INTRODUCTION
Worldwide, cervical cancer is the second most common cancer in women, with the majority of cases (∼80%) occurring in developing countries [Reference Parkin1]. In developed countries, cervical cancer is no longer a leading cause of cancer-related mortality because women undergo routine Pap screening and are treated if cervical intra-epithelial neoplasia (CIN) is diagnosed. Particularly, in the United States, cervical cancer incidence and mortality rates have declined by about 50% over the past three decades (details can be found in the SEER database [2]). However, the disease remains a serious health threat for women.
Human papillomavirus (HPV) is a highly infectious virus; most infections are latent and regress spontaneously without intervention. Neoplasia is a rare event as a complication of HPV infection [Reference Bergeron3]. Based on their association with cervical cancer, HPVs can also be grouped to high-risk and low-risk HPV types. Low-risk HPV (LR-HPV) types include types 6, 11, 42, 43, and 44. High-risk HPV (HR-HPV) types include types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70. Included in the high-risk group are some HPV types that are less frequently found in cancers but are often found in squamous intra-epithelial lesions (SILs). Some authors refer to these HPV types as ‘intermediate risk’ [Reference Burd4]. Pre-cancerous lesions and invasive cancers are associated with a HR-HPV-type infection in 95% of the cases [Reference Bergeron3], while low-risk subtypes are occasionally found in cervical carcinomas [Reference Burd4, Reference Muñoz5]. At least 15 HPV types are human carcinogens that play a central role in the pathogenesis of cervical cancer and other less common cancers, including vaginal, vulvar, anal, penile, and upper aerodigestive tract cancers [Reference Muñoz5, Reference Gillison and Shah6].
Conventional or liquid-based cytology allows the detection of abnormal cytological modifications leading to a biopsy under colposcopy for a definitive diagnosis if either there is persistence of low-grade squamous intra-epithelial lesion (LSIL) present or a diagnosis of high-grade squamous intraepithelial lesion (HSIL) is made [Reference Bergeron3]. The diagnosis of atypical squamous cells of undeterminated significance (ASCUS) corresponds to ill-defined abnormalities of superficial cells and represents 2–3% of all cervical smears. Biopsy shows CIN grades II-III in 5–10% of ASCUS cases. Additionally, LSIL corresponds to mildly abnormal squamous cells and is found in 1–2% of all cervical smears, in most it cases regresses spontaneously, especially in young patients. HSIL corresponds to moderately to severely abnormal squamous cells and represents 0·5% of all cervical smears [Reference Bergeron3].
Studies based on polymerase chain reaction (PCR) technology have been performed in which type-specific as well as general HPV primers have been used. According to their data, HPV DNA sequences were present in 80–100% of patients with cervical cancers and in 60–90% of patients with high-grade CIN [Reference Evander7]. In particular, Walboomers et al. [Reference Walboomers8] demonstrated that 99·7% of patients with cervical cancer were HPV-positive. HPV-16 was the type most frequently present. HPV DNA was demonstrated in 5–49% of cytologically normal smears [Reference Evander7].
Currently underway are international, multicentre phase III trials of a tetravalent HPV- 6/11/16/18 L1 VLP vaccine that includes virus-like particles (VLPs) from HPV-16 and HPV-18 (which cause about 70% of cervical cancers worldwide) [Reference Muñoz5] and VLPs from HPV-6 and HPV-11 (which cause about 90% of genital warts) [Reference Greer9]. However, one difficulty in developing a vaccine is determining which types of HPV to include, since geographical variations in the prevalence of high-risk types are observed [Reference Giuliano10]. According to Muñoz et al. [Reference Muñoz11], a vaccine containing the seven most common HPV types (16, 18, 45, 31, 33, 52, 58) would prevent about 87% of cervical cancers worldwide, with little regional variation. The aim of our study is to describe the prevalence of the different HPV types in women with pre-neoplastic lesions of the cervix in Greece, and to investigate the aetiological role of HPV infection prospectively in the early pathogenesis of cervical carcinoma. These data may assist the public health authorities in planning prophylactic and therapeutic strategies to prevent cervical cancer.
METHODS
Population and specimen collection
All 841 women, who enrolled in the present investigation, aged from 16 up to 72 years (median age 30·0 years), were inhabitants of a primary health-care region in the metropolitan area of Athens, Greece. According to the public record, they were asked to participate in the study during the period 1998–2003; these women were not random but consecutive patients, who were referred due to a history of an abnormal Pap smear, cervical biopsy or to non-specific symptoms for cervical dysplasia, namely genital warts, vaginal discharge, abnormal bleeding, spotting after intercourse and low back pain. Ectocervix and endocervix were sampled separately; exfoliated cervical cells were collected by using a common wooden spatula, rotated with good pressure over the entire ectocervix, whereas an endocervical brush (Cervex Brush, Rovers Medical Devices B.V. Oss, The Netherlands) was used for the endocervix, respectively. The obtained material was then placed in 20 ml of buffer (preserv CytR solution, Cytyc, West Sussex, UK) together with the spatula and the cytobrush. Using ThinPrep cytology, a slide was prepared to be stained according to the Papanicolaou (Pap) procedure. In addition, the remnants of the material were further processed for molecular study. All procedures were performed after obtaining the agreement of all women.
Cytology
A total of 841 smears were obtained for cytological evaluation. They were evaluated at the Department of Cytology, ‘Mitera’ Maternity Hospital of Athens, and abnormal results were categorized according to the 2001 Bethesda Classification System [Reference Burd4].
DNA extraction
Depending on the cellularity, 7–10 ml of cervicovaginal sample from the ThinPrep vial was centrifuged at 3000 rpm for 10 min and washed twice with sterile PBS. The pellet was dissolved in 180 μl of tissue lysis ATL solution, 20 μl proteinase K (20 mg/ml) was added (Qiagen, Hilden, Germany) and the mix was vortexed and incubated at 56°C for 1–3 h or until it became clear. Then the silica-gel mini-columns from QIAamp DNA Mini kit were used for DNA extraction (Qiagen). The DNA was eluted in 150 μl sterile ddH2O and stored at −20°C until analysis.
HPV screening
DNA integrity for each sample was assessed by PCR amplification of the β-globin gene with PC04 and GH020 primers [Reference Bauer, Manos and Persing12]. For HPV detection two different consensus PCR reactions were used: the MY system [Reference Bauer, Manos and Persing12, Reference Hildesheim13] and the GP+ system [Reference de Roda Husman14], both amplifying regions of the L1 HPV gene. Whenever there was a negative or weak positive MY PCR in parallel with a positive GP+ reaction or there was discrepancy between negative MY/GP+ molecular results and a positive clinical assessment of the cytology test, a third multiplex type-specific PCR method was used. It amplified with high sensitivity other regions in the HPV genome for the HPV types most common worldwide (16, 18, 6/11, 31 and 33) [Reference Labropoulou15]. All PCR reactions were performed in a personal Mastercycler (Eppendorf, Germany) using the same cycling programme: after an initial denaturation step of 4 min at 94°C, 40 cycles consisting of 1 min denaturation at 94°C, 1 min annealing at 55°C and 1 min elongation at 72°C and finally 10 min at 72°C (for the GP+ system 40°C was used for annealing). Qiagen Master Mix (2×) was used at 1·5 mm final Mg concentration (total volume 50 μl) along with 3 μl of sample DNA or H2O or appropriate negative and positive controls well characterized by Southern blot and DNA sequencing as in previous studies [Reference Labropoulou15, Reference Kroupis16]. Primers were synthesized at IMBB (FORTH, Greece).
HPV typing
In case of a positive sample in the MY PCR, reactions were performed again in duplicate, the two PCR products were mixed and subjected to restriction fragment analysis: 13 μl of PCR product plus 1·5 μl common NEB buffer 2 and 0·5 μl of each of five restriction enzymes: DdeI, HaeIII, HinfI, PstI and RsaI (New England Biolabs, Beverly, MA, USA) in separate tubes. Incubations lasted 4 h at 37°C and were analysed subsequently in a 2% Nusieve 1:1 agarose gel as previously reported [Reference Bernard17]. In this way, multiple infections could be also resolved [Reference Kroupis16, Reference Bernard17]. Identification of a particular HPV type could be also performed directly from the result of the high sensitivity PCR (GP+ PCR was not used for typing purposes). Assignment of a discovered HPV type to a particular risk category was done according to the most recent epidemiological study [Reference Muñoz5].
Statistical analysis
Frequency distribution tables are used to summarize HPV infection types. Data were analysed by χ2 statistic with Yates' correction accepting a Type I error of 5% (two-sided). The magnitude of association between most common HPV-specific types and cytological diagnosis (negative vs. positive) was evaluated by odds ratios (OR) and the respective 95% confidence interval (95% CI) as calculated by logistic regression analysis standardized for age and number of HPV types in cases with at least one positive HPV type. In order to investigate the concordance between HPV DNA with the Pap test, a McNemar test was applied.
RESULTS
Cytological analysis
In the present study, the Pap test results were normal or showed benign cellular changes in 45·8% (385/841), ASCUS in 23·2% (195/841), LSIL in 27·9% (235/841) and HSIL in 3·1% (26/841) of the cases.
HPV prevalence
We detected HPV in 60% (504/841) of the samples. The prevalence of high-risk HPV types increased with the grade of cervical squamous cell dysplasia (P=0·001), according to the 2001 Bethesda Classification System. In particular, HPV-16 showed a statistically significant increase in prevalence with increasing severity of cervical disease (LSIL/HSIL) (P<0·001), while HPV-51 demonstrated an increase of marginal statistical significance in prevalence with LSIL/HSIL (P=0·069). Interestingly, HPV-58 was inversely correlated with positive cytological findings (logistic regression: OR 0·329, 95% CI 0·112–0·969).
As detailed in Table 1, 27·7% of HPV-positive smears were diagnosed as ASCUS, while 54·2% of the former were diagnosed as LSIL/HSIL. Moreover, HPV DNA was demonstrated in 23·6% of cytologically normal women. Subjects with HPV-negative smears were correlated with negative cytological evaluation (87·2%), cases of weak HPV positivity with ASCUS smears (51·8%), while HPV-positive smears demonstrated a statistically significant association with LSIL/HSIL Pap smears (P<0·0001). The incidence of HPV infection as detected by PCR was 4·4% more frequent than by cytology (95% CI 1·5–7·2%, P=0·003).
Table 1. Distribution of cytological diagnosis and human papillomavirus (HPV) DNA positivity
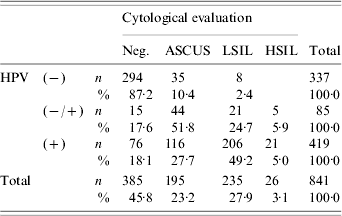
Neg., Negative; ASCUS, atypical squamous cells of undeterminated significance; LSIL, low-grade squamous intra-epithelial lesion; HSIL, High-grade squamous intraepithelial lesion; HPV, Human papillomavirus.
The mean age of the study group was 32·2 years with a range of 16–72 years. Higher rates of LSIL were observed at younger ages (<30, 30–39 years), while ASCUS Pap smears were more frequently detected among older women (⩾40 years) (P=0·036). Age was inversely correlated with positive cytological evaluation (P<0·0001) (logistic regression: OR 0·938, 95% CI 0·91–0·967). Additionally, a clear pattern of decreasing prevalence of HPV with age was observed (P<0·0001); HPV-6 and HPV-16 were inversely correlated with age (P=0·001 and P=0·005 respectively).
HPV type-specific detection
Thirty-four different human papillomavirus types were detected, HPV-16, 53, 6, 31, 51, 66, 58, 61,18 and 33 being the most prevalent types (Table 2). In particular, HPV-16 was the most common HPV DNA detected. Moreover, HPV DNA was detected in 85 samples but typing was not possible for this group of samples. In total, 330 (78·8%) of HPV infections involved a single type, while 89 (21·2%) involved ⩾2 types (18·1%, 3·1% for double and triple infections, respectively). Multiple positivity increased the possibility of HR-HPV types being encountered (P=0·005).
Table 2. Distribution of HPV types detected
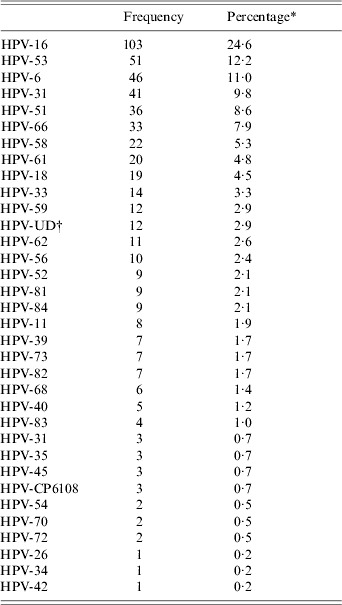
* Percentage among HPV-positive subjects (n=419).
† UD, Undetermined.
Molecular investigations
β-globin PCR was carried out in all of 841 samples (all DNAs were properly prepared). HPV DNA was detected in 333 out of 841 samples by MY PCR and in 383 out of 841 samples by GP+ PCR. A total of 312 samples demonstrated both MY PCR and GP+ PCR positivity, whereas we reported 71 negative or weakly positive MY PCR, in parallel with a positive GP+ reaction; 59 of the latter displayed positive high sensitivity PCR, while the remaining 12 were assigned as undetermined (UD) due to high sensitivity PCR negativity. Fifty-eight samples showed discrepancy between negative MY/GP+ molecular results and a positive cytological evaluation; 15 of the aforementioned samples demonstrated positive high sensitivity PCR [total infected women: 333 MY PCR(+)+71 MY PCR(−)/GP+(+)+15 MY PCR-/GP+(−)/Pap test (+) /high sensitivity PCR(+)=419].
Apart from HPV-positive or -negative samples, a group of samples (n=85) with weak positivity was recognized (Table 1); a weak positive sample was defined as a sample with a weak band in the MY PCR (and negative GP+ PCR) that could not be further typed by high sensitivity PCR. Even if one mixed two or more weak positive MY reactions, a clear restriction pattern could still not be seen; therefore, no HPV subtyping could be performed with certainty.
DISCUSSION
In the present study, women with HPV-negative smears were correlated with negative cytological evaluation. In addition, a statistically significant association of HPV-low viral load smears with ASCUS diagnosis was noticed. Another point of interest concerned the relation between HPV-positive smears with LSIL/HSIL Pap smears. These findings are consistent with the fact that the relationship between HPV infection and cervical neoplasia is a causal one. In particular, the magnitude of the association between HPV and cervical squamous cell carcinoma is higher than that for the association between smoking and lung cancer [Reference Burd4]. Thus, a negative HPV test provides reassurance that cancer is unlikely to develop for several years.
Moreover, HPV DNA was demonstrated in 23·6% of cytologically normal women. Other studies have yielded similar findings [Reference Evander7, Reference Sherman18]. The group of women HPV-positive/cytology-negative corresponds to 5–10% of the screening population [Reference Bergeron3]. Woodman et al. [Reference Woodman19] reported that HPV-positive women with a normal Pap test are at 13-fold increased risk for moderate or severe dyskaryosis. Rozendaal et al. [Reference Rozendaal20] demonstrated that women with normal Pap smears containing HR-HPV genotypes are 116 times more at risk of developing CIN III, in contrast to women without HR-HPV. Additionally, Castle et al. [Reference Castle21] observed that about 15% of women, in annual screening programmes, who concurrently have a negative Pap test and a positive HR-HPV test will have a subsequent abnormal Pap test within 5 years. These studies [Reference Woodman19–Reference Castle21] highlight the central aetiological role of HPV infection in cervical neoplasia by establishing that viral infection precedes the development of disease. Therefore, women with a positive HPV test and a negative Pap test remain at risk and a certain level of anxiety exists in almost all of them.
According to our data, 27·7% of HPV-positive smears were diagnosed as ASCUS. A Pap test result of ASCUS presents a clinical challenge. Only 5–10% of women with ASCUS harbour serious cervical disease (CIN II-III) [Reference Bergeron3], but more than one third of the HSILs in screening populations are identified from ASCUS Pap test results [Reference Manos22]. After an ASCUS diagnosis, HPV DNA identification has been proven more sensitive than follow-up cytology in diagnosing CIN II-III [Reference Bergeron3]. For women who test positive for HR-HPV types, referral to colposcopy is recommended [Reference Burd4]. Moreover, Schlecht et al. [Reference Schlecht23] observed shorter progression transit times from ASCUS to LSIL which was worse in women with non-oncogenic (low-risk) and oncogenic (high-risk) HPV types compared with women who had HPV-negative abnormalities. This observation provides further evidence of the importance of colposcopic evaluation of women with HPV-positive ASCUS smears, particularly those with oncogenic HR-HPV types.
In our study, higher rates of LSIL were observed at younger ages, while ASCUS Pap smears were more frequently detected among older women (⩾40 years). These findings are in accordance with previous studies [Reference Herrero24–Reference Keating and Wang27]. Data from the ‘1991–1995’ National Cervical Cancer Early Detection Programme [Reference Lawson26] indicate that the rates of LSIL were highest among women aged <30 years. This is partly due to the fact that HPV infection rates are higher among sexually active women in their early 20s [Reference Lousuebsakul28]. With regard to ASCUS/SIL ratios, Keating & Wang [Reference Keating and Wang27] reported that there is a statistically significant higher ratio among older women (>46 years) compared with premenopausal women (age ⩽45 years). Furthermore, ASCUS appears to have a particularly low positive predictive value for SIL in perimenopausal women (aged 46–54 years) and this may be attributable in part to air-drying artifact and subtle atrophic changes [Reference Lawson26]. Rader et al. [Reference Rader25] demonstrated that postmenopausal women (⩾55 years) with a diagnosis of ASCUS have a significantly lower, but still real, risk of developing intra-epithelial neoplasia when compared to younger women and suggested that these patients should be managed similarly to the general population.
In the present study, the frequency of HPV infection declined with increasing age. Importantly, other studies also reported this inverse relationship [Reference Herrero24, Reference Burk29, Reference Syrjänen30]. This finding suggests that a biological effect, such as HPV immunity acquired over time and with multiple exposures, may mediate the inverse relationship between age and HPV prevalence. Interestingly, it has been displayed that the lower prevalence of HPV infection in older women compared to younger women appears to be independent of sexual behaviour [Reference Burk29]. Additionally, our findings demonstrated an inverse correlation between age and positive cytological evaluation, being in accordance with the acquisition of incident HR-HPV infection, which is significantly age dependent and contrasts with some previous studies which showed that the acquisition of an abnormal Pap smear result was not significantly age related [Reference Syrjänen30]. In particular, Syrjänen et al. [Reference Syrjänen30] reported that the only significant independent predictor of the incident abnormal Pap smear result was a high viral load. According to our data, increasing age appears to be a protective factor with regard to positive cytological findings.
We detected HPV in 60% of all samples. A slightly increased percentage might have been obtained with a more sensitive nested PCR approach, however, we decided not to implement such a contamination-prone procedure and instead used multiple single-round PCRs. HPV-16 was the most common HPV DNA detected (24·6%). In particular, HPV-16 showed a statistically significant increase in prevalence with increasing severity of cervical disease (LSIL/HSIL) and an inverse correlation with age. Additionally, HPV-51 also demonstrated an increase, of borderline statistical significance, in prevalence with LSIL/HSIL. As far as the prevalence of high-risk types is concerned in the present study, it increased with the progression of CIN grade. This observation is consistent with several findings of previous studies [Reference Kaufman31, Reference Herrero32]. Hwang et al. [Reference Hwang33] demonstrated that HPV-51 was the most prevalent type among various HPV types in LSIL in Korean women, while HPV-16 was the most common, single type in HSIL cases, suggesting a certain type may become dominant over others as the disease progresses. Regarding HPV-16, our study confirmed the strong association of HPV-16 with the risk of SIL. Interestingly, average HPV DNA copy number has been shown to increase significantly with CIN grade for HPV-16 but not for the other HR-HPV types, suggesting a genotype-specific association between HPV-16 DNA load and neoplastic progression [Reference Swan34].
With regard to HPV types, in our study there was a broad diversity of HPV infections with HR-HPV types being the most common types detected. Of importance, no HPV subtyping could be performed in 85 samples of weak positivity; these are usually samples with either emerging or resolving infections with few copies of the viral genome. Additional types might have been identified if we had extended our high-sensitivity method to other types besides the most common worldwide: HPV-16, 18, 6/11, 31 and 33 (e.g. types 53, 51, 66, 58 and 61 – common in our area – as revealed by our study). In total, 78·8% of HPV infections involved a single type, while 21·2% involved ⩾2 types. Although high rates of multiple infections are often detected and may be due to promiscuous sexual behaviour, they are arguably not associated with increased risk of disease above that associated with a single HPV-type infection [Reference Schlecht23, Reference Herrero24, Reference Cavalcanti35, Reference Ho36]. Specifically, Ho et al. [Reference Ho36] did not find a substantially increased risk associated with multiple infections, supporting the view that cervical neoplasia is the result of clonal expansion of a cell infected with a single type of HPV.
Furthermore, our data demonstrated that HPV-58-positive women were more likely to have a normal Pap test result, in contrast to several previous studies which showed a strong association between HPV-58 and risk of HSIL/cancer [Reference Herrero32, Reference Lee37–Reference Gonzalez-Losa39]. In particular, Chan et al. [Reference Chan38] provided epidemiological evidence that HPV-58 variants carrying E7 T20I/G63S substitutions are associated with an increased risk for cervical cancer. Such differences may be partly due to some geographical and/or racial factors. Additionally, in the current study HPV-6 was inversely correlated with age. Regarding HPV-6, Cavalcanti et al. [Reference Cavalcanti35] reported that low-risk HPV-6 and 11 do not seem to play an important role in carcinogenesis, since their prevalences tended to decrease significantly from LSIL to squamous cervical carcinoma and have not been found in premalignant and malignant lesions, except in mixed infections, associated with high or intermediate risk HPVs.
As far as previous studies in Greece are concerned, Labropoulou et al. [Reference Labropoulou15] demonstrated that the prevalence rate of the genital HPV types was in the range previously described for many western countries. Interestingly, she found a higher HPV-18 positivity than that reported for most European countries and also in our study. Moreover, Agorastos et al. [Reference Agorastos40] studied a non-selective sample of 1296 women and reported one of the lowest HPV prevalences ever reported internationally of 2·5%, in contrast to our finding of 60%. This difference is probably attributable to the selective nature of the current cohort, concerning women with pre-neoplastic lesions of the cervix; thus, the respective results cannot be extrapolated to the Greek population at large.
In conclusion, the overall prevalence of HPV infection by PCR in 841 Greek women was 60% and it declined with increasing age. HR-HPV types were the most frequent types of infection and their prevalence was correlated with the degree of cervical dysplasia. In particular, HPV-16 was the most common HPV type detected. HPV-16 and HPV-51 demonstrated a statistically significant increase in frequency with LSIL/HSIL. The higher prevalence and the clinical role of HPV-51 need to be addressed through further studies. HPV-6 and HPV-16 were inversely correlated with age. Surprisingly, our data showed that HPV-58-positive women were more likely to have a normal Pap test result. In addition, our results provided further support for the involvement of HPV in the pathogenesis of this disease. Importantly, HPV DNA was demonstrated in 23·6% of cytologically normal women. Higher rates of LSIL were observed at younger ages, while ASCUS Pap smears were more frequently detected among older women (⩾40 years). Finally, our findings demonstrated an inverse correlation between age and positive cytological evaluation.
DECLARATION OF INTEREST
None.






