The proportion of elderly amongst many Western populations is increasing; for example, the proportion of individuals aged over 65 years in Japan is 20 %, and is estimated to increase to 26 % in 20151. Accordingly, healthcare expenditure is increasing, which becomes a socio-economic issueReference Winker2. Morbidity and mortality by infectious diseases are high in the elderlyReference Klima, Povysil and Teasdale3, where natural defence systems including immune functions are compromised, which give a primary cause of increase in disease risksReference Miller4, Reference Makinodan5. Nutritional status decreases in the elderly, especially in hospitalised and enterally fed elderlyReference Sullivan, Sun and Walls6, Reference Schneider, Le Gall, Girard-Pipau, Piche, Pompei, Nano, Hebuterne and Rampal7, which also causes dysregulation of the immune systemReference Keusch8, Reference Lesourd9. Longevity may be associated with genotype of pro- and anti-inflammatory cytokines, TNF-α and IL-10 productionReference Lio, Scola and Crivello10, suggesting that inflammation, a component of immunity, may also have an influence on health in the elderly. Several research groups have demonstrated that nutritional supplementation using micronutrients and/or fish oil improves immune function and frequency of infectionsReference Fortes, Forastiere and Agabiti11–Reference Chandra13, but the effects are sometimes not or partially observedReference Girodon, Galan, Monget, Boutron-Ruault, Brunet-Lecomte, Preziosi, Arnaud, Manuguerra and Herchberg14, Reference Graat, Schouten and Kok15.
The intestinal microbiota play a key role in providing defence systems called colonisation resistanceReference Wells, Maddaus, Jechorek and Simmons16 to keep out invading pathogenic bacteria. Changes in intestinal microbiota balance are reported in the elderlyReference Mitsuoka, Ohno, Benno, Suzuki and Nanba17, Reference Hopkins, Sharp and Macfarlane18, which may attenuate the host defenceReference Dineen19. Probiotics, defined as live micro-organisms which when administered in adequate amounts confer a health benefit on the hostReference Fuller20, 21, are potential measures to regulate infections in the elderly. Some probiotic bacteria have been shown to improve host immunityReference Gill and Guarner22 and incidence of infections such as infectious diarrhoea in infancyReference Saavedra, Bauman, Oung, Perman and Yolken23 and respiratory infections in children and adultsReference Hatakka, Savilahti, Ponka, Meurman, Poussa, Nase, Saxelin and Korpela24, Reference de Vrese, Winkler and Rautenberg25. It has been shown recently that administration of a Lactobacillus strain for 3 weeks reduced the duration of winter infection in free-living elderlyReference Turchet, Laurenzano, Auboiron and Antoine26. The WHO suggested that probiotics are helpful in normalising nutritional status in children27; however, there is less information of the impact of probiotics on nutritional status in the elderly, especially in combination with other health-status changes including infection, immune status and intestinal microbiota.
Lactobacillus johnsonii La1 (NCC533) is a probiotic strain which is adhesive onto intestinal epithelial cellsReference Bernet, Brassart, Neeser and Servin28 and produces bactericidal compounds against harmful bacteria such as Salmonella Reference Bernet-Camard, Lievin, Brassart, Neeser, Servin and Hudault29. Feeding of fermented milk containing L. johnsonii La1 has been demonstrated to reinforce human leucocyte phagocytic activityReference Schiffrin, Rochat, Link-Amster, Aeschlimann and Donnet-Hughes30, antigen-specific IgA antibody production in healthy adultsReference Link-Amster, Rochat, Saudan, Mignot and Aeschlimann31, and improve intestinal microbiota to enrich bifidobacteria in healthy adultsReference Yamano, Iino, Takada, Blum, Rochat and Fukushima32. The immune modification by the La1 strain also regulates inflammatory responses, the production of pro-inflammatory cytokines in vitro is suppressedReference Haller, Bode, Hammes, Pfeifer, Schiffrin and Blum33, Reference Vidal, Donnet-Hughes and Granato34 and gastric inflammation is reduced by its intake in Helicobacter pylori-infected human subjectsReference Felley, Corthesy-Theulaz and Rivero35, Reference Cruchet, Obregon, Salazar, Diaz and Gotteland36. Therefore, the probiotic strain has potential to improve the health status of elderly, whose immunological status may be compromised and who are prone to suffer from infectious diseases.
To reveal the effect of fermented milk containing the probiotic L. johnsonii La1 on the incidence of infection, immunological and nutritional status, and intestinal microbiota in elderly subjects, we conducted a 12-week feeding trial in a randomised double-blind controlled manner on bed-ridden elderly subjects being fed total enteral nutrition (EN) in hospital, whose food intake was nutritionally standardised.
Materials and methods
Subjects
The trial was conducted in accordance with the Helsinki declaration (2000) and the protocol was approved by the Ethical Committee of Harunaso Hospital (Gunma, Japan). Elderly volunteers were enrolled from bed-ridden in-patients over 70 years of age at Harunaso Hospital suffering from dysphasia with dementia and fed total EN by nasogastric tube or gastrostomy. After obtaining the agreement of the volunteers and their family members on the objectives and the design of the study, seven men and seventeen women (age 75–96 years) were enrolled.
Study design and diet
The study was conducted during the winter season of 2002–3. Subjects were randomly assigned to two groups, the L. johnsonii La1 (LC1) and control groups, and a double-blind controlled study was conducted. The profile of the subjects is shown in Table 1. Test fermented milk (LC1®; Nestlé Japan Ltd, Tokyo, Japan) contained the probiotic strain L. johnsonii La1 (NCC533) at 10Reference Lesourd9 colony-forming units and a non-probiotic strain Streptococcus thermophilus at 10Reference Keusch8 colony-forming units/90 g. In the run-in observation period for the first 12 weeks, all subjects were administered total EN at 3768 kJ (900 kcal)/d (CZ-Hi; Clinico Co., Ltd, Tokyo, Japan). In the intervention period for the subsequent 12 weeks, subjects in the LC1 group (n 12) were administered 90 g (373 kJ (89 kcal)) fermented milk through a tube after feeding of EN at 3395 kJ (811 kcal)/d. The fermented milk was served in a liquid form by mixing it with water in a kitchen, as we did for the control EN preparation, in order to maintain blind conditions. The subjects in the control group were administered the EN diet at 3395 kJ (811 kcal)/d, then administered 373 kJ (89 kcal) of the EN in the same manner as the fermented milk in the LC1 group. The diets in the two groups were nutritionally comparable (Table 2).
Table 1 Characteristics of subjects
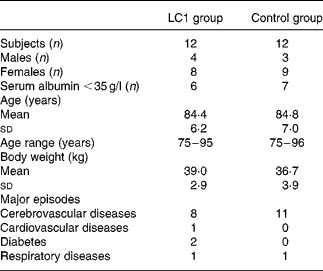
LC1, Lactobacillus johnsonii La1.
Table 2 Daily intake of energy and nutrients
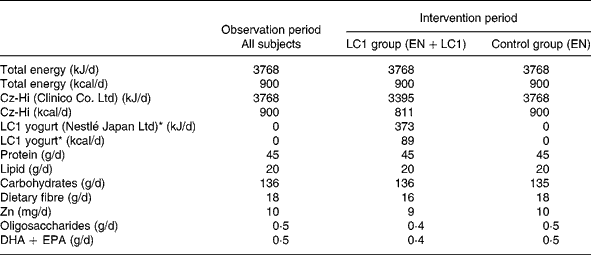
LC1, Lactobacillus johnsonii La1; EN, enteral nutrition.
* LC1 yogurt contains 109 colony-forming units Lactobacillus johnsonii La1/90 g.
All subjects were under nursing care during the entire study period. Health-status parameters including body temperature, faecal condition (the shape of faeces and stool extraction or usage of laxatives for defaecation) and usage of drugs were recorded on a daily basis. Blood and faecal samples were collected before and during the intervention period to assess nutritional and immune status. The number of days of infection was counted when the subjects were diagnosed by a physician as suffering from infections requiring prescribed antibiotics. Infection, for which antibiotics are prescribed, is frequently observed in the elderly, and we did not exclude such subjects in our study design and continued the observation and sampling. A physician comprehensively diagnosed respiratory, intestinal or urinary tract infection taking into account respiratory inflammation, appearance of sputum, coughing and sneezing, turbidity of urine, watery defaecation and fever. Duration of infections was expressed in percentage of days with infections for 12 weeks.
Blood and faecal analysis
Blood samples were collected from the femoral artery at three time points: just before starting the intervention period (week 0), and at 4 and 12 weeks during that period. Serum total protein, albumin, total cholesterol, creatinine, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, C-reactive protein, IgG and IgA were measured using a Hitachi 7600 autoanalyser (Hitachi, Tokyo, Japan), and blood cell numbers were measured with an SE-9000 autoanalyser (Sysmex, Kobe, Japan). Cholinesterase activity was measured using the butyrylthiocholine-5,5′-dithiobis-2-nitrobenzoic acid colorimetric assay. TNF-α was measured using an ELISA kit (Japan Immunoresearch Lab, Takasaki, Japan), and phagocytic activity were measured with a PHAGOTEST kit (Orpegen Pharma, Heidelberg, Germany) using fluorescein isothiocyanate-labelled Escherichia coli and a flow cytometric technique. Phagocytic activity and TNF-α were measured only at 0 and 4 weeks in order to diminish the burden for the subjects.
Faecal microbiota were analysed according to the method of Mitsuoka et al. Reference Mitsuoka, Ohno, Benno, Suzuki and Nanba37 with slight modificationReference Uchino, Kanayama and Hasegawa38. Faecal total bacteria, total anaerobes, total aerobes, Bacteroidaceae, Bifidobacterium, Clostridium, Enterobacteriaceae, Enterococcus and Lactobacillus were counted using CDC anaerobe blood, BBE, lactobacilli-selective, and CCFA agar media (Nippon Becton Dickinson Co., Ltd, Tokyo, Japan), KM-CW egg yolk, DHL, and EF agar media (Nissui Pharmaceutical Co., Ltd, Tokyo, Japan), blood liver and bifidobacteria-selective agar, and sheep blood agar M58 media (Eiken Chemical Co., Ltd, Tokyo, Japan), and CPS IPD agar medium (Biomerieux Japan, Tokyo, Japan). Faecal methicillin-resistant Staphylococcus aureus were also counted using mannitol salt agar medium (Nissui Pharmaceutical Co., Ltd), and OPA Staphyrococcus agar medium (Nippon Becton Dickinson Co., Ltd). Faecal samples were stored anaerobically using Anaero Pack Kenki (Mitsubishi Gas Chemical, Tokyo, Japan) at 4°C and analysed within 24 h after defaecation. Decimal dilution series of faecal samples into anaerobic PBS were prepared and samples were plated on to selective and non-selective media and incubated under anaerobic or aerobic conditions. Bacterial species were identified by colony morphology, gram staining, cell morphology, aerobic growth and biochemical test for sporulation. The three and two subjects in the LC1 and control groups, respectively, who took antibiotics in the 7 d before faecal sampling were excluded from faecal analysis, because the microbiota are known to be significantly disturbed by antibioticsReference Sullivan, Edlund and Nord39.
Statistical analysis
The results are expressed as mean values and standard deviations. Data were analysed with SPSS 11·0 J software (SPSS Japan, Tokyo, Japan). Statistical differences between the LC1 and control groups, and between before and during intervention periods were examined using the Wilcoxon rank sum test. Statistical difference on the appearance ratio of faecal bacteria was examined with the χ2 test. The difference between means was considered significant at P < 0·05.
Results
Clinical observations
All subjects accepted the diet well with or without fermented milk containing L. johnsonii La1 and no adverse health conditions were observed during the entire study period. No subjects received any vaccination in the study periods. Table 3 and Fig. 1 show the duration of infections and the health status of the subjects. There was no difference in duration of infections, diarrhoea or fever during the observation period between the LC1 and control groups. In the LC1 group, the duration of infections (percentage of days) in the observation period was 15·4 (sd 17·3) %which significantly lowered to 5·7 (sd 8·1) % in the intervention period (P = 0·018), and the reduction was larger in the LC1 group than that in the control group (P = 0·047). Respiratory symptoms were most frequently found, and they improved in the LC1 group, but incidence of the others without respiratory symptoms was low and such improvement was undetectable. Decrease in the duration of fever at >37°C tended to be larger in the LC1 group than that in the control group (P = 0·078). In the control group, there were no differences in the duration of infection or fever between the two periods. There were no changes in defaecation conditions including usage of laxatives and watery faecal excretion not caused by laxatives in both groups during the entire study period (Table 3).
Table 3 Infection, body temperature and faecal conditions in elderly subjects(Mean values and standard deviations)

LC1, Lactobacillus johnsonii La1.
* Mean value was significantly different from that of the same group before administration (P < 0·05).
† Mean difference was significantly different from that of the control group (P < 0·05).
‡ The run-in observation period was 12 weeks before starting the intervention period for 12 weeks.

Fig. 1 Durations of infections (a and b) and fever of more than 37·5°C (c and d) in elderly subjects fed Lactobacillus johnsonii La1 (LC1; n 12; a and c) and in control subjects (n 12; b and d). Values are means. * Duration of infections (% of days) during the intervention period was lower than that of the observation period in the LC1 group (P < 0·05).
Nutritional and immunological status
The changes in nutritional status and the other blood biomarkers are shown in Table 4. Hb concentration in blood increased in the LC1 group, and was significantly higher than that of the control group at 4 weeks (P = 0·038). There were no statistical differences between the LC1 group and the control group for other blood biomarkers, but changes were observed within the LC1 group; that is; serum albumin at 12 weeks during the intervention period was higher (P = 0·034) than that before intake (week 0), cholinesterase increased at 4 weeks (P = 0·028) and remained at a similar level at week 12, and total protein tended to increase from 0 to 12 weeks (P = 0·052). Erythrocyte and leucocyte numbers increased at 4 weeks and 12 weeks in the LC1 group, respectively (P < 0·05). However, this was not significant between groups. The ratio of blood cell types remained unchanged. In the control group, we observed a slight increase in serum albumin, white blood cells and IgG, but the changes were not statistically significant, and the other biomarkers showed no changes. Serum albumin was positively correlated with cholinesterase and total cholesterol at each time point (R 0·49–0·67; P < 0·01), but not with total protein. Total cholesterol was slightly increased in the LC1 group, but it was not significant.
Table 4 Blood biomarkers related health, nutritional, and immunological status(Mean values and standard deviations)

LC1, Lactobacillus johnsonii La1; ND, not determined; AST, aspartate aminotransferase; ALT, alanine aminotransferase, BUN, blood urea nitrogen.
Mean value was significantly different from that at 0 weeks: * P < 0·05, ** P < 0·01.
† Mean difference was significantly different from that of the control group (P < 0·05).
‡ Subjects whose basal phagocytic activity was lower than 90 %, LC1 (n 6) and control (n 6).
There were no significant changes in blood immune biomarkers between groups, but the following trends were evident, before and after treatment in the LC1 group. Blood phagocytic activity was normal at not less than 90 % in twelve out of twenty-four subjectsReference Esparza, Sanchez, Barranquero, Sabiono and Merino40. In the LC1 group, there were six subjects whose initial values were lower than 90 %, and they significantly increased from 0 to 4 weeks (P = 0·046; Table 4). The phagocytic activity in subjects with initially high values showed no changes. Neutrophils, the major blood cells providing phagocytic activity, remained unchanged. Serum TNF-α decreased from 0 to 4 weeks in the LC1 group (P = 0·008). Such changes were not observed in the control group (Table 4). There were no differences in serum immune parameters including total IgG, IgA, and C-reactive protein between the groups.
Faecal microbiota
The number and proportion of faecal bacteria are shown in Table 5. Total number of faecal bacteria in the elderly subjects was approximately 10Reference Lio, Scola and Crivello10 colony-forming units/g wet faeces, which was about ten times lower than that usually found in healthy adultsReference Mitsuoka, Ohno, Benno, Suzuki and Nanba37. Bifidobacteria were not found in five out of nineteen subjects (26 %). For both groups, we observed no significant changes in faecal microbiota at any time point. Lactobacillus slightly increased in the LC1 group, but this was not significant. Appearance of methicillin-resistant Staphylococcus aureus decreased from 0 to 12 weeks in the LC1 group (P = 0·016), according to reduction of antibiotic usage.
Table 5 Number and appearance of faecal bacteria in the elderly (log 10 number)*(Mean values and standard deviations)

LC1, Lactobacillus johnsonii La1.
* There were no statistical differences between groups nor between before and after treatments.
† Appearances of methicillin-resistant Staphylococcus aureus in faeces at >102 colony-forming units/g and that of the other bacteria at >103 colony-forming units/g.
Discussion
The present study showed that the duration of infections in hospitalised, enterally fed elderly subjects was reduced by the administration of fermented milk containing L. johnsonii La1 for 12 weeks. Not only nutrition but also exercise and positive emotion are known to influence the immune systemReference Davis, Murphy, Brown, Carmichael, Ghaffar and Mayer41, Reference Kiecolt-Glaser, McGuire, Robles and Glaser42. In the present study, all subjects were bed-ridden in-patients treated with total EN, and suffering from dementia. Their life circumstances were quite similar, nutritionally, mentally and physically. Although the number of subjects in the study was limited, the homogeneous living conditions and health status of the subjects allowed us to demonstrate that feeding of fermented milk containing the La1 strain is a potential way to decrease infections. In the present study, we used the test fermented milk containing not only the probiotic strain L. johnsonii La1, but also the non-probiotic strain S. thermophilus, and their fermentated metabolites, which makes it difficult to conclude whether the anti-infectious effect was provided solely by the probiotic strain. Dunnet-Huges et al. showed that the elevation of blood phagocytic activity in human subjects fed fermented milk containing S. thermophilus is weaker than that containing the La1 strainReference Donnet-Hughes, Rochat, Serrant, Aeschlimann and Schiffrin43. Cruchet et al. reported that the anti-H. pylori effect of probiotic feeding with the La1 strain found in children disappeared when the strain was killed by heat treatmentReference Cruchet, Obregon, Salazar, Diaz and Gotteland36. These findings suggest that the live probiotic strain may efficiently exert some health benefits. It is difficult to distinguish the efficacy of the La1 strain from the other elements in the test fermented milk; however, the La1 strain, whose efficacy is well proven, may more largely contribute or efficiently trigger the effects we found in the present study. Some researchers have shown that nutritional supplementation could work to enhance immunity and suppress infectious diseasesReference Fortes, Forastiere and Agabiti11–Reference Chandra13, but they did not always work sufficientlyReference Girodon, Galan, Monget, Boutron-Ruault, Brunet-Lecomte, Preziosi, Arnaud, Manuguerra and Herchberg14, Reference Graat, Schouten and Kok15 or needed long-term consumptionReference Bogden, Oleske, Lavenhar, Munves, Kemp, Bruening, Holding, Denny, Guariono and Holland44, Reference Bogden, Oleske and Lavenhar45. The EN formula we used for both the test and control groups in the present study was already rich in Zn, vitamins and PUFA, suggesting that probiotic feeding may work on top of such nutritional supplements for the elderly.
An interesting finding shown in the present study was that probiotic feeding improved nutritional status, as shown by the increase in serum albumin. We separately conducted a human trial on healthy elderly living at a nursing home with usual meal services, and observed that the administration of fermented milk containing the La1 strain elevated serum albuminReference Yamori, Sagara, Chen, Yamano and Fukushima46, implying that the use of fermented milk containing the La1 strain to improve nutritional status could be widely adopted by the elderly not only with tube feeding but also with slight malnutrition that frequently occurs in this age groupReference Sullivan47. Serum cholinesterase, a marker of liver function, was also increased by probiotic feeding. These improvements could play a key role in the regulation of infections by revitalising the immune system and organs. Diarrhoea may cause malnutrition, which can be reversed by probiotics through the regulation of diarrhoea by normalising intestinal microbiotaReference Solis, Samartin, Gomez, Nova, de la Rosa and Marcos48. In the present study, however, incidence of diarrhoea was low from the beginning, and we did not find any changes in its incidence or faecal microbiota by the probiotic feeding, indicating that improvement of nutritional status found in the present study was not likely to have occurred through an anti-diarrhoeal effect. Recently, we found that duodenal administration of the La1 strain stimulates the parasympathetic nervous system in vivo Reference Tanida, Yamano, Maeda, Okumura, Fukushima and Nagai49, Reference Yamano, Tanida, Niijima, Maeda, Okumura, Fukushima and Nagai50. Physiological declines of gastrointestinal functions, including not only of immunological but also neurological and metabolic systems, occur in aged populationsReference Firth and Prather51. The probiotic strain may act as an anabolic stimulus to the intestines through the auto-nervous system and may revitalise the digestive and absorption systems. We observed in the present study that the inflammatory blood parameter TNF-α decreased in the probiotic-fed subjects. The anti-inflammatory action of the La1 strain is also shown in an in vitro epithelial cell-culture modelReference Vidal, Donnet-Hughes and Granato34. The decrease in inflammation and fever associated with the regulation of infections might lower the unexpected expenditure of energy and nutrients of the body. These findings imply that the intestinal stimulation and anti-inflammatory effects of the La1 strain might explain the mechanisms of it on the nutritional status improvement.
Immune reinforcement by probiotics may play a key role in the reduction of infections. In the present study, blood phagocytic activity, a biomarker for natural immunity, increased with probiotic feeding in the elderly subjects who had low initial activity. Ageing influences haematopoietic stem cells and lymphocytesReference Linton and Dorshkind52 and we found slight recovery of blood cell numbers in the subjects of the LC1 group. Probiotics, as bacteria, potentially stimulate the host immunity directlyReference Gill and Guarner22, Reference Schiffrin, Rochat, Link-Amster, Aeschlimann and Donnet-Hughes30, after being recognised by the host through antigen-presenting cells such as dendritic cells in the intestinal tractReference Rescigno, Urbano, Valzasina, Francolini, Rotta, Bonasio, Granucci, Kraehenbuhl and Ricciardi-Castagnoli53 and at the Payer's patchesReference Takahashi, Iwata, Yamazaki and Fujiwara54, or through intestinal epithelial cells covering the huge surface of the intestineReference Haller, Bode, Hammes, Pfeifer, Schiffrin and Blum33. The decrease in infections found in the present study may be totally caused by nutritional status improvement, immune enhancement and anti-inflammation, which were triggered by feeding of the fermented milk containing the La1 probiotic strain.
In healthy children and adults, probiotic feeding generally exerts intestinal microbiota improvement and other effects such as immune reinforcement at the same time, and it is therefore difficult to distinguish the direct effect of the probiotic strain from the indirect effect through growth of residential bacteria influenced by probiotic feeding. Interestingly, the present study provides the evidence that feeding of fermented milk containing a single probiotic strain may improve nutritional status and immunity without any help of proportional changes in the residential bacteria. It is a common finding that the administration of lactobacilli results in an increase in bifidobacteriaReference Yamano, Iino, Takada, Blum, Rochat and Fukushima32, Reference Spanhaak, Havenaar and Schaafsma55, where a huge variety of bacterial strains are involved in changing intestinal microbial ecology. While we expected to improve intestinal microbiota in the elderly with poor bacterial mass and diversity, especially in total enterally fed subjectsReference Schneider, Le Gall, Girard-Pipau, Piche, Pompei, Nano, Hebuterne and Rampal7, not just a single strain but a probiotic cocktail or combination with prebioticsReference Guigoz, Rochat, Perruisseau-Carrier, Rochat and Schiffrin56 could be needed to provide the missing links in the chain of bacterial metabolite–growth elements network. In spite of observing no changes in faecal microbiota, the present study showed that probiotic feeding suppressed the appearance of the faecal antibiotic-resistant strain methicillin-resistant Staphylococcus aureus, which may cause opportunistic infectionsReference Siu57. Probiotic feeding could therefore be beneficial in the elderly not only to reduce the incidence of infections but also to reduce the negative impact of antibiotics used in medical facilities. These effects could be directly provided by probiotic feeding, independently from indirect influence through intestinal microbiota.
In conclusion, feeding of the probiotic L. johnsonii La1 on top of a well-designed nutritional supplement may reduce the risk of infections through improvement of nutritional status and immune function in the elderly, contributing to improvement in their quality of life.
Acknowledgements
The present study was conducted with a research grant from the Applied Science Board for Lactic Acid Bacteria (Tokyo). We wish to express our appreciation to Mitsubishi Kagaku BCL Inc. (Tokyo) and FALCO Biosystems Ltd (Kyoto) for carrying out the blood and faecal analyses. We also gratefully thank all volunteers who participated in the study.










