculoskeletal system (see Table 5). Long-term motor and functional interruptions are created by positioning-related facial, neck, skull, and skeletal abnormalities. Using a standardised positioning assessment like the Infant Positioning Assessment Tool 90 guides positioning interventions, communication among all caregivers, and creates a standard for infant positioning; further, it may have predictive capabilities for early identification of acquired positional deformities like torticollis and plagiocephaly. Reference Spilker, Hill and Rosenblum91,Reference Madlinger-Lewis, Reynolds, Zarem, Crapnell, Inder and Pineda92
Developmental therapies work with families and bedside caregivers to help the infant experience a variety of positions, including prone and modified prone, as soon as possible. It is especially beneficial when paired with family holding and skin-to-skin. Additionally, interventions to stretch and strengthen soft tissues through progressive movement experiences help limit contractures, positional deformities, and asymmetrical extended postures. Congenital hand or foot anomalies may require hard or soft splints or taping interventions to facilitate alignment.
Supporting family engagement and education
All infants expect comfort, well-being, and security from their families as the stable, familiar, and predictable caregiver. Reference Als78,Reference Als, Lawhon, Duffy, McAnulty, Gibes-Grossman and Blickman79,Reference Als and Goldson85,Reference Als, Duffy and McAnulty93 Family-centred early motor interventions for hospitalised infants with critical CHD may improve motor development and counteract parental overprotection and stress. Reference Mitteregger, Dirks, Theiler, Kretschmar and Latal94 Establishing care partnerships between the family and developmental therapy professionals fosters parent/family engagement, bonding, attachment, and advocacy during the hospitalisation, which can persist beyond discharge home. Reference Als, Duffy and McAnulty93,Reference Klug, Hall and Delaplane95 Therapeutic interventions occur in collaboration with families (see Table 5).
Conclusion
Beginning in utero, infants with critical CHD often have a different motor developmental trajectory than infants without a heart condition. Infants with critical CHD at 1 year of age commonly have delays in motor milestones and difficulties with motor skills, which may continue and evolve throughout life. This article summarises the early motor skill challenges and the impact of the critical care experience on the infant’s developing motor system. The challenges include limitations related to infant care, pre-operative co-morbidities, and peri-operative fragility. As members of the interdisciplinary healthcare team, occupational therapists, physical therapists, and speech-language pathologists have specialised training to perform standardised and non-standardised assessments to measure and monitor factors that influence motor skill development in the critical care setting. Interventions encompass environmental modifications, facilitating infant self-regulation, motor development activities, musculoskeletal interventions, and family engagement and education. Interdisciplinary collaboration and communication are vital to ensure optimal outcomes for hospitalised infants with critical CHD and their families.
There is currently a knowledge gap in research and dissemination of findings related to the impact of motor therapies and interventions to improve the short and long-term developmental outcomes for hospitalised infants with heart disease. Most of the literature on interventions to support neurodevelopmental outcomes has originated from populations within the neonatal ICU or with healthy infants. Although there is certainly some applicability to these populations, the underlying mechanisms of developmental delays and the unique physiology of newborns with critical CHD mean that one cannot assume these interventions are immediately transferrable between populations. Multi-centre collaboration is a strong tool to support robust prospective research in this vulnerable population.
Acknowledgements
We want to thank Tom Miller of Maine Medical Center for his expert advice, edits, and support of this project. Additionally, we want to thank Karen Uzark of the University of Michigan Mott Children’s Hospital for her time, patience, and guidance during the final edits. Last, we are grateful to the Publications Committee of the Cardiac Neurodevelopmental Outcome Collaborative for their comments and suggestions to improve this manuscript.
Financial support
This project received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.








