Introduction
Diapause is one of the primary mechanisms whereby herbivorous insects synchronize their life cycles with specific phases of host plants (Tauber et al., Reference Tauber, Tauber and Masaki1986; Denlinger, Reference Denlinger2002). Photoperiod, temperature and moisture/humidity strongly affect the physiological processes that take place throughout the different diapause and post-diapause stages of insects (Koštál, Reference Koštál2006). For example, photoperiodism (e.g. exposure to short days of autumn) is most commonly reported in diapause induction and maintenance (Tauber et al., Reference Tauber, Tauber and Masaki1986; Masaki, Reference Masaki1999). For the insects of the temperate zone, exposure to low temperatures (0–10°C) is typically a prerequisite for diapause termination (Saulich and Musolin, Reference Saulich and Musolin2012). Then, high temperatures accelerate post-diapause developmental rates and promote synchronization of adult emergence (Stålhandske et al., Reference Stålhandske, Lehmann, Pruisscher and Leimar2015; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2017). Populations from ecologically different habitats have shown differential responses to environmental factors (particularly temperature and photoperiod) for terminating diapause and/or concluding post-diapause development (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014, Reference Moraiti, Nakas and Papadopoulos2017; Stålhandske et al., Reference Stålhandske, Gotthard, Posledovich and Leimar2014).
Photoperiod is acknowledged to provide the most accurate information for the timing of seasonal events in insect life cycles, particularly for species at high latitudes where autumn temperatures are highly variable (Bradshaw and Holzapfel, Reference Bradshaw and Holzapfel2007; Śniegula and Johansson, Reference Śniegula and Johansson2010; Saunders, Reference Saunders2014). It ‘triggers’ facultative diapause responses well before conditions become unfavourable for survival and/or reproduction (Tauber and Tauber, Reference Tauber and Tauber1976). The diapause-inducing photoperiod is also likely to influence the diapause intensity in many insect species, given that longer scotophases found to induce more intense diapause compared with shorter scotophases (Koštál and Hodek, Reference Koštál and Hodek1997; Nakamura and Numata, Reference Nakamura and Numata2000; Wang et al., Reference Wang, Lin, Chen, Fu and Xue2014). Post-diapause development can also be affected by photoperiod (Wang et al., Reference Wang, Lin, Chen, Fu and Xue2014; Liu et al., Reference Liu, Luo, Zhang, Zhou and Wei2017), even though exceptions exist (Terao et al., Reference Terao, Hirose and Shintani2012; Cheng et al., Reference Cheng, Long, Zhang, Liang and Zhu-Salzman2017). Geographic variation of photoperiodic responses in diapause induction, diapause intensity and/or post-diapause development is common among populations inhabiting different latitudes or among individuals of the same species, based either on genetic factors or on plastic responses (Śniegula and Johansson, Reference Śniegula and Johansson2010; Chen et al., Reference Chen, Chen, He, Xia and Xue2013; Zeng et al., Reference Zeng, Wang and Liu2013; Wang et al., Reference Wang, Lin, Chen, Fu and Xue2014; Lindestad et al., Reference Lindestad, Wheat, Nylin and Gotthard2019). It is important to point out that photoperiod and temperature are usually interacting in inducing, maintaining and/or terminating diapause (Wang et al., Reference Wang, Yang, Zhou, Xu and Lei2009; Chen et al., Reference Chen, Xia, Fu, Wu and Xue2014; Norling, Reference Norling2018).
Compared to both temperature and photoperiod, the role of moisture in diapause developmental processes is less understood (Tauber et al., Reference Tauber, Tauber, Nyrop and Villani1998; Hodek, Reference Hodek2003). In general, moisture is likely to act as a token stimulus that induces, maintains or terminates diapause (Okuda, Reference Okuda1990; Tauber et al., Reference Tauber, Tauber, Nyrop and Villani1998). Nonetheless, moisture is rarely expected to serve as a cue for diapause induction because of spatial and temporal variability in its occurrence (Tauber et al., Reference Tauber, Tauber, Nyrop and Villani1998). However, moisture remains an important factor that triggers resumption of development at the end of dormancy stimulating adult emergence in many insects (Hodek, Reference Hodek2003; Jin et al., Reference Jin, Chen, Zhang, Tian, Feng and Hou2016; Socías et al., Reference Socías, Van Nieuwenhove, Casmuz, Willink and Liljesthrom2016). For example, the larvae of the wheat blossom midges, Contarinia tritici and Sitodiplosis mosellana (Diptera: Cecidomyiidae), need high soil moisture or rainy conditions for pupation after the temperature-regulated diapause development (Cheng et al., Reference Cheng, Long, Zhang, Liang and Zhu-Salzman2017). The decreased rate of adult eclosion under dry soil conditions has been attributed to the ability of dry soil to act as a mechanical barrier preventing adult emergence (Weston and Desurmont, Reference Weston and Desurmont2008; Johnson et al., Reference Johnson, Gregory, McNicol, Oodally, Zhang and Murray2010). Recently, inadequate soil moisture was found to promote prolonged diapause in already diapausing individuals of S. mosellana (Cheng et al., Reference Cheng, Long, Zhang, Liang and Zhu-Salzman2017). On the other hand, exposure to excessive moisture can be detrimental for the resumption of post-diapause development as well (Jin et al., Reference Jin, Chen, Zhang, Tian, Feng and Hou2016). Regardless of the amount of precipitation, the timing of rainfall and the persistence of moist soil may regulate diapause processes of insects, given that diapausing stages may only be sensitive to soil moisture for a short period and different soil textures have various water-retention capacities (Ma et al., Reference Ma, Tian, Zhao, Wei, Hoffman and Ma2017). A combined effect of moisture with temperature on post-diapause development rates has also been reported for Apolygus lucorum (Hemiptera: Miridae) (Jin et al., Reference Jin, Chen, Zhang, Tian, Feng and Hou2016), S. mosellana (Cheng et al., Reference Cheng, Long, Zhang, Liang and Zhu-Salzman2017) and Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) (Tauber et al., Reference Tauber, Tauber and Nyrop1994).
The European cherry fruit fly, Rhagoletis cerasi (Linnaeus) (Diptera: Tephritidae), is a highly destructive pest of cherries (Prunus spp.) (Rosaceae), in Europe and temperate regions of Russia and Asia, and has recently been detected in North America (White and Elson-Harris, Reference White and Elson-Harris1992; Daniel and Grunder, Reference Daniel and Grunder2012; Barringer, Reference Barringer2018). It is an univoltine species that undergoes obligatory winter diapause at the pupal stage in order to synchronize its flight period with the short seasonal period for oviposition in suitable fruits at a local scale (Boller and Prokopy, Reference Boller and Prokopy1976; Daniel and Wyss, Reference Daniel and Wyss2009; Daniel and Grunder, Reference Daniel and Grunder2012). Under field conditions, diapause termination usually takes place from the middle to the end of winter, and then pupae remain in post-diapause quiescence until temperature increases above 5°C, which is known to promote pupae development and adult emergence (Baker and Miller, Reference Baker and Miller1978; Papanastasiou et al., Reference Papanastasiou, Nestel, Diamantidis, Nakas and Papadopoulos2011). The patterns of R. cerasi adult emergence under field conditions differ among populations from ecologically different habitats due to geographical variation in diapause termination and post-diapause developmental rates (Papanastasiou et al., Reference Papanastasiou, Nestel, Diamantidis, Nakas and Papadopoulos2011; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014, Reference Moraiti, Nakas and Papadopoulos2017; Moraiti and Papadopoulos, Reference Moraiti and Papadopoulos2017). Even though patterns of local host fruiting are reliable predictors of the adaptive response of R. cerasi pupae to winter temperatures for diapause termination, they cannot explain the geographical variation in post-winter developmental rates of R. cerasi (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014, Reference Moraiti, Nakas and Papadopoulos2017). Interestingly, inter-annual (temporal) climatic variability results in two types of long life cycles within R. cerasi populations, expressed either as prolonged dormancy due to insufficient chilling (higher chilling temperatures and shorter chilling periods) or as a second, successive, facultative cycle of dormancy driven by an extended exposure to chilling (Vallo et al., Reference Vallo, Remund and Boller1976; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014). Thus, R. cerasi has evolved a complex dormancy strategy based on a combination of local adaptation and diversified bet-hedging strategies for ensuring population survival and reproduction at ecologically different habitats (Moraiti and Papadopoulos, Reference Moraiti and Papadopoulos2017).
The roles of temperature and geographic origin in diapause termination and post-diapause development of R. cerasi pupae have been thoroughly studied (Baker and Miller, Reference Baker and Miller1978; Papanastasiou et al., Reference Papanastasiou, Nestel, Diamantidis, Nakas and Papadopoulos2011; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014, Reference Moraiti, Nakas and Papadopoulos2017; Moraiti and Papadopoulos, Reference Moraiti and Papadopoulos2017); however, the impact of moisture and photoperiod on pupal diapause and post-diapause development is less explored. In this study, we investigated the effects of relative humidity and photoperiod on both diapause termination and post-diapause development of R. cerasi pupae that were exposed to various chilling regimes until adult emergence. Taking into account that R. cerasi pupae overwinter underneath host plants in a soil depth ranged usually from 2 to 5 cm based on soil type (Daniel and Grunder, Reference Daniel and Grunder2012) and that adult emergence is reduced in extremely wet environments and/or wet clay soils (Boller, Reference Boller1966), we examined the hypothesis that relative humidity but not photoperiod is a significant predictor of both diapause termination and post-diapause developmental duration of R. cerasi pupae. Photoperiod is not expected to have an impact on diapause traits because soil can act as a physical barrier for light, even though soils in the field have many cracks and pores in the surface made by plant roots and soil invertebrates, and those openings are likely to allow some light to come through (Gustin and Schumacher, Reference Gustin and Schumacher1989). Sexual differences in post-diapause developmental time were also assessed.
Materials and methods
We used R. cerasi pupae that were recovered from infested sweet cherries collected from an orchard in 2010 located at the experimental field of JKI in Dossenheim in the north part of Baden-Württemberg state, Germany, situated in the upper Rhine valley. This region falls under the ‘humid, warm temperate’ climate (Cfb) of the Köppen and Geiger climate classification: oceanic with warm summers and mild winters, with rainfall distributed throughout the year (Kottek et al., Reference Kottek, Grieser, Beck, Rudolf and Rubel2006; Peel et al., Reference Peel, Finlayson and McMahon2007). Flowering of cherry cultivars usually takes place during April, and the fruit ripening period lasts from beginning of June to the middle of July. In 2007–2010, mean spring temperatures ranged from 8 to 16°C with mean precipitation levels from 35 to 100 mm per month. In 2018, the shortest and longest day in Dossenheim was 8:08 h (start of winter, 22 December) and 16:14 h (start of summer, 21 June). Fruits were placed in plastic boxes with a grid bottom over a layer of dry sand (1 cm thick) allowing mature larvae to pupate at ambient temperature in a rain-protected hall. Pupae were sieved out of the sand twice per week or weekly and stored at room temperature for up to 2.5 months. Then pupae were put in transparent colourless 1.5 ml reaction vials (100 pupae per vial) with holes in the lid and cotton wool between pupae and lid. Light could reach the pupae as the vials were transparent and colourless and humidity could reach the pupae as the lid had holes. Pupae were assigned to the treatments at 4 ± 1°C.
Effect of photoperiod on diapause termination and post-winter development
To determine whether photoperiod is a significant predictor of diapause termination and post-winter development, R. cerasi pupae were exposed to different photoperiod conditions during the chilling period. Specifically, pupae were exposed to continuous light (24L:00D) and dark (00L:24D) conditions as well as to short- (08L:16D) and long-day photoperiod (16L:08D). Newly formed pupae collected in Dossenheim and stored as described above and finally put into vials (n = 100 pupae per vial) before being transferred to a cool chamber (4 ± 1°C, 75–80% RH) for a period ranging from 2 to 8.5 months. In total, 5600 pupae in 56 Eppendorf units were used for this experiment (4 treatments × 14 periods × 100 pupae). To achieve different photoperiod conditions, pupae were put in dark grey plastic boxes (60 × 40 × 34 cm, with two small openings, 3 × 14 cm each, at the top part of opposite sides), wherein a Neon lamp (Radium NL 85W/865, cool day light, spectralux plus; resulting in ~1000 lux at the position where the pupae were stored) and a ventilator to prevent heat up were had been installed. The light periods where regulated by timers. In case of dark conditions (00L:24D), pupae were put in an open box in the cool chamber. Every 15 days, one sample of 100 pupae from each treatment was transferred back to a climate chamber (25 ± 1°C during the light period of 16 h and 18 ± 1°C during the dark period of 8 h, 70 ± 5% RH,) until adult emergence was completed. Upon emergence, adults were sexed and counted for each treatment.
Effect of relative humidity on diapause termination and post-winter development
R. cerasi pupae were exposed to low (4041.7 ± 0.9%), medium (58.3 ± 0.9%) and high (75.6 ± 10% RH) relative humidity conditions during a chilling period ranging from 2 to 8.5 months to assess the effects of humidity on diapause. For this purpose, newly formed pupae from the same population in Dossenheim, stored as described above and finally put into vials (n = 100 pupae per vial) were placed at 4 ± 1°C (with a photoperiod of 00L:24D) for the mentioned chilling periods. In total, 4200 pupae in 42 vials were used for this experiment (3 treatments × 14 periods × 100 pupae). Low and medium humidity regimes were achieved in an exsiccator with MgCl2 and Mg(NO3)2, respectively. The standard humidity in the cool chamber was 75.6 ± 10% RH. Every 15 days, one sample of 100 pupae from each treatment was transferred back to a climate chamber (25 ± 1°C during the light period of 16 h and 18 ± 1°C, 70 ± 5% RH) until adult emergence was completed. Upon emergence, adults were sexed and counted in each treatment.
To estimate the duration of post-winter developmental period, time was recorded from the end of the chilling period to adult emergence (Stålhandske et al., Reference Stålhandske, Lehmann, Pruisscher and Leimar2015; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2017). The post-diapause development of R. cerasi pupae is likely to begin during exposure to chilling and thus an overlapping with the diapause termination phase may occur (AliNiazee, Reference AliNiazee and AliNiazee1988), particularly when chilling include exposure to temperatures ≥5°C (Baker and Miller, Reference Baker and Miller1978). Taking into account that the main criterion for both diapause termination and post-winter development of R. cerasi pupae in each photoperiod and relative humidity treatment is the number of emerging adults, it is of outmost importance to be capable of distinguishing environmental effects on diapause termination from those on post-chilling development. To this end, we assumed that the peak of adult emergence (>60% of pupae gave adults) is a milestone for diapause termination. In this sense, pupae of each treatment maintained in cold for a long enough period (minimum 2 months and maximum 8.5 months) for a proportion >60% of pupae to yield adults were used for assessing photoperiod and relative humidity effects on the duration of post-winter developmental period (for details see Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2017).
Statistical analyses
Binary logistic regression analysis was used to assess the effects of chilling period, relative humidity and photoperiod on adult emergence. The Cox proportional hazard model was used to assess the effects of: (1) photoperiod, (2) relative humidity and (3) sex on the duration of post-winter developmental period of R. cerasi pupae. Significant factors were entered in a multifactorial Cox regression model using a forward stepwise procedure for model selection. All statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
Effect of photoperiod on diapause termination and post-winter development
Diapause termination
Peak of adult emergence after chilling (>60% of pupae yielded adults) was recorded at (i) 4 months in 24L:00D and 08L:16D, and (ii) 4.5 months in 00L:24D and 16L:08D (fig. 1). Thereafter, adult emergence rates remained high (close to 80%) for all treatments, excluding 16L:08D where emergence rates fluctuated a great deal. Binary logistic regression revealed that photoperiod, chilling period and the interaction of photoperiod by chilling period were significant predictors of adult emergence (table 1).

Figure 1. Diapause termination of R. cerasi pupae from Dossenheim population (Germany) after chilling for a period ranged from 2 to 8.5 months. During chilling period, pupae were exposed to: (a) continuous light (24L:00D) (y = −1.2335x 2 + 23.67x − 24.736, R 2 = 0.9745), (b) dark conditions (00L:24D) (y = −1.004x 2 + 20.945x − 22.077, R 2 = 0.9743), (c) short photoperiod (08L:16D) (y = −1.3352x 2 + 25.487x – 31.066, R 2 = 0.9665) and (d) long photoperiod (16L:08D) (y = −1.3839x 2 + 22.904x – 24.877, R 2 = 0.7134).
Table 1. Variables of the binary logistic regression analysis exploring the effects of photoperiod and chilling period on diapause termination of R. cerasi pupae from Dossenheim population (Germany) after chilling for a period ranging from 2 to 8.5 months

Throughout chilling period, pupae were exposed to continuous light (24L:00D) and dark (00L:24D) conditions as well as to short (08L:16D) and long (16L:08D) photoperiod regimes. Dark conditions form the baseline.
Post-winter developmental time
For males, the average duration of post-winter developmental period of pupae that were exposed to short photoperiod (08L:16D), long photoperiod (16L:08D), light conditions (24L:00D) and dark conditions (00L:24D) during chilling ranged (a) from 20 ± 0.3 to 42 ± 2.2 days, (b) from 18 ± 0.3 to 38 ± 2.1 days, (c) from 19 ± 0.3 to 39 ± 0.8 days and (d) from 21 ± 0.2 to 36 ± 2.3 days, respectively. For females, the average duration of post-winter developmental period of pupae that were exposed to short photoperiod (08L:16D), long photoperiod (16L:08D), light conditions (24L:00D) and dark conditions (00L:24D) ranged (a) from 19 ± 0.3 to 35 ± 1.9 days, (b) from 18 ± 0.3 to 34 ± 1.1 days, (c) from 19 ± 0.5 days to 36 ± 2.8 days and (d) from 20 ± 0.2 to 37 ± 1.4 days, respectively (Supplementary Material, tables S1–S4, fig. S1).
Post-winter developmental time was the shortest for pupae that give adults (both males and females) upon peak of adult emergence after chilling under light conditions (for a period ranging from 2 to 8.5 months). Males emerged from pupae that remained in 08L:16D during chilling had the longest post-winter developmental time. On the other hand, long photoperiod conditions during chilling resulted in an extended post-winter developmental time for females (table 2). For all treatments no adults emerged after chilling for a period of only 2 months and females emerged earlier than males. Cox regression analysis revealed that both photoperiod and sex were significant predictors of post-winter development of R. cerasi pupae (table 3).
Table 2. Post-winter development (days ± SE) of R. cerasi males and females from Dossenheim population (Germany)

We used lots of pupae that yielded >60% adult (second period pupae) maintained at 4 ± 1°C for various time intervals before being transferred to room temperature for adult emergence. Throughout chilling period, pupae were exposed to continuous light (24L:00D) and dark (00L:24D) conditions as well as to short (08L:16D) and long (16L:08D) photoperiod regimes. The range is given in parenthesis.
Table 3. Variables of the Cox regression model exploring the effects of photoperiod and sex on the duration of the post-winter development of R. cerasi pupae from Dossenheim population (Germany)

We used lots of pupae that yielded >60% adult (second period pupae) which maintained at 4 ± 1°C for various time intervals before being transferred to a climate chamber for adult emergence. Throughout chilling period, pupae were exposed to continuous light (24L:00D) and dark (00L:24D) conditions as well as to short (08L:16D) and long (16L:08D) photoperiod regimes. Dark conditions and males form the baseline.
Effect of relative humidity on diapause termination and post-winter development
Diapause termination
After chilling emergence rates reached high levels (>60%) for (i) pupae that remained under medium and high relative humidity for 4 months, and (ii) pupae exposed to low relative humidity for 4.5 months (fig. 2). Binary logistic regression analyses revealed that only chilling period (χ 2 = 718.540, df = 2, P < 0.001) was a significant predictor of the proportion of pupae giving adults, as opposed to relative humidity (χ 2 = 0.514, df = 2, P = 0.773).

Figure 2. Diapause termination of R. cerasi pupae from Dossenheim population (Germany) after chilling for a period ranged from 2 to 8.5 months. During chilling period, pupae were exposed to: (a) low relative humidity (y = −1.0776x 2 + 21.311x − 19.566, R 2 = 0.9474), (b) medium relative humidity (y = −1.2157x 2 + 23.62x − 26.011, R 2 = 0.9624) and (c) high relative humidity (y = 1.1065x 2 + 22.157x − 22.533, R 2 = 0.9349).
Post-winter developmental time
For males, the average duration of post-winter developmental period of pupae that were exposed to low, medium and high relative humidity during chilling ranged (a) from 22 ± 0.3 to 39 ± 1.5 days, (b) from 21 ± 0.3 to 41 ± 0.9 days and (c) from 21 ± 0.2 to 39 ± 1.9 days, respectively. For females, the average duration of post-winter developmental period of pupae that were exposed to low, medium and high relative humidity during chilling ranged (a) from 20 ± 0.3 to 38 ± 2.2 days, (b) from 20 ± 0.2 to 37 ± 1.6 days and (c) from 20 ± 0.2 to 35 ± 0.9 days, respectively. For all treatments, no adults emerged after chilling for a period of only 2 months (Supplementary Material, tables S5–S7, fig. S2).
After the peak of adult emergence (60%), high relative humidity during chilling stimulated the fastest post-winter developmental time for both males and females (≈22 days). However, the post-winter developmental period for females and males remained short under low relative humidity conditions (40% RH) as well (table 4). In general, females emerged earlier than males. However, sex differences in post-winter developmental time were limited for adults emerged from pupae that were exposed to high relative humidity during chilling. Cox regression revealed that both relative humidity and sex were significant predictors of post-winter development of R. cerasi pupae (table 5).
Table 4. Post-winter development (days ± SE) of R. cerasi males and females from Dossenheim population (Germany)
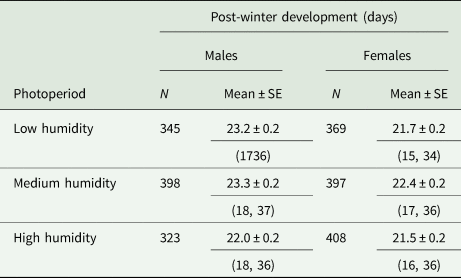
We used lots of pupae that yielded >60% adult (second period pupae) maintained at 4 ± 1°C for various time intervals before being transferred to a climate chamber for adult emergence. Throughout chilling period, pupae were exposed to low, medium and high relative humidity conditions.
Table 5. Variables of the Cox regression model exploring the effects of relative humidity and sex on the duration of the post-winter development of R. cerasi pupae from Dossenheim population (Germany)

We used lots of pupae that yielded >60% adult (second period pupae) which maintained at 4 ± 1°C for various time intervals before being transferred to a climate chamber for adult emergence. Throughout chilling period, pupae were exposed to low, medium and high relative humidity conditions. Males that remain in high humidity form the baseline.
Discussion
Our data revealed that the response of the R. cerasi pupae to photoperiod and relative humidity for concluding obligatory dormancy schedules differs between diapause termination and quiescence post chilling development. Together with chilling duration (under high humidity), photoperiod regulates diapause termination of R. cerasi pupae. On the other hand, different relative humidity regimes had no effect on diapause termination rates as revealed by adult emergence rates. Specifically, pupae from Dossenheim require at least 4–4.5 months under chilling in order to reach peak adult emergence rates (>60% of pupae to give adults), regardless of the photoperiod or relative humidity regime. On the other hand, post-winter developmental rates of pupae that were incubated at (optimum) high temperatures were affected by both relative humidity and photoperiod. High relative humidity or light conditions (24L:00D) were found to accelerate post-winter development of R. cerasi pupae, regardless of sex. Additionally, post-winter development of females remains short under medium relative humidity. Regardless of the photoperiod or humidity treatment, females completed post-winter development faster and thus emerged earlier than males. This result is in line with previous studies on R. cerasi populations (Baker and Miller, Reference Baker and Miller1978; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2017).
Photoperiod effects on diapause traits
Low temperature (winter chilling) is frequently the most important environmental signal for diapause termination in insects of the temperate zone (Tauber et al., Reference Tauber, Tauber and Masaki1986; Zhou et al., Reference Zhou, Gao and Pang2016). For R. cerasi, temperatures ranging from 3 to 10°C have proven to be optimal for pupae diapause termination. Populations from warmer habitats respond adaptively to higher temperatures within the above range. The optimum chilling period for diapause termination of R. cerasi pupae is population-specific in order to ensure timely adult emergence with host fruit availability at a local scale (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014). For sweet cherry cultivars chilling requirements for overcoming endodormancy are specific for each genotype and therefore determine geographic distribution of sweet cherry cultivars. For example, the earlier-flowering cultivars are those with the lowest chilling requirements and are expected to be cultivated in warmer areas (Fadón et al., Reference Fadón, Herrero and Rodrigo2007; Castède et al., Reference Castède, Campoy, García, Le Dantec, Lafargue, Barreneche, Wenden and Dirlewanger2014). Additionally, a minimum cold period (winter chill) is required before spring heat (forcing) can become effective (Heide, Reference Heide2008; Kaufmann and Blanke, Reference Kaufmann and Blanke2019). In line with chilling requirements of sweet cherry trees, R. cerasi pupae regardless of the other stimuli (photoperiod, humidity) did not yield adults after chilling for only 2 months at optimal low temperatures.
Besides chilling, our results revealed that the interaction between the photoperiod and chilling duration (at optimum low temperatures for diapause termination) was also a significant predictor for diapause termination of R. cerasi pupae. Pupae that terminated diapause under long photoperiod conditions exhibited a declining trend in adult emergence rates after the peak of diapause termination. Even though, in the current study, we did not examine the status of the remaining pupae (dead or overlaying pupae that enter prolonged dormancy), our previous study on diapause patterns of Dossenheim pupae revealed that the numbers of overlaying pupae increase after 6.2 months under similar chilling conditions while emergence rates decline (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014). Recently, long photoperiod and continuous illumination under warm conditions at the pupal stage found to extend the dormancy period and increased the proportion of overlaying Rhagoletis pomonella pupae from late-fruiting hawthorn Mexican populations (Rull et al., Reference Rull, Lasa and Aluja2019a), despite the fact that previous studies with North American R. pomonella populations found the larval but not the pupal as the sensitive stage to photoperiod (Prokopy, Reference Prokopy1968). Indeed, North American R. pomonella larvae exposed to continuous light and high temperatures become 100% non-dormant (Prokopy, Reference Prokopy1968). Given that R. cerasi pupae undergo an obligatory diapause and thus photoperiod has no effect on diapause induction, diapause intensity was not expected to be affected by photoperiod, which is usually reported in species following a photoperiod-inducing facultative diapause (Tauber and Tauber, Reference Tauber and Tauber1972; Nakamura and Numata, Reference Nakamura and Numata2000). Whenever diapause termination is regulated by both low temperature and photoperiod, photoperiodic responses to diapause termination can be highly temperature dependent. This suggests that low temperatures remain the key factor for terminating diapause while long daylength has a valid role only in promoting the uniform diapause termination of individuals in a population (Liu et al., Reference Liu, Luo, Zhang, Zhou and Wei2017). In addition, diapause response to both photoperiod and temperature is likely to be genetically different among geographical isolated populations that are located at different latitudes. For example, diapause termination of the multivoltine moth Helicoverpa armigera (Lepidoptera: Noctuidae) is highly sensitive to photoperiod in northern populations and temperature dependent in southern populations (Chen et al., Reference Chen, Chen, He, Xia and Xue2013). However, Filchak et al. (Reference Filchak, Roethele and Feder2001) found no effect of photoperiod on the genetics of R. pomonella. Further studies involving populations from different, distant, geographic areas are required to fully elucidate photoperiodic responses of R. cerasi pupae for diapause termination.
Photoperiod is a significant predictor of post-diapause developmental rates of many insects, including Dendrolimus punctatus (Lepidoptera: Lasiocampidae) (Zeng et al., Reference Zeng, Wang and Liu2013), Cydia pomonella (Lepidoptera: Tortricidae) (Liu et al., Reference Liu, Luo, Zhang, Zhou and Wei2017) and Laodelphax striatellus (Hemiptera: Delphacidae) (Wang et al., Reference Wang, Lin, Chen, Fu and Xue2014). In most cases, long daylength has a valid role in promoting the uniform diapause termination of individuals in a population. However, in our study, light conditions, as opposed to long daylength, seem to accelerate emergence of R. cerasi adults. Regarding other temperate Rhagoletis species, photoperiod, to the best of our knowledge, remain an unexplored factor regarding post-diapause development but other environmental factors, such as latitude and pre-chill duration, have been found to affect the post-diapause development. Specifically, an increased pre-chilling period at room temperature resulted in increased thermal requirement of Rhagoletis completa pupae (Emery and Mills, Reference Emery and Mills2019a), while latitude is suggested to have a negative effect on the thermal requirements of both Rhagoletis mendax and R. pomonella (Dambroski and Feder, Reference Dambroski and Feder2007) as well as R. completa pupae (Emery and Mills, Reference Emery and Mills2019a).
Relative humidity effects on diapause traits
For tephritids pupating in the soil, substrate moisture may influence pupae survival and emergence. Even though humid environments are known to increase survival of R. cerasi and R. pomonella pupae (Wakie et al., Reference Wakie, Yee and Neven2018; Rull et al., Reference Rull, Lasa and Aluja2019b), our results revealed that humidity had no influence on diapause termination of R. cerasi pupae. Response to substrate moisture has been found to be highly variable within Rhagoletis. For instance, Rhagoletis indifferens seems to be tolerant to a wide range of soil moisture regimes since adult emergence rates were found to be high (≥60%) under both dry and moist conditions (Yee, Reference Yee2013). On the other hand, dry soil and medium to low relative humidity (≤60%) prevented the emergence of adults of the apple maggot fly, R. pomonella (Trottier and Townshend, Reference Trottier and Townshend1979), while 80 and 100% RH resulted in 81 and 70% emergence, respectively (Neilson, Reference Neilson1964). For the walnut husk maggot fly, Rhagoletis suavis, 40% RH resulted in 15% adult emergence, whereas those of 90 and 100% resulted in 50–60% adult emergence (Beck, Reference Beck1932). Considering the above findings, it seems that a species-specific response of Rhagoletis pupae to humidity regimes for yielding adults exists.
On the other hand, our results reveal that relative humidity affects the post-winter development of R. cerasi pupae. Specifically, high relative humidity accelerates post-winter developmental rates of pupae for both sexes. High relative humidity is suggested to benefit adult insect emergence by reducing the risk of desiccation of soft-bodied adults (Yee, Reference Yee2013). In addition, emergence of R. cerasi adults often starts after a rainy period which increases soil penetration (Wiesmann, Reference Wiesmann1933). Rainfall and soil moisture were found to reduce the thermal requirements of pupae of R. pomonella (Smith and Jones, Reference Smith and Jones1991), which is less tolerant of low relative humidity than pupae of R. indifferens (Yee, Reference Yee2013). Even though rainfall during months preceding the first adult emergence can accelerate the timing of R. indifferens adult emergence (Song et al., Reference Song, Coop, Omeg and Riedl2003), flies can also emerge earlier when pupae are located in a relatively dry soil as long as relative humidity is high. This implies that relative humidity rather than soil moisture is the key factor regulating adult emergence rates in this species (Yee, Reference Yee2013). Additional field studies revealed that R. completa adult emergence, in walnut orchards in California, was neither affected by spring nor by winter precipitation levels (Emery and Mills, Reference Emery and Mills2019b). It seems therefore that soil humidity that is not always related to the precipitation level and/or soil moisture can serve as a reliable predictor of the post-diapause development of Rhagoletis sp. Indeed, recent studies revealed that humidity at 5 cm beneath the surface of both bare and grass-covered soils (where most Rhagoletis pupate) remained >60%, including summers, regardless of the irrigation status (Yee and Chapman, Reference Yee and Chapman2018). Thus, soil humidity remains sufficiently high even under low soil moisture conditions that are likely to be met in rainfed orchards during spring and summer.
Overall, our results revealed that R. cerasi pupae responds positively to high humidity for concluding post-winter development but the diapause termination processes remained unaffected. Previous studies confirmed that temperature is a significant predictor of both diapause termination and post-diapause development of R. cerasi pupae (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014; Moraiti and Papadopoulos, Reference Moraiti and Papadopoulos2017). Temporal variability of soil temperatures regulates diapause processes of R. cerasi pupae that overwinter within 5 cm of the soil surface, beneath host plants. However, moisture reduces the temporal variability of soil temperatures, which is expected to vary under both high temperature and low humidity conditions due to increased vapour pressure deficit (VPD) (Ashcroft and Gollan, Reference Ashcroft and Gollan2013). As a result, substantial fluctuation in soil temperatures is likely to take place in warm periods of the year that coincide with the post-diapause development period of R. cerasi pupae (Zhang et al., Reference Zhang, Peng, Zhao, Hoffmann, Li and Ma2016). On the contrary, in winter when diapause termination is progressing, VPD remains low, and soil temperature fluctuations are buffered. In addition, rainfall reduces the spatial variability in soil moisture (Buttafuoco and Castrignanò, Reference Buttafuoco and Castrignanò2005) leading to temperature patterns that are determined largely by elevation (Ashcroft and Gollan, Reference Ashcroft and Gollan2012). It is therefore plausible to suggest that humidity has an impact on post-diapause development of R. cerasi pupae through regulating spring soil temperatures fluctuations, as opposed to diapause termination that is mainly driven by spatial variability of chilling temperatures.
Conclusions
In sum, our data show that diapause termination of R. cerasi pupae is affected by low temperature treatments and photoperiod, whereas both relative humidity and photoperiod regimes have an impact on post-winter developmental time of both sexes, regardless of the high temperatures that prevail during this last part of pupae development. Our results underscore the need to thoroughly address the geographical variation in the response of different diapause stages to combinations of environmental factors such as temperature, photoperiod and relative humidity in order to determine plastic and adaptive dormancy responses in R. cerasi. An in-depth understanding of the impact of environmental factors on diapause development processes of R. cerasi pupae from populations located at ecologically and latitudinally different habitats will enable better prediction of population dynamics, and consequently more efficient pest management, especially under the projected changes in temperature and precipitation levels due to climate warming.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485320000073.
Acknowledgements
The present study was partially supported by the International Atomic Energy Agency grand (Coordinated Research Project (CRP) Dg5). We also thank Jürgen Just (JKI Dossenheim) for technical assistance.












