Article contents
Application of iron (III) meso-tetrakis(4-hydroxyphenyl)porphyrin-methylene blue strips for the detection and quantification of H2O2 in aqueous and pharmaceutical fluids
Published online by Cambridge University Press: 04 February 2019
Abstract
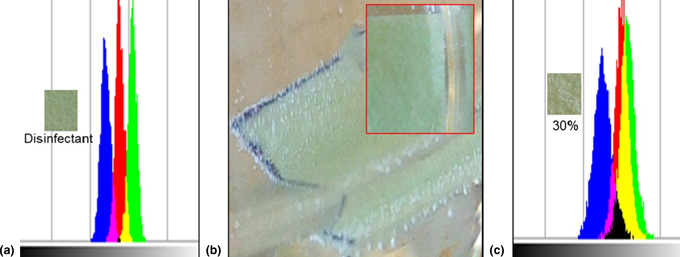
In this report, a facile system consisting of iron (III) meso-tetrakis(4-hydroxyphenyl)porphyrin-methylene blue dye hybrid immobilized on a disposable paper strip was used to detect and sense H2O2 in aqueous solutions via a low-cost pixelometric imaging technique. The new technology was employed to evaluate the level of H2O2 in a self-administering 30%-labeled commercial H2O2 disinfectant solution. The result showed that the concentration of H2O2 in the tested pharmaceutical sample solution deviated from the label by 92.37%. The current approach provides a simple low-cost resolution for the rapid detection and quantification of H2O2 in aqueous solution and pharmaceutical fluids.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by




